Manuscript accepted on : 14-07-2023
Published online on: 08-09-2023
Plagiarism Check: Yes
Reviewed by: Dr. Aparna Gunjal
Second Review by: Dr. Birhanu Babiye
Final Approval by: Dr. Eugene A. Silow
Sumita Sahoo1,2 , Biswajit Rath1
, Biswajit Rath1 , Keshab C. Mondal3
, Keshab C. Mondal3 , Suman Kumar Halder3
, Suman Kumar Halder3  and Arpita Mandal2*
and Arpita Mandal2*
1Department. of Biotechnology, Maharaja Sriram Chandra Bhanja Deo University, Baripada, Odisha, India.
2 Department of Microbiology, Asutosh College, Kolkata, West Bengal, India.
3Department of Microbiology, Vidyasagar University, Midnapore, West Bengal, India.
Corresponding Author E-mail: arpita.mandal@asutoshcollege.in
DOI : http://dx.doi.org/10.13005/bbra/3136
ABSTRACT: Present agriculture sector mostly depend on synthetic fertilizer for better crop. Feather is a rich source of protein and nitrogen. It was degraded by Keratinolytic bacteria Bacillus wiedmanni SAB10 in poultry litter sole media. Feather hydrolysate was produce from solid state fermentation process and fermentation condition was optimized through OVAT (One Variable At A Time) system. In this process feather (1.25%w/v) was fully degraded in poultry litter(1%w/v) with in 48 hrs at pH 10.After the fermentation cell free feather hydrolysate use in rice plant in different concentration and different mode. Liquid feather hydrolysate produced from solid state fermentation contain important amount of protein (3.12mg/ml) and amino acid(792µg/ml) that enhances the rice plant growth in pot trial condition. After application Group D Plants leaves have been reported to have higher levels of total chlorophyll (5.25mg/g of dry wt), IAA (17.23µg/ml)..Carbohydrate contain of rice has increased 1.6 fold than control Following the spraying of feather hydrolysate (300 µl/ml), the phenolic (1.71 fold) and flavonoid (1.52 fold) contents significantly increased.. The novelty of our investigation is we use here two wasted products and convert them a valuable product.
KEYWORDS: Agro ecosystem; Bacillus wiedmanni; Feather hydrolysate; SAB10 Rice plants
Download this article as:| Copy the following to cite this article: Sahoo S, Rath B, Mondal K. C, Halder S. K, Mandal A. Production Optimization of Feather Hydrolysate and Use as a Promising Nitogen-Rich Fertilizer for Rice (Oryza Sativa) Production. Biosci Biotech Res Asia 2023;20(3). |
| Copy the following to cite this URL: Sahoo S, Rath B, Mondal K. C, Halder S. K, Mandal A. Production Optimization of Feather Hydrolysate and Use as a Promising Nitogen-Rich Fertilizer for Rice (Oryza Sativa) Production. Biosci Biotech Res Asia 2023;20(3). Available from: https://bit.ly/3sMndNn |
Introduction
Low commercial value feathers wastes, which are produced in large quantities by chicken slaughterhouses, are difficult to decompose. Every year, 24 billion chickens are killed around the world, producing 8.5 billion tonnes of feathers, of which 350 million tonnes are produced by India alone 1. Currently, wasted feathers and poultry litter are either dumped or burned for disposal, both of which represent significant risks to health and the environment 2. Also waster feather causes several dangerous diseases like fowl cholera, chlorosis, mycoplasmosis 3. The present methods of treating poultry waste are chemical (acid and alkali) and physical (cooking or burning), both of which are costly, dangerous, and also cause contamination of the air, water, and soil 4. In comparison to conventional approaches, microbial bioconversion of poultry wastes have many benefits, in earlier many researchers has largely concentrated only on enzyme purification, enzyme degradation, and characterization of the degradation products, but little is known about its potential uses and outcomes of agriculture sector. As feather is a rich source of hard keratin protein and poultry litter is the high nitrogenous source so it can be efficiently used in plants growth and development. Presently most a vital organic fertilizer gain attention to the researcher that is poultry waste manure. The feather degrading bacterium Bacillus wiedmanni SAB10 (OM510278) hydrolyze the native chicken feather within 48 hours in poultry litter sole media. As feather hydrolysate is good source of amino acid like Leucine, valine, methionine, glycine, cysteine, serine, phenylalanine, tryptophan, lysine, and tyrosine1 is act as a biostimulants to plants and enhanced vegetative growth, Nutrients uptake , stress tolerance 5.
As rice is the common cereal crop produced worldwide. India and china are the most important country to produced rice globally. According to ministry statement in 2022 India produced 130.99 million tones rice and it is higher by 4.40 million tons higher than previous five years. West Bengal, Uttar Pradesh and Punjab are major rice producing states. Among them west Bengal is the largest rice producer state and produced alone 104.99 million tones rice. However, there are a number of strategies to uplift crop. For example, Improving rice yields requires effective nitrogen (N) fertilizer management broadly speaking Fertilizers containing the crucial plant nutrients nitrogen (N), phosphorus (P), and potassium (K) are necessary for effective crops 6. Despite the fact that fertilizers are necessary for agricultural production, using too much fertilizer and using it incorrectly can soil-related problems have been caused by chemically imbalanced NPK ratios and intense rice farming. Concerns such acidification, loss of organic matter, deterioration of the structure, and reductions of soil fertility 7. Crops produced in deteriorated soil are difficult to grow, and the work required to do so is significant. In this present investigation we use biotechnological approaches that utilize microorganisms and their fermentation products stands significant when they appear to be conceptually appropriate in process technology, replacing existing ways. Numerous researchers have earlier studied and created fermented feather hydrolysate for growing various plants. However, investigations have not given attention to the mode of Use, and different concentration of feather hydrolysate. Our investigation mainly focused on valorization of chicken feather, manages the environmental pollution, reduces the use of chemical fertilizer in the field, organic farming and economic development of farmer.
Materials and Methods
Chemicals and raw materials
The reagents, compounds, and solvents employed in this investigation all have acquired from local providers in analytical grade. Chicken feathers were the waste byproduct generated from the poultry processing house at Debra in West Midnapore, West Bengal, India (22.3660° N, 87.5503° E). Raw feathers were washed under tap water repeatedly, air dried, and then stored for future use. Fly ash was collected from a local rice mill .Rice seeds (amrapali) were purchased from local supplier.
Biodegradation of Chicken Feather in waste poultry litter as a sole medium in solid state fermentation and optimization of amino acid production through OVAT(One Variable At A Time) methodology
Waste poultry litter served as the only medium for the fermentation process used to degrade chicken feathers by the Bacillus wiedmanni SAB10. The fermentation process is initiated in flask condition. The fermentation condition were optimized through the OVAT protocol. Fermented chicken feathers that were removed after a specific amount of time during fermentation were used to study feather fibril dissociation and hydrolysis. Briefly, collected chicken feathers from different fermentation times (24, 48, and 72 h) were examined for visible degradation in comparison to controls, and the patterns of detachment and disintegration were recorded by periodically observing the treated feathers under a compound light microscope at 10x magnification.
Effects of cultural conditions on amino acid production in flask
Using waste poultry litter as the only medium, chicken feather as the only substrate, initial medium pH of 6 to 11, incubation temperatures of 25 to 50°C, inoculums volume of 0.5 to 4% (v/v), and incubation period, the optimization of total amino acid production by powerful bacterial strain Bacillus wiedmanni SAB10 was explored (0-72h).
Analysis of degraded end product
A predetermined amount of feather-hydrolysate was taken out at various time intervals (12 h, 24 h, 36 h, 48 h, 60 h, 72 h, and 84 h), passed through four layers of cheesecloth, centrifuged at 10,000 rpm for 5 min at 4 °C, followed by methanol extraction, and then the presence of free amino acids was determined using thin-layer chromatography (TLC) and high-performance liquid chromatography ( Several amino acids were utilized to make standard glycine, including glutamic acid, asparagine, cysteine, threonine, valine, lycine, methionine, hydroxyproline, isoleucine, phenylalanine, tryptophan, leucine, glycine, serine, alanine, tyrosine, arginine, and aspartic acid).
Total protein and amino acid estimation
The amount of protein in the culture supernatant was calculated using the Lowry method according to fermentation time (12-h interval after inoculation) 7. And amino acid was determined by ninhydrin method 9.
Response surface methodology for fermentation process optimization
Box-Behnken design (BBD) based on RSM was employed for optimization of four variable namely water content, pH, time and feather concentration on amino acid production under solid state fermentation. Three levels [low (-1), middle (0) and high (+1)] of each variable encompassing of 27 experimental run were conducted. A polynomial quadratic equation was adopted to evaluate the contribution of each independent variable on the responses (amino acid production).
Small scale pot culture experiment
In this work, rice was employed as an experimental plant. Rice is a well-known cereal crop that is grown all India. In this investigation, ten pots were employed. The typical garden soil in each pot weighs 2 kg and is adequately moist (70% w/v). Only soil is utilized as a control in one pot. As a negative control, one pots contained soil with chemical fertilizer. Another eight pots were used for different combination and dose of feather hydrolysate amino acid concentration.
Group A- 200µl/ml feather hydrolysate with fly ash (Root application)
Group B- 200 µl/ml feather hydrolysate (Foliage spray)
Group C- 300 µl/ml feather hydrolysate with fly ash (Root application)
Group D- 300 µl/ml feather hydrolysate (Foliage spray)
Group E- 400 µl/ml feather hydrolysate with fly ash (Root application)
Group F- 400 µl/ml feather hydrolysate (Foliage spray)
Group G – 500 µl/ml feather hydrolysate with fly ash (Root application)
Group H- 500 µl/ml feather hydrolysate (Foliage spray)
Group I- Commercial Chemical fertilizer
Group J- only soil
Effect of fermented hydrolysate on Rice plant
Phenotypic parameters
Following 90 Days from Showing (DAS), the randomly removed plants were properly washed with tap water, followed by regular saline, and then allowed to air dry. The plants were used to examine a variety of parameters, including shoot length, root length, branches, and overall grain weight of various treatments.
Microscopic examination
In a microscope with a magnification of 10 X also used to investigate the physiological characteristics of the xylem and phloem found in the roots of various treatment plants.
Chemical analysis of rice plant
After spraying the feather hydrolysate on the plants for seven days, fresh leaves were randomly taken from all of the variously treated plants. The collected samples were cleaned twice with normal saline, then they were blotted to dry.
Estimation of chlorophyll content
According to 10 methodologies, the chlorophyll content in the leaves of various treatment plants was estimated.
Estimation of free proline
The free proline present in the rice plant leaves was investigated by the method described by 11.
Estimation of IAA
IAA present in leaves was estimated by the method according to 12.
Estimation of free protein, amino acid and carbohydrates content in grain
Fresh grains were parboiling then upper layer of grain(Rice Husk) was separated. Rice seeds were taken for carbohydrate estimation by 13. The Lowry technique was used to determine the total protein content (lowery, 1951). Using the ninhydrin technique, the total amino acids present in the seeds of various treated plants were estimated 9
Analysis of total phenolic and flavonoid content in rice
To create homogeneous slurries, rice from variously treated plants was pulverised in a mixer grinder. 5 g of slurry were mixed in a 1:10 ratio with several solvents, including methanol, ethanol, and water, for extraction. After that, the flavonoid content was assessed using 14method. And the phenolic content estimated by the folin-Ciocalteu method.
Microbial analysis in soil
Rice plants were randomly selected from each treatment group to determine the amount of beneficial microorganisms that were present in the plant roots. The rice plant was gently pulled from the ground, its roots were cut into 1-cm pieces, and the excess soil was gently shaken off. To remove bacteria and fungi from the roots .5 g were put into tubes with 45 ml of sterile, distilled water and sonicated for 15 minutes. To count all of the microorganisms in the liquid, successive dilutions were used. For phosphate solubilizers, Pikovskaya’s medium was employed; for N 2 fxers, Ashby N-free sucrose medium was used. Dubey & Maheshwari et al. Colony forming units (CFU) per gm of dry matter were used to express the quantity of phosphate-solubilizing and nitrogen-changing bacteria.
Statistical analysis
Data is presented as the mean of values in the figures and tables. Each experiment was carried out separately in triplicates. They estimated standard deviation from means. To statistically support our findings, we performed the student’s t-test. P values under 0.05 were regarded as significant.
Result
The microbial population gradually evolves in response to environmental pollution and climate change, developing some capacity for reproduction and colonization in quiet environments. This is actually due to a few remarkable enzymes that can transform resistant molecules into beneficial metabolites for both themselves and other organisms. One of these enzymes to breakdown keratinous waste is keratinase. Feathers and litter from chickens are among the richest sources of keratin. Such waste products may biodegrade and then be used as fertilizer in crop production. Our research is primarily concerned with commercializing the liquid fertilizer produced using a newly discovered bacteria and a cost-effective technique.
Analysis of feather hydrolysate
After 48 hours completely degraded feather hydrolysate was taken for centrifugation and then cell free supernatant was measured for protein content 3.12mg/ml , amino acid 792µg/ml .
Optimization of amino acid production through OVAT method and analysis of feather degradation pattern
In laboratory scale solid state fermentation condition bacterium produce 775.33µg/ml amino acid within 48 hrs fermentation period with 1%(w/v) poultry litter concentration and 1.25% (w/v) feather at pH 10 in a flask at 400 C temperature( Fig 1a). By studying the feather degradation patterns under a microscope, this phenomenon is further proved (Fig 1b,1c, 1d).
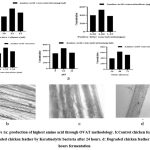 |
Figure 1a: production of highest amino acid through OVAT methodology b: Control chicken feather. c: Degraded chicken feather by Keratinolytic bacteria after 24 hours. |
d: Degraded chicken feather after 48 hours fermentation
RSM optimization of amino acid production
ANOVA of the quadratic model suggested the model was significant (F-value of 67.64, P-value <0.0001). The statistical measurements like coefficient of variation (CV) and coefficient of determination (R2) were taken under consideration, and estimated to be 3.19 and 0.9875, respectively which confirm adequacy of the RSM model. The model also exhibited statistically insignificant lack-of-fit (P = 0.4958). Adequate precision value of 26.2731 indicated that the models can be used to navigate the design space. The response surface and contour plots were plotted to study the interactions of major factors for the production of amino acids. The foremost significant mutual interactions for amino acids production by Bacillus wiedmanni SAB10 were represented in Fig 2.
Through numerical optimization solutions with higher desirability suggested that the maximum production of amino acid(795.07 µg/ml) can be achieved when water of 11.34 ml, pH of 10.89, 55.79 h time and 1.28 % feather concentration will provide in the flask, which was further validated by performing fermentation with the specified conditions. The result revealed that the experimental result (797µg/ml) is in good agreement with the predictive result. All the results provide in table 1. Production of microbial metabolites needed standardization of the physico-chemical environment that trigger the metabolite production followed by scale up.
 |
Figure 2: Response surface plot showing interaction between different variable for amino acid production |
Table 1: Experimental design used in the RSM studies of four independent variables for amino acid productions
| Run | Water | pH | Time | Feather | Actual AA(µg/ml) | Predicted AA(µg/ml) |
| 1 | 11 | 9 | 48 | 1.5 | 712 | 719.67 |
| 2 | 10 | 10 | 72 | 1.25 | 613 | 610.50 |
| 3 | 12 | 9 | 48 | 1.25 | 663 | 686.25 |
| 4 | 11 | 9 | 48 | 1 | 642 | 650.17 |
| 5 | 10 | 10 | 48 | 1 | 665 | 666.92 |
| 6 | 11 | 10 | 48 | 1.25 | 792 | 775.33 |
| 7 | 10 | 10 | 24 | 1.25 | 404 | 425.33 |
| 8 | 11 | 9 | 72 | 1.25 | 572 | 551.75 |
| 9 | 11 | 10 | 72 | 1 | 510 | 518.58 |
| 10 | 10 | 11 | 48 | 1.25 | 723 | 697.25 |
| 11 | 12 | 10 | 72 | 1.25 | 614 | 599.67 |
| 12 | 11 | 11 | 72 | 1.25 | 675 | 693.42 |
| 13 | 12 | 10 | 48 | 1 | 665 | 659.58 |
| 14 | 12 | 10 | 24 | 1.25 | 446 | 455.50 |
| 15 | 11 | 11 | 24 | 1.25 | 392 | 407.75 |
| 16 | 12 | 10 | 48 | 1.5 | 712 | 705.58 |
| 17 | 12 | 11 | 48 | 1.25 | 765 | 758.42 |
| 18 | 11 | 9 | 24 | 1.25 | 531 | 508.08 |
| 19 | 10 | 9 | 48 | 1.25 | 724 | 728.08 |
| 20 | 11 | 10 | 24 | 1 | 466 | 453.42 |
| 21 | 11 | 10 | 48 | 1.25 | 777 | 775.33 |
| 22 | 10 | 10 | 48 | 1.5 | 678 | 678.92 |
| 23 | 11 | 11 | 48 | 1 | 712 | 711.33 |
| 24 | 11 | 10 | 24 | 1.5 | 394 | 382.92 |
| 25 | 11 | 11 | 48 | 1.5 | 701 | 699.83 |
| 26 | 11 | 10 | 48 | 1.25 | 757 | 775.33 |
| 27 | 11 | 10 | 72 | 1.5 | 637 | 647.08 |
Pot trial
Phenotypic Character analysis
The yield in rice plants is greatly increased in the current experiment by spraying fermented feather hydrolysate. In Group D condition spraying with fermented feather hydrolysate increased near 1.69 folds overall grain weight than control plant, and it also increased 1.18 fold than other treated condition. (Table -2 ). So the foliar spray was chosen as the application method for feather hydrolysate. Earlier, several researchers claimed that applying foliage to various crop production methods produces superior results 14. Selection of dose and mode of application were selected based on another phenotypic and biochemical character of plants such as shoot length, root length, Sheaf of paddy, number of branching and overall grain yield. In Group C and D shoot length (1.34 & 1.37 fold) and root length (1.55 & 1.66 fold) significantly higher than control group J. (Table- 2). The sheaf of paddy in Group D plants increased near 1.4 fold than untreated plants.( Fig 3) Microscopic analysis showed that size of xylem and phloem look healthier in Group D plants. (Fig 4).
Table 2: Morphological character of rice plants
| Groups | Shoot length (cm) | Root length(cm) | Sheaf of paddy(cm) | No of Branching | Total weight of paddy(gm) | Total weight of Rice(gm) |
| Gr- A | 74.16±
0.23a |
3.5±0.49a | 18.14±0.68a | 43 ±
0.63a |
46.83±
0.62a |
29.26±
0.60a |
| Gr- B | 70.66±
0.47a |
3.50±
0.62a |
21.1±
0.69a |
46 ±
0.49a a |
41.33±
0.47a |
30.83±
0.61a |
| Gr-C | 90.33±
0.47b |
4.4
±0.66b |
20.16±
0.55a |
51±
0.40b |
49.33±
0.41a |
34.83±
0.38b |
| Gr- D | 91.29±0.50b | 4.43±0.73b | 28.03±0.77b | 51±0.73b | 54.03±0.81b | 38.13±0.28b |
| Gr- E | 78.33±0.47a | 3.7±0.74a | 20.267±0.71a | 46±0.49a | 44.67±0.47a | 30.81±0.58a |
| Gr- F | 68.86±0.57a | 2.88±0.41c | 19.48±
0.68a |
46±
0.37a |
48.4±
0.43a |
34.04±
0.31b |
| Gr- G | 73.36±
0.57 a |
3.69±
0.72a |
19.12±
0.58a |
47±
0.67a |
53.16±
0.62b |
37.18±
0.47b |
| Gr – H | 72.49±
0.36 a |
2.43±
0.70c |
18.1±
0.56a |
41±
0.51a |
41.83±
0.62a |
28.91±
0.34a |
| Gr- I | 81.43±
0.41c |
3.01±
0.79a |
22.23±
0.57b |
49±
0.69a |
45.66±
0.47a |
32.10±
0.45b |
| Gr- J | 66.8±
0.58a |
2.46±
0.63c |
19.93±
0.73a |
41±
0.59a |
39.68±
0.48c |
28.13±
0.70a |
Each value is mean ±SD of triplicates means with different subscript letter within each column are statistically significant at P< 0.05
 |
Figure 3: Measurement and Visual analysis of paddy sheaf |
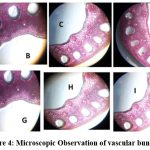 |
Figure 4: Microscopic Observation of vascular bundle |
Chlorophyll Content analysis
The pigments most necessary for photosynthesis are chlorophylls. Rice plants treated with feather hydrolysate in Group D have higher chlorophyll content (5.05±0.78mg/gm) followed by other treated groups. (Table -3 ).
Estimation of Proline
Accumulation of proline in plants leaves was more similar in Group C and D (1.06±0.78 mg/gm & 1.02±0.79 mg/gm) where as control Group J had proline content (1.14±0.73mg/gm). all the data given in table 3.
Analysis of IAA synthesis
The most essential phytohormone IAA content was significantly higher in Group D plants (17.03±0.60µg/ml) followed by other treatment groups. It was nearly 2.4 fold higher than control Group J.(Table- 3 ). The primary phytohormone that promotes plant growth and development, including cell plasticity and tissue elongation, is IAA 20. Previous studies indicated that tryptophan amino acid, which is the precursor of IAA production, is present in feather hydrolysate 21. So it enhances the IAA production in the plants that helps in plant growth.
Table 3: Biochemical analysis of rice plants leaves
| Groups | Chlorophyll(mg/gm) | Proline(mg/gm) | IAA(µg/ml) |
| Gr- A | 2.10±0.79a | 1.01±0.72a | 10.4±0.64a |
| Gr- B | 3.18±0.78b | 0.74±0.64b | 11.02±0.59b |
| Gr-C | 3.94±0.63b | 1.06±0.78a | 13.68±0.49b |
| Gr- D | 5.05±0.78c | 1.02±0.79a | 17.03±0.60b |
| Gr- E | 4.01±0.67b | 1.06±0.75a | 14.8±0.51b |
| Gr- F | 2.89±0.55a | 1.04±0.77a | 10.97±0.63a |
| Gr- G | 3.21±0.75b | 1.04±0.75a | 14.46±0.66b |
| Gr- H | 3.61±0.56b | 1.03±0.77a | 11.43±0.73b |
Each value is mean ±SD of triplicates means with different subscript letter within each column are statistically significant at P< 0.05
Carbohydrate, Protein and amino acid content analysis in Rice
Rice is the major source of carbohydrate that provides energy to the human. Carbohydrate content is higher in Group D (21.56±0.42mg/gm) than any other treatment groups. (Table- 4).Analysis of protein estimation clearly indicates that Group D (15.04±0.15mg/gm) showed higher protein synthesis than any other treatment group and the result showed similar train in case of Amino acid content.(78.09±0.64µg/gm ).
Estimation of phenolic and flavonoid content
The most powerful natural antioxidants are polyphenols, which are extensively present in plant species 21. In our present investigation phenolic and flavonoid content were high in Group D (22.09±0.75mg/gm & 18.18±0.41 mg/gm) plants than untreated and all treated groups. ( Table- 4 ).
.Table 4: Biochemical assessment of rice grain
| Groups | Total Carbohydrate (mg/gm) |
Total protein (mg/gm) |
Total Amino acid (µg/gm) |
Total Phenolic content(mg/gm) |
Total Flavonoid content(mg/gm) | ||||
| AE | ME | EE | AE | ME | EE | ||||
| Gr-A | 10.5 ±0.64a |
6.78 ±0.48a |
51.89 ±0.59a |
9.33 ±0.57a |
9.98 ±0.64a |
10.1 ±0.77a |
4.95±
0.66a |
8.9±
0.57a |
7.43 ±0.53a |
| Gr- B | 10.23 ±0.77a |
9.08 ±0.74a |
58.91 ±0.49a |
11.08 ±0.68b |
12.82 ±0.57b |
14.05 ±0.60b |
7.4±
0.43a |
9.01±
0.66a |
7.57±
0.42a |
| Gr- C | 20.18 ±0.36b |
11.97 ±0.57b |
72.09 ±0.76b |
19.14 ±0.78b |
20.21 ±0.78b |
17.65 ±0.40b |
14.02±
0.73b |
13.01333±
0.34b |
12.993±
0.45b |
| Gr- D | 21.56 ±0.42b |
15.04 ±0.15b |
78.09 ±0.64b |
22.09 ±0.75c |
21.56 ±0.43c |
21.05 ±0.760c |
17.25±
0.67b |
18.18±
0.41b |
16.55±
0.77b |
| Gr- E | 17.55 ±0.69b |
10.91 ±0.54a |
65.81 ±0.52c |
17.07 ±0.18b |
18.03 ±0.77b |
15.84 ±0.59b |
11.82±
0.57b |
12.78±
0.63b |
10.94±
0.65a |
| Gr- F | 11.75 ±0.45b |
9.08 ±0.68a |
61.86 ±0.57c |
14.17 ±0.61b |
15.19 ±0.73b |
12.84±
0.53b |
8.61±
0.53a |
9.92±
0.57a |
7.52±
0.66a |
| Gr- G | 14.76 ±0.45c |
8.88 ±0.53a |
68.78 ±0.49c |
17.80 ±0.63b |
18.91 ±0.56b |
16.65±
0.70b |
13.38±
0.76b |
14.4±
0.77b |
11.147±
0.72b |
| Gr- H | 14.06 ±0.68c |
11.79 ±0.55b |
63.62 ±0.79c |
17.28 ±0.41b |
19.04 ±0.71b |
18.41±
0.63b |
11.84±
0.51b |
14.11±
0.32b |
10.18±
0.68a |
| Gr- I | 11.311 ±0.41c |
8.07 ±0.67a |
57.17 ±0.79a |
12.03 ±0.73b |
12.92 ±0.58b |
10.92±
0.57a |
6.03±
0.74a |
7.84±
0.38a |
5.12±
0.79 |
| Gr- J | 9.11 ±0.70a |
6.18 ±0.73a |
51.72 ±0.72a |
8.55 ±0.62a |
8.86 ±0.60b |
6.86±
0.48a |
4.23±
0.77a |
4.84±
0.50a |
3.13±
0.72a |
Each value is mean ±SD of triplicates means with different subscript letter within each column are statistically significant at P< 0.05
Soil microbial population analysis
After treatment with feather hydrolysate, the microbial population on the photosphere and rhizosphere did not significantly change, but it was very intriguing to note that the fermented hydrolysate affected the growth of PGP bacteria in the rhizosphere and phyllosphere while only slightly inhibiting the growth of fungal strains in the phyllosphere. A comparable outcome was previously reported 25. Our hypothesis is that using this liquid fertilizer reduced leaf microbial diversity variance and increased the amount of beneficial microbes. (fig- 5 & 6).
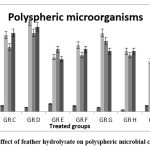 |
Figure 5: Effect of feather hydrolysate on polyspheric microbial community |
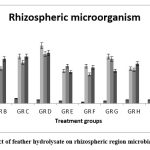 |
Figure 6: Effect of feather hydrolysate on rhizospheric region microbial community |
Discussion
Using only waste poultry litter as a support medium and under extremely high pH conditions, the bacterium is capable of growing and producing keratinase, an enzyme that rupturing the disulfide bonds as well as peptide bonds between amino acid residues in the keratin protein present in feathers, and degrading chicken feathers.
Response surface methodology (RSM) is one of the globally accepted statistical tools to search the optimum conditions in multifactorial bioprocess which is techno-economically proficient than univariate optimization strategies. Box-Behnken design (BBD) based on RSM was employed to find out optimum level of four variables on amino acid production during fermentation of feather by Bacillus wiedmanni SAB10.
The availability of Zn to the plants, as well as nutrients like N, Mg, S, Ca, Fe, and Mn, is necessary for the production of chlorophyll 16. These metals were sufficiently present in the feather hydrolysate, which may have increased the chlorophyll concentration. It is well established that plants with more chlorophyll content are physically healthier, and that plants with insufficient nutrient inputs exhibit poor development and chlorosis 17.
According to earlier research, plants accumulate proline as a defence mechanism against adverse environmental conditions 18 the accumulation of proline may also take place for developmental goals under non-physiologically stressful circumstances, 19.according to some data. As a metabolite and signal molecule for flower growth and enhancement as well as increased demand during protein synthesis, proline buildup can play a significant function.
According to earlier research, plants accumulate proline as a defence mechanism against adverse environmental conditions 18 the accumulation of proline may also take place for developmental goals under non-physiologically stressful circumstances, 19.according to some data. As a metabolite and signal molecule for flower growth and enhancement as well as increased demand during protein synthesis, proline buildup can play a significant function.
The primary phytohormone that promotes plant growth and development, including cell plasticity and tissue elongation, is IAA 20. Previous studies indicated that tryptophan amino acid, which is the precursor of IAA production, is present in feather hydrolysate 21. So it enhances the IAA production in the plants that helps in plant growth.
Carbohydrate, Protein and amino acid result clearly indicate that feather hydrolysate contain essential amino acid that is the responsible for the upliftment of the biochemical properties of rice and induce the protein assimilation in the grain that also increased biomass of rice.
Polyphenols play a role in plants’ defence mechanisms against biotic and abiotic stress and influence plant hues. Since polyphenols are present in all plant organs, they are essential to the human diet because they provide as a secondary dietary supply of different antioxidant phytochemicals22. The antioxidant effects of polyphenols may be related to their redox characteristics, which enable them to function as reducing agents or hydrogen/electron donors . Antioxidants are beneficial to human health and lower the risk of cardiovascular disease and cancer23. Reactive oxygen species-related cell damage is also repaired 24. Since synthetic antioxidants are harmful and carcinogenic, natural polyphenol antioxidants from plant sources have come to light as having preventive properties against several degenerative disorders. Prior studies have shown increases in polyphenol levels in crops treated with organic waste, indicating that organic waste may trigger modifications that encourage the accumulation of antioxidants1.
Conclusion
Utilizing microbial fermentation processes has a part in all newly emerging fields of research. In the past, feather wastes were merely placed, dumped, and used as land fill. As a fertilizer, it is regarded as slow-release. For the first time, it has been proven that the hydrolysate displayed a potential influence on boosting plant development after fermentation with the proper bacteria. Modulation of the quality and composition of the soil, including the main nutrients and microbial composition, mediates the effects that promote plant growth. The hydrolysate can be thought of as an inexpensive, long-lasting fertilizer that promotes plant development because even a single dose has demonstrated prolonged effects. The production of feather hydrolysate from feather and poultry litter will increase the agricultural sector’s viability and sustainability and may really help to lessen the need for chemical fertilizers. The suggested method for the first time highlighted the volatilization of feather wastes as a substitute step toward the construction of an eco-friendly and economically advantageous method of making sustainable fertilizer. The outcomes showed that this technique will serve as a bioenhancer to lessen the use of chemical fertilizers in agriculture and to solve the problem of treating or disposing of poultry waste. With such economical methods, resistance waste could be reduced and a worthwhile product could be produced, opening up several possibilities for further study and massive commercial manufacturing.
Acknowledgements
Authors greatly acknowledge to the Dept. of Microbiology, Asutosh College, Kolkata-700 026, West Bengal, India for providing the research space to elaborate this study. We are also thankful to the Dept. of Biotechnology, Raja Sri Ram Chandra Bhanja Deo University, Baripada- 757 003, Odisha, India for their assistance in detailing the research study.
Conflict of Interest
All the authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Funding sources
The research work is financially supported by the Science and Engineering Research Board (SERB) [Grant No.-SRG/2020/000873], Govt. of India.
References
- Gurav R.G, Jadhav J.P. A novel source of biofertilizer from feather biomass for banana cultivation. Environmental Science and Pollution Research., 2013; 20(7):4532-4539.https://doi.org/10.1007/s11356-012-1405-z.
CrossRef - Bose A, Pathan S, Pathak K, Keharia H. Keratinolytic protease production by Bacillus amyloliquefaciens 6B using feather meal as substrate and application of feather hydrolysate as organic nitrogen input for agricultural soil. Waste and biomass valorization., 2014; 5: 595-605.
CrossRef - Agrahari S, Wadhwa N. Degradation of chicken feather a poultry waste product by keratinolytic bacteria isolated from dumping site at Ghazipur poultry processing plant. Int J Poult Sci., 2010; 9(5): 482-489.
CrossRef - Ramnani P, Gupta R. Optimization of medium composition for keratinase production on feather by Bacillus licheniformis RG1 using statistical methods involving response surface methodology. Biotech. Appl. , 2004; 40: 491–496.
CrossRef - Aroca Ricardo et al. “Drought, abscisic acid and transpiration rate effects on the regulation of PIP aquaporin gene expression and abundance in Phaseolus vulgaris plants. Annals of botan., 2006; 98(6): 1301-1310.
CrossRef - Moe K, Htwe A. Z, Thu T.T.P, Kajihara Y, Yamakawa T. Effects on NPK status, growth, dry matter and yield of rice (Oryza sativa) by organic fertilizers applied in field condition. Agriculture., 2019; 9(5):109.
CrossRef - Zhong W.H, Cai Z.C. Long-term effects of inorganic fertilizers on microbial biomass and community functional diversity in a paddy soil derived from quaternary red clay. Applied Soil Ecology., 2007; 36(2-3): 84-91.
CrossRef - Lowry O.H, Rosebrough N.J, Farr A.L, Randall R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem., 1951; 193: 265–275.
CrossRef - MOORE , STEINH. A modified ninhydrin reagent for the photometric determination of amino acids and related compounds. J. Biol. Chem., 1954; 211(2): 907-13.
CrossRef - Arnon D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol., 1949; 24:1-15.
CrossRef - Bates L., Waldren R.P., Teare I.D. Rapid determination of free proline for water stress studies. Plant Soil. 1973; 39:205-207.
CrossRef - Malik K, Sindhu S.S. Production of indole acetic acid by Pseudomonas sp.: effect of co-inoculation with Mesorhizobium sp. Cicer on nodulation and plant growth of chickpea (Cicer arietinum). Physiol. Mol. Biol. Plants., 2011; 17(1):25–32.
CrossRef - PlummerT. An Introduction to Practical Biochemistry. third Edition. pp-179. 1990 .
CrossRef - Chang C.C, Yang M.H, Wen H.M, Chern J.C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. food drug anal., 2002; 10(3).
CrossRef - Sadak S, Abdelhamid M.T, Schmidhalter U. Effect of foliar application of amino acids on plant yield and some physiological parameters in bean plants irrigated with seawater. Acta. biol. Colomb.,2015; 20(1): 141-152.
CrossRef - Neales T.F. Components of the total magnesium content within the leaves of white clover and perennial ryegrass. Nature., 1956; 177: 388-389.
CrossRef - Zarco-Tejada P.J, Miller J.R, Morales A, Berjón A, Agüera J. Hyperspectral indices and model simulation for chlorophyll estimation in open-canopy tree crops. Remote sensing of environment., 2004; 90(4): 463-476.
CrossRef - Verbruggen N, Hermans C. Proline accumulation in plants: a review. Amino acids., 2008; 35: 753-759.
CrossRef - Mattioli R, Costantino .P, & Trovato M. Proline accumulation in plants: not only stress. Plant signaling & behavior., 2009; 4(11): 1016-1018.
CrossRef - Teale W.D, Paponov I.A, Palme K. Auxin in action: signaling, transport and the control of plant growth and development. Nature reviews Molecular cell biology., 2006; 7(11): 847-859.
CrossRef - Tamreihao K, Mukherjee S, Khunjamayum R, Devi L. J, Asem R.S, Ningthoujam D.S. Feather degradation by keratinolytic bacteria and biofertilizing potential for sustainable agricultural production. Journal of basic , 2019; 59(1): 4-13.
CrossRef - Karaman Ş, Tütem E, Başkan K.S, Apak R. Comparison of total antioxidant capacity and phenolic composition of some apple juices with combined HPLC–CUPRAC assay. Food Chemistry., 2010; 120(4): 1201-1209.
CrossRef - Le K, Chiu F, & Ng K. Identification and quantification of antioxidants in Fructus lycii. Food chemistry., 2007; 105(1): 353-363.
CrossRef - Kura B, Szeiffova Bacova B, Kalocayova B, Sykora M, Slezak J. Oxidative stress-responsive microRNAs in heart injury. International Journal of Molecular Sciences., 2020; 21(1): 358.
CrossRef - Tang T, Zhang Y, Wang F, Mao T, Guo J, Guo X, You J. Paenibacillus terrae PY8 controls Botrytis cinerea and modifies the endophytic microbial community of the medicinal plant, Paris polyphylla. Biological Control., 2022; 169: 104888.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





