Manuscript accepted on : 19-07-2023
Published online on: 08-09-2023
Plagiarism Check: Yes
Reviewed by: Dr. Ana Golez
Second Review by: Dr. Nagham Aljamali
Final Approval by: Dr. Ahmad Ali
Immobilization of Bee Pollen Extract on Polyethylene Terephthalate (PET) Fabric for Wound Dressing
Chetna Bhat1 , Jahnavi Jeswani1
, Jahnavi Jeswani1 and Myrene Roselyn Dsouza1*
and Myrene Roselyn Dsouza1*
Department of Biochemistry, Mount Carmel College Autonomous, Bengaluru, India.
Corresponding Author E-mail:myrene83@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/3139
ABSTRACT: Bee pollen is used in the apitherapeutic treatment as it demonstrates anti-inflammatory, immunostimulant, antimicrobial, and local analgesic activities and also facilitates the granulation process of burn wound healing. In this study, pure bee pollen synthesized by the giant honeybee Apis dorsata dorsata was investigated for the presence of phytochemicals. The extraction of bioactives was done using 30% ethanol and 70% methanol. To develop wound dressing fabric with biomedical applications, phenolics derived from bee pollen were covalently bound to amino polyethylene terephthalate (PET) fabric by immobilization using polyethylene glycol diglycidyl ether (PEGDGE) as a cross-linker. Alterations in the chemical configuration were studied using ATR-FTIR spectra and the contact angle of 122° in unmodified PET decreased to 110° and 98° post amination and immobilization. Antibacterial activity of the immobilized fabric was observed against bacteria and yeast isolated from burn wounds. Thus, the study revealed that PET fibrous mats in the presence of bee pollen could be considered potential wound dressing materials due to their enhanced processing capabilities and suitable structural properties. As not much research has been conducted on bee pollen previously, this study sets out to examine its efficacy and therapeutic utility in connection to burn wound healing capabilities.
KEYWORDS: Antibacterial activity. Apis dorsata; Bee pollen; Immobilization; Phenolics; PEGDGE
Download this article as:| Copy the following to cite this article: Bhat C, Jeswani J, Dsouza M. R. Immobilization of Bee Pollen Extract on Polyethylene Terephthalate (PET) Fabric for Wound Dressing. Biosci Biotech Res Asia 2023;20(3). |
| Copy the following to cite this URL: Bhat C, Jeswani J, Dsouza M. R. Immobilization of Bee Pollen Extract on Polyethylene Terephthalate (PET) Fabric for Wound Dressing. Biosci Biotech Res Asia 2023;20(3). Available from: https://bit.ly/3sQAnbT |
Introduction
Apitherapy is gaining more popularity among modern and conventional treatment modalities as it utilizes the medicinal and therapeutic benefits of pharmacologically active fractions derived from the bee hive1. Studies suggest that the antioxidant, immunomodulation, re-epithelialization and antibacterial properties of bee pollen have rightly confirmed its application in wound healing1,2. Another significant fact is that bee pollen is far less expensive, has a potent anti-inflammatory potential, has a quicker healing time, and reduces both the duration and degree of illness-related suffering. Surprisingly, the phenolic component of bee pollen, kaempferol, possesses the capacity to hinder the activity of two enzymes: hyaluronidase and elastase. As a result, swelling, inflammatory reactions, and transudates are reduced. Skin becomes moisturized and tight as blood circulation in the vessels improves3,4. The anti-inflammatory action of phenolics may also result from quercetin, which inhibits the activity of histidine decarboxylase and thus reduces the histamine level in the organism. In addition, it suppresses the cascade of arachidonic acid metabolism, resulting in a decrease in pro-inflammatory prostaglandins and thereby has an anti-inflammatory impact, alleviates localized pain, and prevents platelet aggregation6.
The antibacterial properties reported in bee pollen are brought on by active substances like flavonoids and phenolic acids, which result in complexes with the cell wall of bacteria, disturb cell wall integrity, block ion channels, and reduce the flow of electrons in the electron transport chain. The inhibition of bacterial RNA Pol by phenolics including flavonol, flavanone, galangin, pinocembrin, and caffeic acid phenethyl ester may be another method by which bee pollen exhibits antibacterial activity. The utilization of topical application of the apitherapeutic agent in burn wound therapy is implied by the advantages and benefits of bee pollen ointment in the treatment of burn wounds7. The study’s clinical and histological observations led to the inference that bee pollen positively impacts the cellular processes that promote reepithelization and wound closure.
Typically, substrates of polymers are utilized in numerous applications of bee pollen (BP) extract in biomaterials. Bee pollen ethanolic extract (BPEE) was added to gelatin-based films to provide them with antibacterial capabilities8. With increasing amounts of bee pollen used for coating, they demonstrated improved in vivo activity of wound healing. Limited research on the incorporation of BP extracts into fabrics has been published in the literature. Ethanolic extract of bee pollen (EEBP) immobilized onto nonwoven fabrics for the treatment of burn wounds by using scattering and immersing techniques significantly improves microbial barriers9. Bee pollen extracts can be incorporated into cotton fabric10 as the functional groups present in bee pollen interact with hydroxyl groups of the derivates of cellulose.
However, when it comes to synthetic fabrics incorporating BP, less usage has been reported to date. Few synthetic fabrics, such as polyethylene terephthalate (PET) fabric are used for their exceptional mechanical characteristics, biodegradability, and affordability11. However, its use in the creation of biomedical materials is constrained by its chemical inertness and hydrophobic nature. Therefore, such fabrics require chemical or physical modification for their incorporation. For instance, Zhang12 used NaOH to etch polyester fabric, form pits and thereafter embedded magnetic particles inside those pits. Following that, they combined these elements along with cyclodextrin to create a controlled release of menthol for the treatment of dermatological disorders.
The wound-healing potential of BP is ascribed to its extensive and diverse phenolic content, along with antioxidant, anti-inflammatory and antibacterial properties. The aim of this investigation is to immobilize the phenolic compounds of bee pollen on PET fabric for the production of biomaterial for wound healing. This novel fabric is a possible contender for numerous applications, including wound healing bandages and surgical fibres because of its moisture retention and cell regeneration properties, respectively. Consequently, a new technical textile and a product with superior added value have been introduced.
Materials and Methods
Sample collection
Raw unprocessed BP was acquired from Apis dorsata dorsata that commonly forage in Acacia mangium forests of the Himalayan range. 100% Polyethylene terephthalate (PET) fabric was purchased as an antiseptic wound pad. Other reagents such as ethylenediamine (for amination of the fabric), tetrahydrofuran and Poly(ethylene glycol) diglycidyl ether (for immobilization of bee pollen extract on modified fabric) were supplied from Sigma-Aldrich.
Extraction of bee pollen
Phenolic components present in BP were extracted by various extraction solvents to check the extractability percentage of each solvent. The solvents selected for the extraction of BP were 30% ethanol, 70% ethanol, 70% methanol and 20% v/v polyethylene glycol (PEG). Ten g of finely ground bee pollen powder was added to 150 ml of the four solvents taken in a 250 ml beaker. Then, the mixtures were subjected to ultrasound for 5 minutes in a bath. Following sonication, the mixtures were stirred at 1500 rpm at 25°C for 1.5 h on a magnetic stirrer. Afterwards, they were filtered separately, and the filtrates were collected. To obtain a viscous mixture, they were vaporized on a hot plate at 60°C for 30-40 minutes and then the respective mixtures were collected and stored in tightly closed bottles at 4oC.
Modification of the PET fabric
Typically, a PET fabric of dimension 3.5 cm × 2.6 cm was taken and aminated using a mix of ethylenediamine (EDA) in distilled water (1:3) in a glass bottle secured tightly. The following steps (Fig. 1.) were performed out for amination:
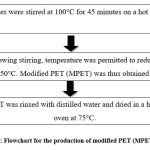 |
Figure 1: Flowchart for the production of modified PET (MPET) fabric.
|
Immobilization of BP extracts on MPET fabric
Bee pollen extracts were immobilized by employing the amine groups of the modified fabric. For this, tetrahydrofuran (THF) and poly(ethylene glycol) diglycidyl ether were used as reagents.THF, a stable compound with a boiling point that is comparatively low with exceptional solubility, was utilized to effectually dissolve and react with MPET and extracts. As a cross-linker between MPET and extracts, poly(ethylene glycol) diglycidyl ether (PEGDGE) was used. The following steps (Fig. 2.) were performed for immobilization:
 |
Figure 2: Flowchart for immobilization of Bee pollen extracts on MPET fabric
|
ATR-FTIR studies and contact angle determination
The structural changes in the following samples i.e., 100% PET, MPET, 30% ethanolic extract (EEBP-PET) and 70% methanolic extract (MEBP-PET) were investigated by Attenuated Total Reflectance-Fourier Transform Infrared Spectroscopy (ATR-FTIR) using Thermo Scientific Nicolet iS50 FTIR Spectrometer (USA).
Revival of microbial cultures
The freeze-dried microbial cultures ampoules brought from Microbial Type Culture Collection and Gene Bank (MTCC), CSIR, Chandigarh, India were disinfected with alcohol and the cotton plug was removed carefully. Around 0.3-0.4 ml of Nutrient Broth was added to make a suspension of the culture and finally, the complete suspension was added to 200 ml of the prepared Nutrient Broth medium. This was done to revive the cultures and hence grow pure cultures of the given organisms in a shaker. The analysis was performed for the given four organisms, viz. Pseudomonas aeruginosa, Klebsiella pneumoniae Staphylococcus aureus, and Candida albicans were incubated in a shaker at respective optimal incubation conditions.
Isolation and screening of microorganisms
After incubation, 10 µl of the respective microbial cultures were streaked on Nutrient Agar (NA) and Muller Hinton Agar (MHA) plates using the quadrant streaking method. The Petri plates with the bacteria were incubated at 37°C while the Candida was incubated at room temperature until visible growth was obtained. Agar slant cultures were also prepared to preserve the pure cultures of the selected organisms.
Agar diffusion plate test
Following incubation, the isolated bacterial and fungal colonies were spread on Nutrient agar and Muller Hinton agar plates. Cut circularly, the immobilised fabrics were then deposited on a lawn culture of bacteria and fungi and incubated for 48 h. Antibacterial activity was recorded as the diameter of the zone of inhibition (ZOI), which was calculated by subtracting the diameter of the observed zone from that of the disc.
Results and Discussion
Characterization tests
The ATR-FTIR spectra were performed to monitor chemical alterations in the structure of the PET fabric due to the treatment with ethylenediamine (Fig. 3.). Sharp peaks at 1715 cm-1 and 1253 cm-1 were ascribed to the carbonyl group of the ester unit and aliphatic -CH2 groups were accountable for the sharp peaks at 2878 and 2946 cm-1 in the spectrum. The distinctive peaks seen in the MPET spectrum are diagnostic of PET (Fig. 3.). Amine peaks occurred at a frequency of about 3284 cm-1, while a carbonyl signal originating from PET was seen at 1742 cm-1. The peak at 1632 cm-1 is due to the bending vibrations of secondary amines while that of 1562 cm-1 is ascribed to the bending vibrations of primary amines. These findings make it quite evident that PET amination was completed without any problems.
The ATR-FTIR spectra of MPET and MEBP-PET are shown side by side in Fig.3. for easy comparison. Up to 20% of the mass of MEBP has been attributed to flavonoid derivatives. Pinobenchin, pinocembrin, chrysin, and galangin are the most often used derivatives10. Structure-wise, they feature carbonyl and hydroxyl groups in addition to aromatic rings. The results of analyses of their morphologies can be found reported in scholarly literature10.
An important peak in the MEBP spectra appears at a frequency of about 3290 cm-1 due to the -OH in the flavonoid structures11. The -OH peak is thought to coincide with the peaks of aromatic -CH groups, which are anticipated to reach about 3010 cm-1. The sharp peaks at 2946 cm-1 and 3980 cm-1 are attributable to the elongation of aliphatic -CH groups, as is the case of tectochrysin and pinostrobin, other flavonoid derivatives14. Carbonyl groups in flavonoid derivatives generate the 1738 cm-1 band. At 1637 cm-1, the stretching vibration of aromatic C=C was identified as the peak. According to Anjos12, the peaks near 1367 cm-1 can be ascribed to -CH2 group and its bending vibration. A noticeable peak occurs at about 1010 cm-1, and its origin may be traced back to the stretching of the C-O-C group. These results are similar to those obtained for other flavonoid derivatives.
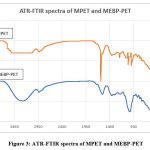 |
Figure 3: ATR-FTIR spectra of MPET and MEBP-PET
|
In the spectra of MEBP-PET, the disappearance of the primary amine peak at 1550 cm-1 is observed quite clearly. It indicates that the -NH2 group reacted with the PEGDGE molecule at its epoxy ring. The broad shoulder at approximately 1642 cm-1 in Fig. 4.1 can be ascribed to two distinct secondary amines in the EEP-MEBP structure. It is suggested that the carbonyl peaks caused by flavonoids and PET13 coincide. In addition, when compared to MPET, the broadening of the peak at approximately 3320 cm-1 is due to the -OH groups in flavonoids. The comparison supports this hypothesis. On the basis of these results, it would appear that the immobilization of MEBP on PET was accomplished successfully and to the desired extent.
Contact angle measurement
The surface hydrophobicity observed in MPET fabric due to modification and immobilization was analysed using contact angle (CA) measurements. Unmodified PET fabric has a CA value of 122o and thus demonstrates hydrophobicity15. The contact angle value for MPET declined to 110o and is attributed to the amine groups by ethylenediamine. MEBP-PET was more hydrophobic on account of the presence of -OH groups on the PET surface arising from flavonoids. This was evidenced by the CA of 98o. It can thus be concluded that the amination of PET resulted in the successful immobilization of MEBP on the fabric.
Extraction of bee pollen
Phenolic components in BP were extracted using several extraction solvents to check the extractability percentage of each solvent. From the various solvents used, 30% ethanol and 70% methanol provided the largest zone of inhibition for S. aureus and P. aeruginosa (Table 1) and were chosen for subsequent immobilization of modified PET fabric. The source, geographic origin, and extraction technique of BP will influence the activity of extracts. Using more polar solvents will enhance the extraction of polar molecules, whereas non-polar organic and oily solvents will extract non-polar molecules. A good extraction yield of both non-polar and polar compounds is made possible by organic polar solvents. The development of modified fabrics still requires reproducible antibacterial activity, which can only be obtained through extract standardization17.
Agar diffusion plate test
The zone of inhibition (ZOI) of bacterial and fungal colonies following incubation with the immobilized fabrics cut in circular shapes is depicted in Table 2.
Table 1. Depiction of the volume and ZOI after vaporization for various solvent systems employed in the extraction of BP.
|
Bee pollen extracts |
The volume collected after vaporization (ml) |
ZOI (cm) |
|
|
S. aureus |
P. aeruginosa |
||
|
30% EE |
3.2 |
1.3 |
1.2 |
|
70% EE |
1.7 |
1.1 |
0.9 |
|
70% ME |
3.0 |
1.4 |
1.3 |
|
20% PEG extract |
0.5 |
not distinct |
not distinct |
Table 2: Depiction of the ZOI of different microorganisms with extraction solvents immobilized on PET fabric.
|
No. |
MTCC_ID |
Genus |
Species |
Incubation (h) |
BP extracts |
ZOI (cm) |
|
1 |
647 |
Pseudomonas |
aeruginosa |
24 |
30% EE |
1 |
|
1.1 |
647 |
Pseudomonas |
aeruginosa |
24 |
70% ME |
1.2 |
|
2 |
1144 |
Staphylococcus |
aureus sub sp. aureus |
24 |
30% EE |
1.3 |
|
2.2 |
1144 |
Staphylococcus |
aureus sub sp. aureus |
24 |
70% ME |
1 |
|
3 |
183 |
Candida |
albicans |
48 |
30% EE |
0.6 |
|
3.3 |
183 |
Candida |
albicans |
48 |
70% ME |
0.8 |
|
4 |
39 |
Klebsiella |
pneumoniae sub sp. pneumoniae |
48 |
30% EE |
0.9 |
|
4.4 |
39 |
Klebsiella |
pneumoniae sub sp. pneumoniae |
48 |
70% ME |
0.8 |
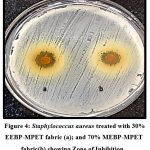 |
Figure 4. Staphylococcus aureus treated with 30% EEBP-MPET fabric (a); and 70% MEBP-MPET fabric(b) showing Zone of Inhibition.
|
It was interpreted that Candida albicans and Pseudomonas aeruginosa showed a high ZOI with 70% methanolic extract treated with modified PET Fabric whereas for Klebsiella pneumoniae and Staphylococcus aureus 30% Ethanolic extract treated with modified PET fabric showed great results of ZOI. Using an agar diffusion plate assay, the antibacterial activity of MPET was evaluated against S. aureus, C. albicans, P. aeruginosa, and K. pneumonia. The fabric was not used as a control due to the inability of proper placement on account of its high twisting behavior. It is already well-established that PET fabric lacks antibacterial properties13. The result obtained demonstrates conclusively that the MPET fabric acquires antibacterial properties. The antibacterial activity of BP against both gram positive and negative bacteria varies depending on the source of the BP and the extraction method, as stated numerous times in scientific literature18,19. The diffusion of the antibacterial agent from the MPET fabric into the agar media causes the formation of the ZOI13.
Conclusion
The PET fabric underwent a chemical transformation to incorporate functional groups required for subsequent immobilization. ATR-FTIR spectroscopy provided evidence for both the alteration and immobilization of the extract components. Both processes lead to a rise in the hydrophilicity of the substance, as revealed by the CA measurements. It was perceived that the immobilization had a subsequent therapeutic effect and the MPET is adequate for use in biomedical applications. When tested against bacteria and candida, we determined that the MPET exhibits antibacterial properties. As a consequence, a novel type of natural-origin technical textile that has the capability to be used as a biomedical material has been developed.
Acknowledgement
The authors are grateful to Mount Carmel College, Autonomous, Bengaluru for the provision of facilities needed for conducting this project. This is part of the Major Research Project (MCC/RC-MRP-27/2021-22 08/) sanctioned by the Institute.
Conflict of Interest
There are no conflict of interest.
References
- Trinh, T.X., Nguyen-Van, Long., Anh, L., et al. A Comprehensive Review of Natural compounds for Wound Healing: Targeting Bioactivity Perspective. Int J Mol Sci. 2022; 23(17):9573.
CrossRef - Liang, Y., Zhang, H., Guo, B. Antibacterial biomaterials for skin wound dressing. Asian J Pharm Sci. 2022;17(3):353-384.
CrossRef - Abdul-Hafeez, M.M., Hamouda, S.M., Honey and propolis for wound healing. Int J Complement Alt Med. 2023;16(1):12–17.
CrossRef - Dzik K.A., Stojko S.E., Adamek W.I., Stojko R. Experimental observations of the apitherapeutics use in the treatment of burn wounds. Anales Academiae Medicae Silesiensis, 2003; 54:15–21.
- Vassev K.K., Olczyk P., Kaźmierczak J., Mencner L., Olczyk K. Bee pollen: Chemical composition and therapeutic application. eCAM, 2015; 297425.
CrossRef - Kanashiro A., Souza G.J., Kabeya M.L., Azzolini S.C.E.A., Lucisano-Valim M.Y. Elastase release by stimulated neutrophils inhibited by flavonoids: importance of the catechol group. Zeitschrift für Naturforschung C, 2007; 62(5-6): 357–361.
CrossRef - Olczyk, P., Koprowski, R., Kaźmierczak, J., Mencner, L., Wojtyczka, R., Stojko, J., Olczyk, K., & Komosinska-Vassev, K. Bee Pollen as a Promising Agent in the Burn Wounds Treatment. Evidence-based complementary and alternative medicine : eCAM, 2016; 8473937.
CrossRef - Reyes, L.M., Reyes, L., Landgraf, M., Landgraf, M., Sobral, P.J.A. Gelatin-based films activated with red propolis ethanolic extract and essential oils. Food packaging and shelf life, 2021; 27:100607.
CrossRef - Rogina-Car, B., Rogina, J., Bajsic, E.G., Budimir, A., ‘Propolis – Eco-friendly natural antibacterial finish for nonwoven fabrics for medical application. Journal of Industrial Textiles, 2020; 49:1100-1119.
CrossRef - Sharaf, S., El-Naggar, M.E. Wound dressing properties of cationized cotton fabric treated with carrageenan/cyclodextrin hydrogel loaded with honey bee propolis extract. International Journal of Biological Macromolecules, 2019; 133:583-591.
CrossRef - Geckil, T., Onal, Y., Ince, C.B., Moisture Resistance of Bituminous Hot Mixtures Modified with Waste PET, Journal of Polytechnic Politeknik Dergisi, 2021; 24:461-471.
CrossRef - Zhang, H., Li, X., Mao, N.T., Sun, R.J., Xu, J. Fabrication of magnetized polyester fabric grafted with -cyclodextrin for controlled release of menthol, Journal of Industrial Textiles, 2018; 47:1060- 1082.
CrossRef - Gumus, O.Y., & Yssaad, I. Immobilization of propolis extract on PET fabric for biomedical applications, Politeknik Dergisi, 2022; 25(3): 1299-1307.
CrossRef - Przybyłek, I., & Karpiński, T. M. Antibacterial Properties of Propolis. Molecules (Basel, Switzerland), 2019; 24(11), 2047.
CrossRef - Anjos, O., Guiné, R., Santos, A., Paula, V., Pereira, H. & Estevinho, L. Evaluation of FT-Raman and FTIR-ATR spectroscopy for the quality evaluation of Lavandula Honey. Open Agriculture, 2021; 6(1), 47-56.
CrossRef - Li, Q., Zhang, S., Mahmood, K., Jin, Y., Huang, C., Huang, Z., Zhang, S., Ming, W. Fabrication of multifunctional PET fabrics with flame retardant, antibacterial and superhydrophobic properties, Progress in Organic Coatings, 2021; 157:106296.
CrossRef - Šuran, J., Cepanec, I., Mašek, T., Radić, B., Radić, S., Tlak Gajger, I., Vlainić, J. Propolis Extract and Its Bioactive Compounds—From Traditional to Modern Extraction Technologies, Molecules, 2021; 26:2930.
CrossRef - Ayesha, F., Ayesha, N., Myrene, R. D., Evaluation of in vitro antimicrobial activity of Indian honey on burn wound isolates. Journal of Chemical and Pharmaceutical Research, 2016; 8(3):1027-1034.
- El-Seedi, H.R., Eid, N., Abd El-Wahed, A.A., Rateb, M.E., Afifi, H.S., Algethami, A.F., Zhao, C., Al Naggar, Y., Alsharif, S.M., Tahir, H.E., Xu, B., Wang, K., and Khalifa, S.A.M., Honey Bee Products: Preclinical and Clinical Studies of Their Anti-inflammatory and Immunomodulatory Properties. Nutr., 2022; 8:761267.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





