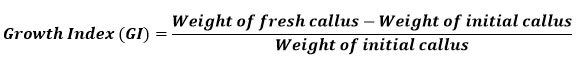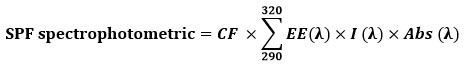Manuscript accepted on : 25-08-2023
Published online on: 03-10-2023
Plagiarism Check: Yes
Reviewed by: Dr Sabyasachi Banerjee
Second Review by: Dr. Anoma Dongsansuk
Final Approval by: Dr. Muhammad Hamayun
Jasmonic Acid: Enhancing SPF Potential in Butea monosperma Floral Variants Callus Cultures
Manali Maruti Sasane  and Indu Anna George*
and Indu Anna George*
Department of Life Sciences, University of Mumbai, Vidyanagri, Kalina, Santacruz East, Mumbai. Maharashtra, India.
Corresponding Author E-mail: indu.george@lifesciences.mu.ac.in
DOI : http://dx.doi.org/10.13005/bbra/3143
ABSTRACT: Butea monosperma (Lam.) Taub. or "Flame of the forest" (Fabaceae) is famous for its bright scarlet flowers. A rare variant – Butea monosperma var lutea, with chrome yellow flowers has been sighted in various locations in the country. The current study explored the differences between the two varieties of Butea monosperma (Lam.) Taub in terms of callus growth (monitored for 60 days) evaluated for total phenolic and flavonoid content, SPF and the effect of jasmonic acid on these parameters. A good correlation of 0.73 between SPF and TFC was obtained with the application of jasmonic acid. Assessment of phytochemical composition of callus from both varieties revealed the presence of different isoflavones, flavones, flavanones, chalcones classes of flavonoids that could possibly be responsible for good SPF. The growth index was the highest for the red variety in media without the elicitor, contrary to the results of the yellow variety, where 1 µM JA was found to increase the growth index. Differences were also noticed in the total phenolic content (173.84 mg GAE/g and 165.65 mg GAE/g) total flavonoid content (10.50 mg QE/g and 6.31 mg QE/g) and Sun Protection Factor (19 and 14.5) between the red and yellow variant callus respectively.
KEYWORDS: Butea Monosperma Variants; Elicitation; Flavonoid; Jasmonic Acid; Phenolic; SPF
Download this article as:| Copy the following to cite this article: Sasane M. M, George I. A. Jasmonic Acid: Enhancing SPF Potential in Butea monosperma Floral Variants Callus Cultures. Biosci Biotech Res Asia 2023;20(3). |
| Copy the following to cite this URL: Sasane M. M, George I. A. Jasmonic Acid: Enhancing SPF Potential in Butea monosperma Floral Variants Callus Cultures. Biosci Biotech Res Asia 2023;20(3). Available from: https://bit.ly/3EZObnh |
Introduction
Sunlight, particularly ultraviolet A (UVA) and ultraviolet B (UVB) rays can trigger the production of free radicals, harming mitochondrial enzymes and plasma membranes as well as lowering skin’s antioxidant levels. 1. Moreover, UVB of sunlight interacts with cellular chromophores in the skin’s upper epidermis, leading to DNA damage, which is associated with photoaging, photo immunosuppression, photphotocarcinogenesis and skin burns 2. It is necessary to produce sunscreen formulations to heal sunburn, suntan, skin cancer and premature skin aging as well as to enhance the degree of Sun Protection Factor because of the detrimental effects of UV radiation 3. Protective agents in the sunscreen or body lotion mitigate the effect of these UV rays on the skin. These agents could also cause adverse effects that range from hypersensitivity, irritant dermatitis or severe allergic reactions 4,5. A suggestion from WHO that medicinal plants could be the best source for a variety of drugs, coupled with a reports such as 80% of a population surveyed preferred plant-based drugs and products which contain bioactive secondary metabolites prompted most researchers to screen phytochemical constituents to develop more efficient and desirable therapeutic agents 6. Some of the major phytoconstituents are polyphenols, flavonoids, terpenes, catechol and alkaloids. Among numerous plant chemicals, flavonoids have received the greatest attention for their potential use as sun filters as their structures have cyclic and aromatic rings, which absorb UV light, in the range 240–285 nm and 300–550 nm. Flavonoids, one of the largest family of natural compounds and have numerous essential impacts on plants, mostly in terms of defence against diseases and UVB radiation 7.
Butea monosperma (Lam.) Taub commonly known as Palash, is a medium-sized deciduous tree of the Fabaceae family 8. It grows in the tropical and subtropical areas of the Indian subcontinent. The tree bears a beautiful canopy of orange-red flowers during early summer (Fig 1) earning its name ‘Flame of the forest’ 9. A majority of flowers have flavonoids that are responsible for their brilliant colours 7. Phytochemical analysis of Butea monosperma indicated the presence of various flavonoid pigments like 7,3’,4’-trihydroxyflavone, aurones, butein, butrin, chalcones, coreopsin, isobutrin, isomonospermoside, monospermoside, palasitrin, and sulphuresin, in its flowers 10. Several other secondary metabolites like glycosides and kino oil are present in leaves whereas bark is a rich source of polyphenols. Due to this wide range of bioactive molecules, this plant has been extensively used in Ayurveda, Unani and Indian folk medicine. A comprehensive study of Butea monosperma revealed that its flowers, leaves, seeds, stem and bark have a wide range of pharmacological functions namely anticonvulsive 11, hepatoprotective 12, antidiabetic 13, antioxidant, anti-inflammatory 14, wound healing 15, chemoprotective and anticancer activity 16. The considerable sunscreen activity of Butea monosperma flower and leaf extracts, when used individually or as a polyherbal preparation 17,18 indicated its promise in cosmeceuticals.
A rare yellow flowered variant of Palash (Butea monosperma var lutea) has been sighted at different locales in India. This is a lesser-known variety which has been overlooked and not listed in the IUCN red list but declared as globally endangered medicinal plant 19. This variety is extremely rare in nature which piqued interest about them. The significant use of the scarlet flowered palash in herbal medicines leads to the pertinent query whether the yellow variant would be as effective.
Although the flowers of Butea monosperma are rich in bioactive secondary metabolites, the pharmaceutical and cosmetics industry would be challenged by their seasonal availability. These limitations can be overcome by in vitro callus culture which offers a great advantage of producing and enhancing the bioactive secondary metabolites under controlled environmental conditions with high levels of uniformity and without any pathogenic contamination 20. Several studies have been conducted in recent years to enrich the yield of secondary metabolite in cell cultures by optimising culture requirements, adding precursors, and examining signal transduction pathways that target secondary metabolite production. Utilization of elicitors as one of the key strategies for increased production of secondary metabolites in cell culture 21. Elicitors are physical (cold shock, hyperosmotic stress, pulsed electric field, UV and ultrasound), chemical (heavy metals, pesticides, and plant defence signalling compounds), or biological (microbial cell components and poly and oligosaccharides) factors that influence enzyme which respond to stress. Plant hormones Jasmonic acid (JA) and methyl jasmonate (MeJA: methyl ester of Jasmonic acid), have been indicated as key signalling molecules in the elicitation of secondary metabolites like alkaloids, terpenoids, steroids, saponin, phenolics and flavonoids in the elicitation of secondary metabolites like alkaloids, terpenoids, steroids, saponin, phenolics and flavonoids 22, 23
This study was aimed to develop a callus culture from both the varieties of Butea monosperma (Red and Yellow), monitor the secondary metabolite production in terms of total phenolic content (TPC), total flavonoid content (TFC), evaluates of callus extracts for its Sun Protection Factor and its correlation with TPC and TFC. This study also examines the influence of elicitors on callus growth, TPC, TFC, and SPF activity. The phytochemical composition of phenolics and flavonoids that could be responsible for the possible sunscreen agent was determined through HR-LCMS. A unique report yet of callus culture, growth kinetics and phytoconstituents of the rare Butea monosperma var lutea (BMVL) has been presented in this article.
Materials and methods
Reagents and chemicals
The plant growth regulators were obtained from HiMedia. TDZ and the elicitor Jasmonic acid were sourced from Sigma Aldrich. Quercetin and Gallic acid were procured from HiMedia and Sigma Aldrich respectively. Aluminium chloride was acquired from SD Fine Chemical limited.
Collection of explants
Fresh and young leaves of Butea monosperma (Lam.) Taub (BM) were collected from Bombay Port Trust botanical garden (Sagar Upavan), Colaba, Mumbai, and identified. A small sampling of Butea monosperma var lutea (BMVL) was acquired from the Indian Institute of Natural Resins and Gums (IINRG), Ranchi, Jharkhand with appropriate authentication. The seeds of the yellow variety of Butea were germinated and grown in the glass house of the Department of Life Sciences, University of Mumbai.
Sterilization of plant materials
Fresh, young leaves of both varieties of Butea monosperma were used for the establishment of callus culture. Leaves pieces of approximately 1 cm2 were washed with tap water for 10 min, followed by Tween 20 for 15 mins, an antifungal treatment with 4% Bavistin (w/v) for 20 min and subsequent rinse with 70% ethanol for 1 min was followed by surface sterilization with 0.05% HgCl2 solution. The sterilant were washed off the explants thoroughly with sterile distilled water (three rinses of approximately 15 min each) to remove all traces of the sterilant.
Inoculation and culture conditions
The surface-sterilized explants were blotted dry (using sterile blotting paper) and the edges were carefully trimmed using sterile surgical blades. Explants of approximately 1 cm2 in size were placed in the culture bottles with 15 ml of medium available for each explant. The explants were cultured at a light intensity of 1000 Lux, a photoperiod of 16/8 h (light/darkness) and a temperature of 25±2°C.
Media for callus induction
The basal medium of choice was Woody Plant Medium (WPM) with 3% sucrose and 0.8% agar. Several combinations of plant growth regulators (PGRs) were tested for the optimal induction of callus and its continued growth. The rate of callus induction on full strength WPM basal medium was compared to that on WPM basal medium augmented with Plant Growth Regulators such as auxins ((α-Naphthalene acetic acid (NAA) and 2,4-dichlorophenoxyacetic acid (2,4-D)) and cytokinins ((Thidiazuron (TDZ), kinetin, Isopentyl adenine (2-iP) and N6 Benzyl aminopurine (BAP)), in concentrations ranging from 2-10 µM. The medium in all cases was adjusted to pH 5.8 and then autoclaved at 121°C for 20 min.
Growth analysis
Callus induction frequency (percentage), proliferation and nature of callus (size, colour and texture of callus) were the parameters observed for growth and the results were documented after 8 weeks. Then, for improved callus growth, PGRs that each individually produced callus with a frequency of 50% or greater were utilised in conjunction. Further studies were assayed in media that incorporated the best combination.
Well-grown exponential phase callus was used to determine the growth index (GI). The pre-weighed callus was inoculated in culture vessels containing 16 ml of standardized medium for each explant. The growth index (GI) was evaluated for 2 months. Random samples (3) were collected at weekly intervals. The fresh weight of the callus was recorded before it was dried for two to three weeks in a hot air oven at 40 to 50 °C until its weight remained constant. The dried callus was used for further studies. The growth index of the callus was calculated using the formula below 24:
Elicitation
Jasmonic acid, the elicitor, was filter-sterilized with a 0.22 µm Nylon Syringe filter (Qualisil) and added aseptically into the selected autoclaved media such that its final concentration in the medium was 1 µM, 5µM or 10µM.
Determination of Total Phenolic Content, Total Flavonoid Content and Sun Protection Factor
Preparation of extracts
The callus was extracted using a cold percolation extraction method. Completely dried callus was crushed into a fine powder using a mortar and pestle. Extracts were prepared by mixing 20 mg dried powder with 5 ml of absolute methanol in a clean and dry test tube. This mixture was sonicated for 15 min and then rested for 24 h at room temperature. A Whatman filter paper no. 1 was used to filter the mixture, and the filtrate was collected in a fresh test tube. The residue on the filter paper was flushed repeatedly with 5ml of methanol and the filtrates were collected, each time, till the filtrate was colourless. The collected filtrates were pooled and further concentrated, at 40 °C, in a hot air oven, till the volume of methanol that remained was 3 ml. The concentrated extracts were used for the determination of the total phenolic, total flavonoid content, HRLCMS and SPF.
Total phenolic content (TPC)
Follin-Ciocalteau reagent was used to determine the TPC of all the extracts as previously described in the literature with slight modifications 25. Callus extract (200 µl) was mixed with 600 µl of water and 200 µl of diluted FC reagent (1:1, with distilled water) and incubated for 5 mins. 1ml of 8% (w/v) sodium carbonate solution was added to this mixture and further incubated for 30 min in a dark chamber. The products of the reaction were diluted by adding 3 ml of water and the absorbance was noted at 765 nm using a UV-Vis 1800 Shimadzu Spectrophotometer. A standard graph of Gallic acid (1mg/ml; phenolic standard) with a concentration range from 80 to 400 µg/ml, in steps of 80 µg/ml was used to estimate the total phenolic compounds and expressed as milligram of gallic acid equivalents per gram (mg GAE/g). The regression coefficient (r2) of the graph obtained was 0.96.
Total flavonoid content (TFC)
With slight modifications, the aluminium chloride colorimetric test published by Liyanaarachchi GD et al. 26 was used to evaluate the total flavonoid concentration of the callus extracts. An aliquot of the control, the extract or the standard solution was mixed with 2% aluminium chloride solution (1:1; v/v) and incubated for an hour at room temperature after which the absorbance for all the reactions was determined at 420 nm on UV/Visible 1800 Shimadzu Spectrophotometer. Quercetin (flavonoid standard) was used in a concentration range of 10 – 50 µg/ml, in steps of 10 µg/ml, to establish a standard graph. The r2 of the standard graph obtained was 0.98. The TFC of the extracts was estimated using the quercetin standard and represented as milligrams of quercetin equivalents per gram (mg QE/g).
HRLC-MS for Qualitative analysis of secondary metabolites in callus
Different compounds present in the methanolic extracts were determined by High Resolution Liquid Chromatography Mass Spectrometry (HR-LCMS, Agilent Technologies) that had a quaternary pump and a diode array detector (DAD). The column was coupled with an MS Q-TOF mass spectrometer equipped with an electrospray ionization interface (ESI). Fractions were injected in a C-18 column. The solvents used were A: 0.1% Formic Acid in water; B: 90% Acetonitrile + 0.1% Formic Acid in water. The elution gradient was isocratic: 5% B for 1 min, 5% to 100% over 20 mins, 100% B for 5 mins and re-equilibrium of the column with the flow rate of 300 µl/min. Spectra were recorded in positive and negative ionization mode between m/z 120 to 1200. This data was obtained from the SAIF laboratory, IIT, Bombay.
SPF activity
The concentrated stock solution, as described above, was diluted (1:9 v/v) using methanol as a diluent. A spectrum of the extract was acquired by scanning the solution’s absorbance in the wavelength range of 290 nm to 320 nm at intervals of 5 nm against methanol as a reference. The Mansur equation 27 was used to calculated the SPF
EE – erythemal effect spectrum, I – solar intensity spectrum, Abs – absorbance, CF – correction factor (10). EE (λ) × I(λ) is constant.
Statistical analysis
All observations and results thus obtained were analysed using GraphPad Prism software version 6.0. Each treatment was done in triplicate and the results were presented as mean ± SD. The significant difference between the mean values was analysed using the Analysis of Variance (ANOVA) followed by Tukey’s test for multiple comparisons (p <0.05).
Results
Callus induction
The callus induction was initiated on the explants within two weeks of inoculation and characteristics such as texture and colour were recorded after four weeks. The maximum BM callus induction was observed in media supplemented with 6 µM BAP (80 %) while BMVL explants responded best (63 %) in media fortified with 6 µM TDZ. However, these percentages increased to 100% percent callus induction when the combinations 6 µM BAP and 4 µM NAA for BM callus and 6 µM TDZ and 10 µM NAA for BMVL callus were used. BM callus was white, cream and friable where as that of BMVL was green and friable (Fig 1).
 |
Figure 1: (a) Fully grown plant of Butea monosperma (Lam.) Taub bearing flowers (b) Flowers
|
Growth kinetics analysis
Well-grown exponential phase callus of both BM and BMVL was inoculated on 6 µM BAP + 4µM NAA (6B4N) and 6 µMTDZ + 10 µM NAA (6TN10) medium respectively to determine their growth kinetics. A sample of callus was harvested each week and the fresh weight (thereby the growth of callus) was observed over 8 weeks (Fig 2). A distinctive growth pattern for both BM and BMVL emerged when the growth index was plotted against the number of days of culture. The highest GI achieved was 4.12 ± 1.83 and 3.9 ± 0.38 in media augmented with 6B4N (BM) and 6TN10 (BMVL) respectively. The addition of the elicitor JA had a detrimental effect on the growth of BM and BMVL callus in a dose-dependent manner with the exception that 1 µM JA which improved the GI (4.13 ± 1.5) of the BMVL callus.
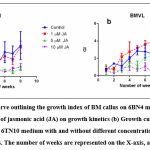 |
Figure 2: (a) Growth curve outlining the growth index of BM callus on 6BN4 medium with and without different concentrations of jasmonic acid (JA) on growth kinetics
|
Total phenolics (TPC) and total flavonoid content (TFC)
Accumulation of TPC and TFC was recorded concomitant with the progression of the growth curve of BM and BMVL callus on 6B4N and 6TN10 medium respectively and the effect of jasmonate (1 µM, 5 µM and 10 µM JA) on their accumulation was determined (Fig 3) A negative correlation between TPC and GI was seen of BM callus when treated with 10 µM JA. The maximum levels TPC and TFC accumulation have been noted in Table 1. TPC and TFC accumulation levels in BMVL callus, remained unaffected by the JA treatment whereas a dose-dependent accumulation of TFC was observed in the BM callus (Fig 4).
Table 1: Maximum TPC, TFC and SPF of BM and BMVL callus.
|
|
BM |
BMVL |
|
Maximum TPC (mg GAE/g) |
173.84 ± 9.3 |
165.65 ± 56.32 |
|
Jasmonic acid (conc) |
5 µM |
0 |
|
Time |
2nd week |
8th week |
|
Maximum TFC (mg QE/g) |
10.5 ± 1.49 |
6.31 ± 3.3 |
|
Jasmonic acid (conc) |
10 µM |
0 |
|
Time |
6th week |
8th week |
|
SPF |
19.0 ± 3.22 |
14.5 ± 1.03 |
|
Jasmonic acid (conc) |
5 µM |
0 |
|
Time |
7th week |
6th week |
Table 1. Table summarizes Mean ± SD values of maximum Total Phenolics Content (TPC), Total Flavonoid Content (TFC) and Sun Protection Factor (SPF) observed during the growth cycle of BM and BMVL callus based on a weekly interval.
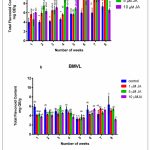 |
Figure 4: (a) TFC during the growth cycle of BM callus observed at a weekly interval. The number of weeks are represented on the X-axis, and TFC on the Y-axis.
|
HRLC-MS for Qualitative analysis of secondary metabolites in callus
 |
Figure 5: (a) Chromatogram of BM callus in positive ionization mode (b) Chromatogram of BM callus in negative ionization mode
|
HPLC fingerprinting provided a quick analysis of secondary metabolites especially flavonoids compounds in BM and BMVL callus extracts. The chromatogram of BM and BMVL callus extracts (Fig 5) revealed the presence of eleven and eight flavonoids respectively. Some secondary metabolites were common to both extracts whereas others were unique. The details have been shown in (Table No. 2). An interesting component of BMVL callus extract, belonging to spingoid family, was Phyto-sphingosine which is naturally occurring phospholipid in skin cells with an important role in skincare.
Table 2: Phytochemical constituents of BM and BMVL callus extracts by HR-LCMS.
|
Name of Compound |
Class of compound |
Molecular Formula |
Molecular weight
|
|
BM and BMVL Bowdichione, |
Isoflavones |
C16 H10 O6 |
298.04 |
|
Sayanedine |
Isoflavones |
C17 H14 O5 |
298.08 |
|
Formononetin |
Isoflavones |
C16 H12 O4 |
268.07 |
|
Sophorol |
Isoflavonone |
C16 H12 O6 |
300.06 |
|
Chrysin |
Flavone |
C15 H10 O4 |
282.08 |
|
Homoferreirin |
Flavonone |
C17 H16 O6 |
316.09 |
|
BM Butin |
Flavonone |
C15 H12 O5 |
272 |
|
Apigenin 7,4′- dimethyl ether |
Dimethoxyflavone
|
C17 H14 O5
|
298.08 |
|
6-Hydroxy-2-(4- hydroxyphenyl)-5,7-dimethoxy 4H-1-benzopyran-4-one |
Dimethoxyflavone
|
C17 H14 O6
|
314.07 |
|
Neohesperidin dihydrochalcone |
Chalcone |
C11 H14 N2 O6 |
612.20 |
|
BMVL Isoliquiritigenin |
Chalcone |
C15 H12 O4 |
256.07 |
|
5,6,2′-Trimethoxyflavone |
Flavone |
C18 H16 O5 |
312.09 |
|
Phytosphingosine |
Phospolipid |
C18 H39 N O3 |
317.29 |
Table 2 List of phytoconstituents identified in the methanolic extracts of BM and BMVL callus by HR-LCMS
SPF
 |
Figure 6: (a) TPC, TFC and SPF activity during the growth cycle of BM callus observed at a weekly interval on control medium. (b) Effect of 1 µM JA (c) Effect of 5 µM JA (d) Effect of 10 µM JA.
|
The SPF activity of BM and BMVL was calculated using Mansur’s equation and plotted against the weeks of culture and compared with the Growth index and graphically represented in (Fig 6 and 7) The variation in the SPF activity was not significant across the treatments. The maximum SPF of the BM callus grown in the control medium was 16.4 on the 28th day. The addition of the elicitor (5 µM JA) to the 6B4N enhanced the SPF to 19 (Table 1). The highest SPF activity shown by BMVL callus was 14.6 but the addition of elicitor decreased the SPF activity.
 |
Figure 7: (a) TPC, TFC and SPF activity during the growth cycle of BMVL callus observed at a weekly interval on control medium. (b) Effect of 1 µM JA (c) Effect of 5 µM JA (d) Effect of 10 µM JA.
|
The coefficient of correlation between TPC and SPF of BM callus was 0.47 and that between TFC and SPF was 0.58 which increased to 0.73 after elicitation with 5 µM JA. The coefficient of correlation between TPC and SPF and that of TFC and SPF of BMVL callus extracts were 0.088 and 0.017 respectively.
Discussion
Previous research on callus culture in Fabaceae suggested that a good proliferative callus was obtained with the synergistic action of auxins and cytokinins The interdependent balance between cytokinin and auxin encourages cell division for the creation of callus 28. All explants of BM showed callusing when a combination of 6µM BAP and 4µM NAA (6B4N) was supplemented in WPM medium. Similarly, the BMVL explants when inoculated on media supplemented with 6 µM TDZ and 10 µM NAA (6T10N) in this combination resulted in 100% callus induction. This outcome matched the earlier study on callus induction from the nodal segments of Butea monosperma 29. The combination of the cytokinin BAP and the auxin NAA has been shown to produce highest callus induction on cotyledon and hypocotyl explants of Glycyrrhiza glabra 30. Reports on the callus production of Butea monosperma var lutea were unavailable in the literature reviewed.
Previous reports regarding the growth kinetics of callus suggested that a typical growth curve pattern is sigmoidal with five distinct phases: lag, exponential, linear, stationary and deceleration phases 31,32 .32Thus, the growth pattern exhibited by the callus from both explants in this study was limited to either an exponential phase (BMVL; up to the 8th week) or an exponential phase tending towards a stationary phase in the later stage (BM; day 7 to day 42). The lack of a visible lag phase in the growth pattern of both cases could indicate a quick adaption of callus to the new nutrient source. Similar patterns were also reported by Pratap M.et al.33 where the exponential growth of Picrorhiza kurroa Royle ex Benth callus was between the 14th to 21st days of culture and Pan Y. et al.31 in their study on Bletilla striata which showed the exponential phase between 7 to 12 days and reached the highest on day 36.
Alterations in the fresh biomass of callus were noted in jasmonate (elicitor) treated BM and BMVL callus. BM callus when exposed to 1 µM, 5 µM and 10 µM JA concentration showed a modest increase in fresh biomass production (2.4-fold, 2.3-fold, 1.4-fold respectively) as compared to the control medium which showed 12-fold increase in the biomass on the 3rd week of the callus culture. Increasing levels of the elicitor positively inhibited the growth of both the callus types. This finding was in consonance with the reports of inhibiting effect of JA, MeJA and GA on the rate of biomass production described in the study on Artemisia absinthium L cell suspension cultures 34 and MeJA on as red-fleshed apple (Malus sieversii f niedzwetzkyana) and Thevetia peruviana cell cultures 35, 36. A study on Taxus explained that the jasmonates tend to impede the progression through the cell cycle, trapping cells in the G1 phase, thus reducing the number of actively dividing cells 37 thereby decreasing the biomass accumulation.
Several studies have indicated that maximum secondary metabolite (the common source of bioactivity) accumulation occurred when the growth of the culture was low especially at the stationary phase. Hence, the trend of TPC and TFC of BM and BMVL callus was traced along with the growth index, in this study. In both cases, the TPC and TFC accumulation curves showed the expected converse correlation with that of the growth index. The reported TPC in Butea monosperma tincture was 84.9 mg GAE/g and the TFC of its flowers was 57.3 mg RE/g 9. The TPC of callus extracts observed from both varieties after 8 weeks of culture (128.95 mg GAE/g (BM), 165.65 mg GAE/g (BMVL)) in this investigation was higher than TPC reported in Butea monosperma tincture but the TFC recorded in this study was considerably low (7.32 mg QE/g (BM), 6.31 mg QE/g (BMVL)) when compared to that of Butea monosperma flower extracts. The TFC of the callus extracts in the current study was low in comparison to the hydroethanolic leaf extracts (65.15 mg RE/g) and the methanolic leaf extracts (30.02 mg CE/g) of Butea monosperma whereas the TPC was more comparable to the TPC of both these extracts (125.25 mg GAE/g and 210.59 mg GAE/g respectively) 38,39.
Jasmonates have been shown to elicit the production of phenolic and flavonoid compounds in Habenaria edgeworthii (jasmonic acid 40), Allium cepa L. (methyl jasmonate 41), Zingiber officinale Rosc. (salicylic acid 42), phenylpropanoid and naphthodianthrones in Hypericum perforatum L (jasmonic acid 43). The results of this study suggested that TPC and TFC buildup with jasmonate treatment was faster and a dose-dependent accumulation of TFC was observed in the BM callus which was the highest (enhanced by 75 %) when compared to other treatments as well as that in control. A similar study conducted on Thevetia peruviana 3 µM MeJA showed enhanced TPC and TFC accumulation by 49% and 105% as compared to the control culture at the 96h and 72h post elicitation 36. However, all tested concentrations of JA failed to improve TPC and TFC accumulation in BMVL callus.
Flavonoids and other polyphenols, valued for their ability to scavenge free radicals set off by UV radiation 39 and the plants arsenal against harmful UV radiations, would be biosynthesized in their response to exposure to the Sun’s UV rays and would serve efficiently as Sun Protection Factors 44. SPF is a quantitative measurement of the product’s efficacy against UV 17. The high-protecting products are those that have an SPF value of more than 15 and confers 93 % protection from the UV B radiations 4. The SPF potential calculated for BM and BMVL extracts was a maximum of 16.4 and 14.4 respectively. The SPF activity of potent antioxidant and photoprotective plant polyphenols screened in literature such as curcumin, quercetin, resveratrol and safranal were reported as 11.58, 14.81, 21.53 and 10 respectively 3. Manisha Sutar et.al. 2020 reported the SPF of methanolic extracts of Butea monosperma flower was 2.5 at 40 µg/ml concentration 45. Similarly, the SPF value obtained for an herbal mixture containing the aqueous, methanolic or ethanolic extracts from different parts of plants such as Aloe vera gel, Asparagus racemosa, Butea monosperma, Cyperus rotundus, Ficus bengalensis, Hibiscus-rosa-Sinensis Rubia cordifolia and Terminalia arjuna, was in the range of 2.14 to 12.97 17.
Earlier research on some medicinal Brazilian plants suggested a correlation between the SPF activity of a plant and its flavonoid-phenolic content 46. Zahra Hashemi et al. reported a correlation coefficient of 0.469 between SPF and phenolic; and 0.355 between SPF and flavonoid content medicinal plants 47. The coefficient of correlation between TPC and SPF and TFC and SPF of BM callus was found to be 0.47 and 0.58 respectively while such correlations were not observed in BMVL callus. This indicated that an increase in the phenolic and flavonoid content in the callus culture would enhance the SPF potential of BM callus.
The fact that there was a positive correlation between the TFC and SPF of the BM callus extract and an adequate SPF activity in the BMVL callus despite no correlation to the TFC prompted phytochemical profiling of both BM and BMVL callus using HR-LCMS technique. The HPLC fingerprinting data of both BM and BMVL varieties included amino acids, glycosides, alkaloids, terpenoids, anthraquinones, flavonoids and phenols but the present examination focused on flavonoid compounds in BM and BMVL callus extracts. Ratchanaporn Chokchaisiri et al.48, fractionated the ethanolic extracts of Butea monosperma flowers using quick column chromatography and isolated compounds like formononetin (an orange solid), isoliquiteringenin (as pale-yellow solid), butin (as orange solid) along with several other flavonoids. All these compounds were also present in the BM and BMVL callus extracts. The chalcone isoliquiritigenin, implicated in the yellow colour of Japanese yellow wood49, was detected in the callus extracts of the yellow variety of Butea monosperma and could have the same function in BMVL as well. The presence of formononetin and isoliquiteringenin in BMVL and formononetin in BM callus extracts may be the cause of the characteristic colour of the extracts. Butin, one of the most important bioactive present in Butea monosperma, believed to have many medicinal properties such as antioxidant and anti-inflammatory agent, antibacterial, anti-cancerous agent, apoptotic factor, immuno-modulatory agent, protein synthesis inducer, potent signal transducer and sensitizer, 50 was detected in the BM callus extracts.
Previous data on the phytochemical screening and profiling Butea monospserma var lutea was unavailable in the literature scanned in this study. The HRLC-MS analysis, in this investigation, detected more than 100 compounds in the callus extracts of BMVL and literature indicated that they play a major role as antioxidant and anti-inflammatory agents protecting the skin by inhibiting pro-inflammatory cytokines at the site of lesions. BMVL callus also showed the presence of Phyto- sphingosine which could be of a therapeutic benefit in a variety of inflammatory and hyperproliferative cutaneous diseases.
Conclusion
An in vitro callus culture of both Butea monosperma varieties was successfully established with 100 % callus induction within 4 weeks. The screening and evaluation of secondary metabolite production in the callus, in this study, confirmed the presence important skin protecting flavonoids such as formononetin, butin and isoliquiteringenin in the callus extracts of Butea spp. The phenolic and flavonoid content could be further enhanced with the application of jasmonate in BM cultures. However, the application of elicitor resulted in limited production biomass. The callus cultures also showed good SPF values. Interestingly, the TFC of BM callus showed a positive relationship with the SPF which indicates its potential in the cosmetic industry.
Acknowledgment
The authors would like to thank Dr. Lohot, Indian Institute of Natural Resins and Gums for providing the rare yellow flower variant of Butea monosperma. The authors would also like to acknowledge Dr. E. N. Murthy for providing the flowers of Butea monosperma var lutea. MS expresses her gratitude to Mumbai University for providing her with a non-NET fellowship. The authors, MS and IG, would also like to acknowledge the BPT for their help in the collection of the plant material of Butea monosperma (Lam.) Taub. The authors would like to express their gratitude to the Department of Life Sciences, University of Mumbai for supporting their research activities. Authors would also like to thank SAIF, IIT, Bombay for the HRLCMS analysis.
Conflict of interest
No potential conflict of interest was reported by the authors.
Funding Sources
No funding was received to assist with the preparation of this manuscript.
References
- Rojas, J. O. H. N., Londoño, C. E. S. A. R., & Ciro, Y. The health benefits of natural skin UVA photoprotective compounds found in botanical sources. Int J Pharm Pharm Sci, (2016).8(3), 13-23.
- Cavinato M, Waltenberger B, Baraldo G, Grade CVC, Stuppner H, Jansen-Dürr P. Plant extracts and natural compounds used against UVB-induced photoaging. Biogerontology. 2017;18(4):499-516. doi:10.1007/s10522-017-9715-7
- Donglikar M.M, Deore S.L. Development and evaluation of herbal sunscreen. Pharmacogn J. 2017;9(1):83-97. doi:10.5530/pj.2017.1.15
- Napagoda M.T, Malkanthi B.M.A.S, Abayawardana S.A.K, Qader M.M, Jayasinghe L. Photoprotective potential in some medicinal plants used to treat skin diseases in Sri Lanka. BMC Complement Altern Med. 2016;16(1):1-6. doi:10.1186/s12906-016-1455-8
- Ebrahimzadeh M.A, Enayatifard R, Khalili M, Ghaffarloo M, Saeedi M, Charati J.Y. Correlation between sun protection factor and antioxidant activity, phenol and flavonoid contents of some medicinal plants. Iran J Pharm Res. 2014;13(3):1041-1048. doi:10.22037/ijpr.2014.1554
- Padghan S.V. Phytochemical and Physicochemical screening of different extracts of Butea monosperma flowers. Int. Res. J. of Science & Engineering. 2018;(63628):161-164.
- Cefali L.C, Ataide J.A, Fernandes A.R, et al. Evaluation of in vitro solar protection factor (Spf), antioxidant activity, and cell viability of mixed vegetable extracts from dirmophandra mollis benth, ginkgo biloba L., ruta graveolens L., and vitis vinífera L. Plants. 2019;8(11):1-13. doi:10.3390/plants8110453
- Vaidya A, Pandita N. Comparative Pharmacognostic and Phytochemical Studies of Flower , Leaf and Stem Extracts of Butea monosperma. Asian Journal of Biomedical and Pharmaceutical Sciences 2017;7(63):10-18.
- Baessa M, Rodrigues M.J, Pereira C, et al. A comparative study of the in vitro enzyme inhibitory and antioxidant activities of Butea monosperma (Lam.) Taub. and Sesbania grandiflora (L.) Poiret from Pakistan: New sources of natural products for public health problems. South African J Bot. 2019;120(July):146-156. doi:10.1016/j.sajb.2018.04.006
- Mazumder P.M, Das M.K, Das S. Butea Monosperma (LAM.) Kuntze – A Comprehensive Review. Int J Pharm Sci Nanotechnol. 2011;4(2):1390-1393. doi:10.37285/ijpsn.2011.4.2.2
- Kasture V.S, Kasture S.B, Chopde C.T. Anticonvulsive activity of Butea monosperma flowers in laboratory animals. Pharmacol Biochem Behav. 2002;72(4):965-972. doi:10.1016/S0091-3057(02)00815-8
- Kaur V, Kumar M, Kaur P, Kaur S, Pal A. ScienceDirect Hepatoprotective activity of Butea monosperma bark against thioacetamide-induced liver injury in rats. Biomed Pharmacother. 2017;89:332-341. doi:10.1016/j.biopha.2017.01.165
- Somani R, Kasture S, Singhai A.K. Antidiabetic potential of Butea monosperma in rats. Fitoterapia. 2006;77(2):86-90. doi:10.1016/j.fitote.2005.11.003
- Krolikiewicz-Renimel I, Michel T, Destandau E, et al. Protective effect of a Butea monosperma (Lam.) Taub. flowers extract against skin inflammation: Antioxidant, anti-inflammatory and matrix metalloproteinases inhibitory activities. J Ethnopharmacol. 2013;148(2):537-543. doi:10.1016/j.jep.2013.05.001
- Sumitra M, Manikandan P, Suguna L. Efficacy of Butea monosperma on dermal wound healing in rats. Int J Biochem Cell Biol. 2005;37(3):566-573. doi:10.1016/j.biocel.2004.08.003
- Choedon T, Shukla S.K, Kumar V. Chemopreventive and anti-cancer properties of the aqueous extract of flowers of Butea monosperma. J Ethnopharmacol. 2010;129(2):208-213. doi:10.1016/j.jep.2010.03.011
- Singh M, Sharma V. Spectrophotometric Determination of Sun Protection Factor and Antioxidant Potential of an Herbal Mixture. Br Biotechnol J. 2016;10(3):1-8. doi:10.9734/bbj/2016/21434
- More, B. H., Sakharwade, S. N., Tembhurne, S. V., & Sakarkar, D. M. Evaluation of Sunscreen activity of Cream containing Leaves Extract of Butea monosperma for Topical application. International Journal of Research in Cosmetic Science, (2013).3(1), 1-6.
- Mahender, A., Mahesh, D. M., & Murthy, E. N. In vitro seed germination and development of Butea monosperma (Lam.) Taub. Var. lutea (Willt.): a step for rehabilitation. International Journal of Multidisciplinary and Current Research.(2014.2, 297-301.
- Park D.E, Adhikari D, Pangeni R, Panthi V.K, Kim H.J, Park J.W. Preparation and characterization of callus extract from Pyrus pyrifolia and investigation of its effects on skin regeneration. Cosmetics. 2018;5(4). doi:10.3390/cosmetics5040071
- Zafar N, Muzamil A.M, Dipti A, Basit T. Aluminum chloride elicitation ( amendment ) improves callus biomass growth and reserpine yield in Rauvolfia serpentina leaf callus. Plant Cell, Tissue Organ Cult. 2017;130(2):357-368. doi:10.1007/s11240-017-1230-7
- Wang, J., Qian, J., Yao, L., & Lu, Y. Enhanced production of flavonoids by methyl jasmonate elicitation in cell suspension culture of Hypericum perforatum. Bioresources and Bioprocessing. 2015;2, 1-9.
- Hariprasath L, Jegadeesh R, Arjun P, Raaman N. South African Journal of Botany In vitro propagation of Senecio candicans DC and comparative antioxidant properties of aqueous extracts of the in vivo plant and in vitro-derived callus. South African J Bot. 2015;98:134-141. doi:10.1016/j.sajb.2015.02.011
- Shah M, George I.A. Pigment elicitation and sun protection factor of callus induced from Cassia tora seedling explants. Plant Cell Tissue Organ Cult. 2020;143(1):201-210. doi:10.1007/s11240-020-01913-3
- Saeed, N., Khan, M. R., & Shabbir, M. Antioxidant activity, total phenolic and total flavonoid contents of whole plant extracts Torilis leptophylla L. BMC complementary and alternative medicine. 2012;12, 1-12.
- Liyanaarachchi GD, Samarasekera JKRR, Mahanama KRR, Hemalal KDP. Tyrosinase, elastase, hyaluronidase, inhibitory and antioxidant activity of Sri Lankan medicinal plants for novel cosmeceuticals. Ind Crops Prod. 2018;111(January):597-605. doi:10.1016/j.indcrop.2017.11.019
- Dutra, E. A., Oliveira, D. A. G. D. C., Kedor-Hackmann, E. R. M., & Santoro, M. I. R. M. Determination of sun protection factor (SPF) of sunscreens by ultraviolet spectrophotometry. Revista Brasileira de Ciências Farmacêuticas, (2004). 40, 381-385.
- Nazir, S., Jan, H., Tungmunnithum, D., Drouet, S., Zia, M., Hano, C., & Abbasi, B. H. Callus culture of Thai basil is an effective biological system for the production of antioxidants. Molecules, (2020). 25(20), 4859.
- C. Kumari RS. In vivo and in vitro production of flavonoids from Butea monosperma.World Journal of Pharmaceutical Research. 2017;6(8):1951-1956. doi:10.20959/wjpr20178-9130
- Safari, M., Zebarjadi, A., & Chaghamirza, K.. Study of callus induction of Glycyrrhiza glabra as an important medicinal plant. In The Second International Conference on Agriculture and Natural Resources2013;pp. 483-485.
- Pan Y, Li L, Xiao S, et al. Callus growth kinetics and accumulation of secondary metabolites of Bletilla striata Rchb. F. Using a callus suspension culture. PLoS One. 2020;15(2):1-14. doi:10.1371/journal.pone.0220084
- Silva, A. L. C. D., Caruso, C. S., Moreira, R. D. A., & Horta, A. C. G. Growth characteristics and dynamics of protein synthesis in callus cultures from Glycine wightii (Wight & Arn.) Verdc. Ciência e Agrotecnologia, (2005). 29, 1161-1166.
- Partap M, Kumar P, Ashrita, Kumar P, Kumar D, Warghat AR. Growth Kinetics, Metabolites Production and Expression Profiling of Picrosides Biosynthetic Pathway Genes in Friable Callus Culture of Picrorhiza kurroa Royle ex Benth. Appl Biochem Biotechnol. Published online 2020. doi:10.1007/s12010-020-03391-x
- Ali M, Abbasi BH, Ali GS. Elicitation of antioxidant secondary metabolites with jasmonates and gibberellic acid in cell suspension cultures of Artemisia absinthium L. Plant Cell Tissue Organ Cult. 2015;120(3):1099-1106. doi:10.1007/s11240-014-0666-2
- Sun J, Wang Y, Chen X, et al. Effects of methyl jasmonate and abscisic acid on anthocyanin biosynthesis in callus cultures of red-fleshed apple (Malus sieversii f. niedzwetzkyana). Plant Cell Tissue Organ Cult. 2017;130(2):227-237. doi:10.1007/s11240-017-1217-4
- Mendoza D, Cuaspud O, Arias JP, Ruiz O, Arias M. Effect of salicylic acid and methyl jasmonate in the production of phenolic compounds in plant cell suspension cultures of Thevetia peruviana. Biotechnol Reports. 2018;19(63):e00273. doi:10.1016/j.btre.2018.e00273
- Patil RA, Lenka SK, Normanly J, Walker EL, Roberts SC. Methyl jasmonate represses growth and affects cell cycle progression in cultured Taxus cells. Plant Cell Rep. 2014;33(9):1479-1492. doi:10.1007/s00299-014-1632-5
- Srivastava MP, Tiwari R, Sharma N. Assessment of phenol and flavonoid content in the plant materials. J New Biol Reports. 2013;2(2):163-166.
- De Silva, D. S. N., Bandara, L. L., Samanmali, B. L. C., Ratnasooriya, W. D., Pathirana, R. N., & Abeysekara, W. P. K. Investigation of sun screening and antioxidant activity of M. indica ver “Willard”. Journal of Pharmacognosy and Phytochemistry, (2019).8(4), 1130-1133.
- Giri L, Dhyani P, Rawat S, et al. In vitro production of phenolic compounds and antioxidant activity in callus suspension cultures of Habenaria edgeworthii: A rare Himalayan medicinal orchid. Ind Crops Prod. 2012;39(1):1-6. doi:10.1016/j.indcrop.2012.01.024
- Iqbal, M. S., Iqbal, Z., & Ansari, M. I. Enhancement of total antioxidants and flavonoid (quercetin) by methyl jasmonate elicitation in tissue cultures of onion (Allium cepa L.). Acta Agrobotanica, (2019). 72(3).
- Ali, A. M. A., El-Nour, M. E. M., & Yagi, S. M. Total phenolic and flavonoid contents and antioxidant activity of ginger (Zingiber officinale Rosc.) rhizome, callus and callus treated with some elicitors. Journal of genetic engineering and biotechnology, (2018).16(2), 677-682.
- Gadzovska S, Maury S, Delaunay A, Spasenoski M, Joseph C, Hagège D. Jasmonic acid elicitation of Hypericum perforatum L. cell suspensions and effects on the production of phenylpropanoids and naphtodianthrones. Plant Cell Tissue Organ Cult. 2007;89(1):1-13. doi:10.1007/s11240-007-9203-x
- Priyanka, S., Mary, S. R. I., Nandini, H. S., Kutty, A. V. M., & Kiranmayee, P. A pilot study on sun protection factor of plant extracts: An observational study. Asian J. Pharm. Clin. Res, (2018). 11(4), 67-71.
- Sutar, M. P., & Chaudhari, S. R. Screening of in vitro sun protection factor of some medicinal plant extracts by ultraviolet spectroscopy method. Journal of Applied Biology and Biotechnology, (2020).8(6), 48-53.
- Nunes AR, Rodrigues ALM, de Queiróz DB, et al. Photoprotective potential of medicinal plants from Cerrado biome (Brazil) in relation to phenolic content and antioxidant activity. J Photochem Photobiol B Biol. 2018;189:119-123. doi:10.1016/j.jphotobiol.2018.10.013
- Hashemi Z, Ebrahimzadeh MA, Khalili M. Sun protection factor, total phenol, flavonoid contents and antioxidant activity of medicinal plants from iran. Trop J Pharm Res. 2019;18(7):1443-1448. doi:10.4314/tjpr.v18i7.11
- Chokchaisiri R, Suaisom C, Sriphota S, Chindaduang A, Chuprajob T, Suksamrarn A. Bioactive flavonoids of the flowers of Butea monosperma. Chem Pharm Bull. 2009;57(4):428-432. doi:10.1248/cpb.57.428
- Colors W, Review MA. Natural Product Communications. Published online 2015. doi:10.1177/1934578X1501000332
- Chauhan SS, Mahish PK. Flavonoids of the flame of forest-butea monosperma. Res J Pharm Technol. 2020;13(11):5647-5653. doi:10.5958/0974-360X.2020.00984.1
Abbreviations
|
BAP |
N6 Benzylaminopurine |
|
NAA |
α-Naphthalene acetic acid |
|
TDZ |
Thidiazuron |
|
2-iP |
Isopentyl adenine |
|
2,4-D, |
2,4-dichlorophenoxyacetic acid. |
|
BM |
Butea monosperma |
|
BMVL |
Butea monosperma var lutea |
|
6B4N |
6 µM BAP + 4 µM NAA |
|
6TN10 |
6 µM TDZ + 10 µM NAA |
|
TPC |
Total Phenolic Content |
|
TFC |
Total Flavonoid Content |

This work is licensed under a Creative Commons Attribution 4.0 International License.