Manuscript accepted on : 30-05-2023
Published online on: --
Plagiarism Check: Yes
Reviewed by: Dr Manita Paneri
Second Review by: Dr. Ichrak Jaouadi and Dr. Hiren B. Soni
and Dr. Hiren B. Soni
Final Approval by: Dr. Susana Rodriguez-Couto
A Recent Advancement Towards Herbal Biomass-Assisted Synthesis of Silver Nanoparticles
Ruchi Shivhare1* and Neelam Jain2
and Neelam Jain2
1Oriental University, Indore, Madhya Pradesh, India.
2Oriental University, Sanwer Road, Jakhya opposite Revati Range Gate No. 1, Indore, Madhya Pradesh, India.
Corresponding Author E-mail:shivharer4@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/3134
ABSTRACT: Using plant extracts in metal nanoparticle production is a straightforward, practical, cost-effective, and ecologically benign alternative to the use of harmful chemicals. As a consequence, several approaches for the quick manufacture of silver nanoparticles that are acceptable to the environment and use aqueous extracts of plant materials including leaves, bark, and roots have been published recently. In this paper, recent advancements in the area of environmentally friendly manufacturing of silver nanoparticles (AgNPs) using diverse plant extracts are highlighted and expanded upon, along with the potential use of these materials as antibacterial agents. A thorough analysis of the potential effects of phytochemical concentrations in plant extracts, extraction temperatures, extraction solvents, reaction temperatures, reaction times, reaction pHs, and precursor concentrations. Furthermore provided are extensive details on the potential mechanism by which AgNPs have strong antibacterial action and induce cell death in pathogens by interacting with their cell walls. In addition, whereas chemical methods for shape-controlled synthesis are widely established, controlling the shape of biologically generated AgNPs has several advantageous effects on its activities.
KEYWORDS: Bioreduction; Nanomaterials; Phytoconstituents; Plant Extract; Silver Nanoparticle
Download this article as:| Copy the following to cite this article: Shivhare R, Jain N. A Recent Advancement Towards Herbal Biomass-Assisted Synthesis of Silver Nanoparticles. Biosci Biotech Res Asia 2023;20(3). |
| Copy the following to cite this URL: Shivhare R, Jain N. A Recent Advancement Towards Herbal Biomass-Assisted Synthesis of Silver Nanoparticles. Biosci Biotech Res Asia 2023;20(3). Available from: https://bit.ly/458YJvE |
Introduction
Nanotechnology is an expanding area of study concerned with the manufacture of nanoparticles (NPs) and nanomaterials (NMs) for use in a variety of industries, including electrochemistry, catalysis, pharmaceuticals, biomedicine, food technology, sensors, cosmetics, and so on. NPs are solid particles on the atomic or molecular size that have advantageous physical characteristics compared to bulk molecules. Metal and metal oxide NPs have been extensively studied in the scientific and technical communities due to their unique characteristics. These characteristics include a high surface-to-volume ratio and stable dispersion in solution. Because of these characteristics, metal oxides NPs are more effective in killing bacteria 1.
Textiles, electronics, and personal care products are just some of the many industrial items that use NPs that have been modified or created. New drugs, either as stand-alone NPs or in synergistic combination with existing antibiotics, have been actively employed across a range of medical specialties as the number of bacteria resistant to conventional antibiotic treatment rises. These days, NPs are used in molecular imaging to provide high-resolution diagnostic images. Furthermore, contrast compounds are imprinted onto NPs for the detection of cancer and atherosclerosis 2. Additionally, several nano-based drugs have been created all around the globe since the first nanotherapeutic was authorized by the FDA in 1990. Scientists around the start of the twentieth century used a variety of physicochemical techniques, including as reduction, milling, etc., to synthesis NPs and improve their effectiveness. However, traditional methods are not eco-friendly since they need the use of expensive and sometimes harmful chemicals. Because of their stability, biocompatibility, therapeutic versatility, and low cost, metal and metal oxide NPs produced biogenically using aqueous plant extract and bacteria are gaining increasing attention among scientists. This has made bio-inspired NP synthesis an important area of study in nanoscience and nanotechnology 3.
Plant extracts, microorganisms, and other methods have been used to produce several metal oxide NPs. Plant biomass is widely employed as a catalyst in chemical synthesis, biodiesel generation, and NP synthesis owing to its abundance, renewability, and eco-friendliness. Silver nanoparticles (AgNPs) have caught the interest of the scientific community due to their potential applications in a variety of fields, including chemistry, microbiology, cell biology, food technology, parasitology, and pharmacology. AgNPs’ chemical and physical characteristics are modified by their shape. AgNPs have been synthesized by a wide variety of techniques, including sol-gel, chemical vapor deposition, hydrothermal, microwave-assisted combustion, and thermal decomposition 4. Recently, there has been a lot of interest in the biogenic manufacture of AgNPs, which uses biomaterials like plant extract and microbes as reducing agents. Several biomolecules, such as those present in plant extracts (Figure 1), are capable of oxidizing Ag+ to Ag0. These include ketones, flavonoids, tannins, aldehydes, phenolic acids, proteins, and carboxylic acids. The result of this process is AgNPs. UV-visible spectroscopy is a simple and commonly used analytical method that might be used to monitor AgNP production. Surface plasmon resonance (SPR) is the collective vibration of the conducting electrons in the outermost orbital of metal NPs in response to an electromagnetic field at certain wavelengths. Stimulation of AgNP surface plasmon resonance (SPR) results in the colloidal solution’s color and absorbance. SPR peaks about 435 nm are often used to demonstrate the reduction of silver nitrate into AgNPs. While the absorbance spectra of anisotropic particles may show two or more SPR bands, those of spherical NPs generally show just one. When there is no peak between 335 and 560 nm in UV-Vis spectra, it is commonly thought that NPs are not aggregating 5.
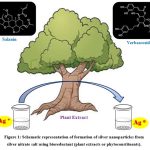 |
Figure 1: Schematic representation of formation of silver nanoparticles from silver nitrate salt using bioreductant (plant extracts or phytoconstituents).
|
This article will also show novel approaches to producing AgNPs with potential human health benefits. An abundance of studies has been devoted to both the biogenic manufacture of silver nanoparticles utilizing a wide range of plants and their use in inhibiting bacterial growth. It also explained how the size and form of AgNP affect their efficacy against different types of harmful bacteria. As NPs have the predisposition to form enormous aggregates that precipitate, limiting their efficiency, success in the synthesis of metal NPs depends not only on their size and shape but also on their stability.
Protocols for the Biosynthesis of AgNPs
AgNPs are produced via a straightforward, one-step biological process that yields no harsh or dangerous byproducts, making them economical, effective, and ecologically safe. Recent years have seen much research into the biosynthesis of AgNPs of diverse sizes, morphologies, stability, and antibacterial efficacy in plants and microbes. 6. A variety of plant parts, including roots, leaves, fruits, rhizomes, flowers, etc. have been successfully employed to create AgNPs. Plant parts are taken from various sources, properly cleansed using ordinary water, and then distilled to eliminate dirt and other unwanted components. After that, the components are either utilized fresh to form extract or dried and crushed to generate powder 7. As prolonged high-degree heating may induce phytochemical degradation in the biomass extract, the chopped pieces or powdered plant components are cooked in deionized water or alcohol for a few hours at a temperature below 60°C to create the extract. As a metal precursor, plant extracts with different pH levels were added to solutions with different concentrations of Ag salt. These mixtures were then heated at different temperatures to create AgNPs 8. Due to the dual roles played by the extract’s biomaterials—as a reducing agent and a stabilizing agent during synthesis—chemical stabilizers are unnecessary. Visual color changes or UV-Vis spectroscopy may be used to monitor AgNP synthesis, with a noticeable peak at roughly 430-450 nm induced by the surface plasmon resonance (SPR) of AgNPs. After the successful synthesis of AgNPs, the resulting mixture is centrifuged at high rpm to separate the NPs, followed by solvent washing and drying in a low-temperature oven 9.
Factors Affecting Nanoparticle Formation
Even after decades of research, understanding antibacterial characteristics and conducting an efficient green synthesis of AgNPs remain challenging processes. Nonetheless, we may make certain assumptions based on the literature that might result in AgNPs with potent antibacterial activity. The following factors should be taken into account during the creation of AgNPs, the investigation of green synthesis and antibacterial activity is intricate, and hence presents a challenge
Composition of plant extract
During the reduction of Ag+ to Ag0, several biomolecules, including flavonoids, ketones, aldehydes, tannins, carboxylic acids, phenolic acids, and plant protein, are predicted to be oxidized. In addition, the biomolecules that serve as a capping agent for the manufactured AgNPs control both their stability and their size. The first step towards successful AgNP production is an analysis of the biomolecules in the plant extract and how well they cap the AgNP. Typically, the average particle size is reduced and the capping activity is increased as NPs become more stable. Yet sometimes, this is not the case. As a consequence, specific interactions between produced NPs and biomolecules must be taken into account individually 10.
Concentration of plant extract
The created AgNPs’ form and size are dependent on the amount of plant extract utilized. Further research is required since its possible that NP synthesis doesn’t even happen at low doses. In most cases, the color of the produced AgNPs shifts, and there is a notable UV-Vis absorption at 430 cm-1. As the concentration of the extract increases, a huge number of NPs appear at that location. Nevertheless, subsequent reduction on the surface of the prepared nuclei may occur at high concentrations of extract due to the presence of excess reducing agents 11. While a desirable amount of extract may provide well-dispersed AgNPs with strong antibacterial activity, this is not always the case.
AgNO3 concentration
When AgNO3 concentrations rose, more AgNPs formed, which meant that all of the Ag+ had been converted to Ag0. This was easily seen by boosting UV-Vis spectroscopy intensity. After all of the AgNO3 has been used, equilibrium is attained. As a consequence, it is necessary to assess the equilibrium between AgNO3 and the extract’s lowering agent concentration 12.
Solvent for extraction
The efficiency of extracting the necessary biochemicals for AgNP synthesis is strongly reliant on the extraction solvent used since different biochemicals in plants have differing degrees of solubility in different solvents. Ethanol, methanol, and their mixtures with water are all recognized to be excellent solvents for phenolic compounds (ethanol-water or methanol-water). These are the preferred extraction solvents as a consequence, along with pure water 13.
Temperature and extraction time
Extraction temperature is also another important consideration for the efficient production of biogenic AgNPs. Its good knowing that raising the extraction temperature and lengthening the extraction time makes biochemicals more soluble. As a consequence, a stronger reducing agent will be produced by extracting more chemicals at a higher temperature. Nonetheless, it is possible to extract non-reactive biochemicals or to break down biochemicals over an extended period of time at a higher temperature 14.
pH
The pH of plant extracts may change the electrical charges of the biomolecules, which may change the kind of their capping and stabilizing affinities and, as a consequence, the creation of NP. An increase in pH often causes NPs to produce at a quicker pace and with a more uniform size distribution. Nevertheless, under acidic conditions, production and aggregation slowed, resulting in bigger NPs. However, the precipitation of AgOH at high pH levels might be undesirable. If an external buffer is utilized, it is recommended that the pH be kept at 7 15.
Response
UV-Vis spectrometer readings show a redshift is thought to indicate that the size of the NPs is increasing with time. To maintain steady small NPs, it is crucial to pay careful attention to the reaction16.
Reaction temperature
While the chemical reduction of AgNO3 to AgNPs normally requires a high temperature, the RT reaction is the best choice from the perspectives of cost and green chemistry. With a few exceptions, the RT approach, however, often produces spherical NPs that are less susceptible to microbes when it comes to the generation of green NPs. Meanwhile, there is a great need for the synthesis of different NPs to serve specific purposes. According to a literature review, cubic, pentagonal, hexagonal, triangular, and rod-shaped nanowire AgNPs are most often produced above the reaction temperature (RT), but other variables like capping agents and stabilizer concentration must also be taken into account. As a consequence, when creating NPs with different forms for a particular function, notably as a powerful antibacterial 17, the reaction temperature must be taken into account,not only does warmth make the process go faster, but it also makes the NPs smaller.
Plant-Mediated Biogenic Synthesis of AgNPs
Aesculus hippocastanum
Küp et al. (2020) have demonstrated that special AgNPs mediated by this plant have the strongest antibacterial action (zone of inhibition of 20 mm) against the Gram-negative bacterium Pseudomonas aeruginosa. Remarkably, although having a significant influence on every bacterial strain tested, AgNPs had no affect on the fungus strains Candida albicans ATCC 10231, C. tropicalis ATCC 13803, and C. krusei ATCC 1424. For the microorganisms under investigation, the Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) of AgNPs were 0.19-12.5 μg/mL and 1.56-25 μg/mL, respectively 18.
Amaranthus gangeticus
Kolya et al. (2015), AgNPs made from A. gangeticus Linn leaf extract shown inhibitory effectiveness against Gram-positive bacteria, Gram-negative bacteria, and fungal species19.
Andrographis echioides
Elangovan et al. (2015) produced AgNPs with cubic, pentagonal, and hexagonal forms and diameters ranging from 68.06 nm to 91.28 nm using A. echioides leaf extract, and then they examined their bactericidal activity against various microbes. The findings demonstrated a high ZOI 20 in the case of Escherichia coli (28 mm) and Streptococcus aureus (23 mm) in 100 μg/mL AgNPs concentration 20.
Andrographis paniculata
Sinha and Paul (2015) showed that a leaf extract of this plant produces AgNPs with a distinctive cubic shape. Research on different AgNP forms is of great interest because of the shape-dependent effects of AgNPs against microorganisms. AgNPs demonstrated potent antibacterial efficacy against the Gram-negative bacterium P. aeruginosa with a high ZOI of 21.3 mm and a very low MIC of 3.125 mL mL-1. The author discovered a lower ZOI (16.6 mm) in E. coli because researchers discovered that other Gram-negative bacteria, including E. coli, had a thicker peptidoglycan layer than P. aeruginosa 21.
Artemisia vulgaris
Rasheed et al. (2015) stated that AgNPs mediated by A. vulgaris were discovered for the first time in 2017. AgNPs displayed significant inhibitory effects against the pathogens tested, according to antimicrobial tests, with S. aureus having the highest value (18 mm inhibition zone) 22.
Catharanthus roseus
Al-Shmgani et al. (2017) made AgNPs from this plant. Methods such as color change, UV-Vis spectrum, X-ray Diffraction (XRD), Fourier-transformed Infrared (FTIR) spectroscopy, and Atomic Force Microscopy (AFM) have been used to validate the biosynthesis of AgNPs. The color of the leaf extract changes from yellowish to reddish-brown after adding 2 mM AgNO3 and heating at 70°C for 3 minutes, indicating the generation of NPs. Crystalline NPs with grains ranging in size from 10 nm to 88 nm, with a mean size of around 49 nm, are visible using AFM. According to scientists, created AgNPs penetrated microorganisms’ cells and interfered with ATP synthesis, DNA replication, the production of reactive oxygen species (ROS), and cell structural damage 23.
Croton bonplandianum
Khanra et al. (2016) demonstrated that AgNPs produced by this plant are very powerful against bacteria. The minimal inhibitory dosages of generated AgNPs for E. coli, P. aeruginosa, and S. aureus were found to be 50, 45, and 75 μg/mL, respectively. It has been shown that Gram-positive bacteria with a thick cell wall (S. aureus) are less susceptible to cell wall deterioration than Gram-negative bacteria with a thin cell wall, such as E. coli and P. aeruginosa24.
Curcuma longa
Sathishkumar et al. (2010) made AgNPs from C. longa leaf extract and tested the antibacterial effectiveness of cotton fabric coated with AgNPs. The loading of AgNPs on the cotton fabric was confirmed by SEM analysis, which was helped by the EDX analysis. According to scientists, cotton fabric loaded with AgNPs manufactured from C. longa has shown excellent resistance to harmful microorganism development and may be utilized for a number of applications in medical patients and medical staff to fight microbial infection 25.
Jatropha curcas
Bar et al. (2009) created AgNPs, J. curcas leaves were collected from the Micro model complex. SEM measurements showed that the diameter of NPs was between 50 nm and 100 nm, while TEM analysis showed that there was variation in the shape and size of the particles (20–50 nm). The microbial cell had been fully killed, according to TEM analysis. Based on ZOI data, the synthesized NPs’ antibacterial properties were assessed, and the pattern of sensitivity was discovered to be E. coli > P. aeruginosa > B. cereus > S. enteric > L. monocytogenes > S. aureus 26.
Lantana camara
Using leaf extract from this plant, Ajitha et al. (2015) showed how to produce spherical AgNPs with the use of ultrasound. By reducing reaction time and improving reaction rate, ultrasonication is used to improve the biosynthesis of AgNPs. The generated AgNPs were shown to have effective antibacterial action against both Gram-positive bacteria and Gram-negative bacteria 27.
Maclurapomifera
In 2017, spherical AgNPs were made using this plant by Azizian-Shermeh et al. (2017). The produced NPs’ ZOI against E. coli (0.1 mg/mL concentration) was 23.4 mm, which is higher than the ZOI of the well-known antibiotic drug ampicillin 28.
Mentha aquatica
In the most recent syntheses of ultra sound-assisted AgNPs of size 8 mm, Mentha aquatica leaf extract was utilized by Nouri et al. (2020) as a reducing and capping agent. These are the smallest biogenic AgNPs ever found that we are aware of. Although NPs could be generated at RT, ultrasonography markedly sped up the reaction time to only 10 minutes as opposed to 1 hour at RT. According to the researchers, the phenolic chemicals in the Menthaaquatica leaf extract are oxidized in an alkaline environment to Quinone, which provides free electrons for the reduction of the Ag+ ion to Ag0 and the formation of the desired AgNPs. Due to their exceptionally tiny size, the AgNPs exhibited a very low MIC of 2.2 mg/mL for P. aeruginosa, confirming their outstanding efficiency against the tested pathogen 29.
Mukia maderaspatana
Leaf extract from Mukia maderaspatana was employed in the biosynthesis of AgNPs with a size range of 58 nm-458 nm by Samad et al. (2021). When tested against human pathogens such as B. subtilis, K. pneumonia, S. typhi, and S. aureus, the antibacterial efficiency of the synthesizedNP conjugated to ceftriaxone was compared to the pathogen-inhibitory effectiveness of the free NP and antibiotic. The AgNPs conjugated with ceftriaxone displayed the highest inhibitory activity in compared to the other AgNPs 30.
Prosopis farcta
At room temperature (RT), Miri et al. (2015) biosynthesized AgNPs with an average size of 10.8 nm using Prosopis farcta extract. Gram-positive (S. aureus, B. subtilis) and Gram-negative (E. coli, P. aeruginosa) microorganisms were used to test the antibacterial activity of the generated AgNPs using the disc diffusion method. The results showed that the inhibitory diameter increased for each pathogen evaluated, showing that manufactured AgNPs cause cellular injury to bacteria and may thus be used as nano-antibiotics31.
Skimmia laureola
So far, a number of leaf extracts from the plant S. laureola have been employed in the biosynthesis of AgNPs by Ahmed et al. (2015). A 38 nm-diameter spherical AgNP with the antimicrobial properties of E. coli, K. pneumoniae, P. aeruginosa, P. vulgaris, and S. aureus was shown to be able to be produced by S.laureola32.
Solanum nigrum
AgNPs with an average size of 3.46 nm were recently made by Jinu et al. (2017) using a plant leaf extract from the S. nigrum species. With NPs as small as 1.74 nm, this is one of the smallest biogenic AgNPs yet to be found. By using SPR bands at 442 nm in UV-visible spectroscopy, AgNP generation was confirmed. Interestingly, AgNPs had superior antibacterial action compared to AuNPs and PdNPs. According to HRTEM results, this may be explained by the fact that AgNP nanoparticles cap more effectively than Au or PdNPs, producing well-dispersed, small AgNPs with less agglomeration. The polyphenols in the S. nigrum extract create a bad environment around the particles, defeating the van der Waals force of attraction and inhibiting AgNP aggregation. AgNPs exhibited a ZOI of 22 mm against E. coli at a dosage of 10 μg/mL, whereas Au NPs and Pd NPs had ZOIs of 20 mm and 19 mm, respectively. AgNPs’ enhanced antibacterial activity may be due to many factors, including their smaller size (3.46 nm) as compared to Au (9.39 nm) and PdNPs (21.55 nm), which the authors attribute to their effective capping 33.
Trichoderma viride
Using T. viride extract, another fascinating investigation on the shape-dependent activity of biogenic AgNPs was reported by Elgorban et al. (2016). The scientists discovered that pentagonal and hexagonal NPs showed higher antibacterial activity than spherical NPs when their diameters were equivalent. Pentagonal, hexagonal, and spherical AgNPs were produced using a variety of physical variables, including temperature, pH, and reaction time. At neutral pH, spherical NPs were seen under all reaction conditions. After 72 hrs of incubation at pH 5.0 and 9.0, rectangular and pentagonal/hexagonal NPs were generated at 40°C. In general, lengthier reactions result in bigger NPs, while higher temperatures invariably produce smaller NPs. Additionally, it was found that triangular-shaped AgNPs had better antimicrobial activity than spherical and rod-shaped AgNPs because they have a higher percentage of facet (1 1 1) with a high atomic density, which improves the efficiency of Ag binding to sulfur-containing components, compared to spherical and rod-shaped particles’ high percentage of (1 0 0) facets34.
Urtica dioica
A remarkable research on the creation of AgNPs using U. dioica leaf extract was released by Jyoti et al. (2016), and it showed that the herb had a positive synergistic effect with well-known antibacterial drugs. Remarkably, the generated AgNPs not only displayed potent antimicrobial activity against a wide range of microbes, but they also worked well with medicines to increase their antibacterial power. The combination of amoxicillin and AgNPs against S. marcescens demonstrated the synergistic impact of AgNPs by causing a 17.8 fold increase in ZOI 35. Table 1 described some common plants used for the biosynthesis of AgNPs.
Table 1: Some common plants used for biosynthesis of AgNPs.
|
S. No. |
Plant |
Plant Part |
Size of AgNPs (nm) |
References |
|
1. |
Abutilon indicum |
leaf |
5-25 |
18 |
|
2. |
Acalypha indica |
leaf |
20-30 |
19 |
|
3. |
Allium cepa |
whole plant |
33.6 |
20 |
|
4. |
Allium sativum |
whole plant |
3-6 |
21 |
|
5. |
Andrographis paniculata |
leaf |
13-27 |
22 |
|
6. |
Astragalus gummifer |
gum |
12-14 |
23 |
|
7. |
Azadirachta indica |
leaf |
5-35 |
24 |
|
8. |
Bacopa monniera |
leaf |
10-30 |
25 |
|
9. |
Camellia sinensis |
leaf |
40 |
26 |
|
10. |
Carica papaya |
fruit |
15 |
27 |
|
11. |
Citrus lemon |
fruit |
2-10 |
28 |
|
12. |
Citrus sinensis |
peel |
35 |
29 |
|
13. |
Datura metel |
leaf |
16-40 |
30 |
|
14. |
Glycyrrhiza glabra |
root |
20-30 |
31 |
|
15. |
Murraya koenigii |
leaf |
10 |
32 |
|
16. |
Ocimum sanctum |
leaf |
10-20 |
33 |
|
17. |
Piper betle |
leaf |
5-30 |
34 |
|
18. |
Piper longum |
leaf |
17-41 |
35 |
|
19. |
Piper nigrum |
flower |
5-50 |
36 |
|
20. |
Swietenia mahogany |
leaf |
10-25 |
37 |
|
21. |
Zingiber officinale |
leaf |
10-30 |
38 |
Future Perspectives
The larger the NPs are, the more their surface area comes in touch with the microbial cell, increasing the NPs’ antibacterial properties. The following is a list of the antibacterial activity shown by AgNPs: spherical > triangular > pentagonal > hexagonal > cubic > nano-rod [36]. Due to its sharp edge and majority stable facet, the triangular one had the greatest amount of activity. Hexagonal, cubic nano-rods have a bend edge compared to triangular shape NPs, which may reduce their efficiency against microbes 37. Spherical form NPs with no sharp edges and mostly facets had the least antibacterial effects. Gram-negative bacteria (like E. coli) have a cell wall made of lipopolysaccharides on the outside and a peptidoglycan layer (7-8 nm) beneath, whereas Gram-positive bacteria (like S. aureus) possess peptidoglycan (20-80 nm) layer. Recent research has demonstrated that Gram-positive bacteria are less susceptible to the antimicrobial effects of AgNPs than Gram-negative bacteria. Yet this isn’t always the case 38. As a result, further investigations are needed to understand the function of lipopolysaccharides in Gram-negative bacteria, which may play a role in protecting them from certain AgNPs. Additionally, the interaction between AgNPs and biomolecules needs to be studied, as it may affect how NPs interact with cell walls. As a consequence, it is still difficult to figure out the interaction’s underlying mechanism 39. The synergistic interactions between AgNPs and several well-known antibiotics may greatly increase the antibacterial efficacy of AgNPs. The battle against a swarm of newly discovered, highly contagious, multidrug-resistant bacteria 40, opens up a whole new realm of possibilities. The mechanism of AgNPs’ interactions with drugs as well as the shift in the mode of attack brought on by the synergistic interaction with microbes must be completely comprehended and experimentally confirmed in order to have a better understanding 41.
Conclusion
Research in the area of biomass-assisted nanomaterial’s synthesis will very certainly go on to receive a great deal of attention in the years to come given the numerous benefits of using plant extracts to greenly synthesize AgNPs and their exceptional antibacterial properties whether used alone or in combination with antibiotic medications. This article presents a comprehensive review of several biogenic methods for the manufacture of AgNPs using non-toxic cum affordable phytochemicals and ecologically friendly approaches. Also highlighted is the AgNPs’ antibacterial susceptibility to a range of dangerous pathogens. Therefore, a deeper understanding of the individual phytochemicals, their concentrations, and their interactions is necessary to develop shape-selective biogenic NPs.
Conflict of Interest
There is conflict of interest
Funding Sources
There are no funding source.
References
- Yadav SR, Hodiwala AV, Patil P, Thakur M. Physiochemical characterization & antibacterial properties of biologically synthesized silver nanoparticles from aqueous extracts of ginger. J Med Pharm Allied Sci. 2021;10(4):3328-3333.
CrossRef - Nanophytoremediation for textile dye degradation using abutilon indicum silver nanoparticles: photo catalytic activities. J Med Pharm Allied Sci. 2021;10(6):3960-3964.
CrossRef - Green adeptness in the synthesis and stabilization of copper nanoparticles using aqueous root extract of SchreberaswietenioidesRoxb, and its catalytic application. J Med Pharm Allied Sci. 2022;11(1):4233-4240.
CrossRef - Physiochemical characterization of silver nanoparticles using rhizome extract of Alpinia galanga and its antimicrobial activity. J Med Pharm Allied Sci. 2022;2(2):219-223.
- In-vitro cytotoxic potential of selenium nanoparticles biosynthesized using Embeliaribes fruits. J Med Pharm Allied Sci. 2021;10(6):3534-3539.
- Mustapha T, Misni N, Ithnin NR, Daskum AM, Unyah NZ. A Review on Plants and Microorganisms Mediated Synthesis of Silver Nanoparticles, Role of Plants Metabolites and Applications. Int J Environ Res Public Health. 2022;19(2):674.
CrossRef - Hiba H, Thoppil JE. Medicinal herbs as a panacea for biogenic silver nanoparticles. Bull Nat Res Centre. 2022;46(1):1-5.
CrossRef - Panda BS. A Review on Synthesis of Silver Nanoparticles and their Biomedical Applications. Lett. Appl. NanoBioScience. 2022;11:3218-31.
CrossRef - Ahmed O, Sibuyi NR, Fadaka AO, Madiehe MA, Maboza E, Meyer M, Geerts G. Plant Extract-Synthesized Silver Nanoparticles for Application in Dental Therapy. Pharmaceutics. 2022;14(2):380.
CrossRef - Mahapatra DK, Kumar Bharti S, Asati V. Nature inspired green fabrication technology for silver nanoparticles. CurrNanomed. 2017;7(1):5-24.
CrossRef - Dadure KM, Mahapatra DK, Haldar AG, Chaudhary RG, Potbhare AK. Phytofabrication of nickel-based nanoparticles: focus on environmental benign technology and therapeutic perspectives. InBiogenic Sustainable Nanotechnology 2022 (pp. 41-57). Elsevier.
CrossRef - Mahapatra DK, Tijare LK, Gundimeda V, Mahajan NM. Rapid Biosynthesis of Silver Nanoparticles of Flower-like Morphology from the Root Extract of Saussurealappa. Res Rev J Pharmacog. 2018;5(1):20-4.
- Mahapatra DK, Bharti SK. Research Progress and New Insights in Biosynthesis of Silver Nanoparticles with Particular Applications. ChemNanosciNanotechnol. 2019:195-240.
CrossRef - Haldar AG, Mahapatra DK, Dadure KM, Chaudhary RG. Phytofabrication of metal oxide/iron-based and their therapeutic and their therapeutic potentials: in-depth insights into the recent progress. InBiogenic Sustainable Nanotechnology 2022 (pp. 185-216). Elsevier.
CrossRef - Telrandhe R, Mahapatra DK, Kamble MA. Bombaxceiba thorn extract mediated synthesis of silver nanoparticles: Evaluation of anti-Staphylococcus aureus activity. Int J Pharm Drug Anal. 2017:376-9.
- Mahapatra DK, Haldar AG, Dadure KM. Highlights of decade long progress of nano-selenium fabricated from plant biomass: insights into techniques and mechanisms. InBiogenic Sustainable Nanotechnology 2022 (pp. 217-226). Elsevier.
CrossRef - Mahapatra DK, Bharti SK. Recent Advances in Bioorganism-Mediated Green Synthesis of Silver Nanoparticles: A Way Ahead for Nanomedicine. Res Method ApplChemBiol Eng. 2019:275-89.
CrossRef - Küp FÖ, Çoşkunçay S, Duman F. Biosynthesis of silver nanoparticles using leaf extract of Aesculushippocastanum (horse chestnut): Evaluation of their antibacterial, antioxidant and drug release system activities. Mater Sci Eng. 2020;107:110207.
CrossRef - Kolya H, Maiti P, Pandey A, Tripathy T. Green synthesis of silver nanoparticles with antimicrobial and azo dye (Congo red) degradation properties using Amaranthusgangeticus Linn leaf extract. J Anal Sci Technol. 2015;6(1):1-7.
CrossRef - Elangovan K, Elumalai D, Anupriya S, Shenbhagaraman R, Kaleena PK, Murugesan K. Phyto mediated biogenic synthesis of silver nanoparticles using leaf extract of Andrographisechioides and its bio-efficacy on anticancer and antibacterial activities. J PhotochemPhotobiol. 2015;151:118-24.
CrossRef - Sinha SN, Paul D. Phytosynthesis of silver nanoparticles using andrographispaniculata leaf extract and evaluation of their antibacterial activities. SpectrLett. 2015;48(8):600-4.
CrossRef - Rasheed T, Bilal M, Iqbal HM, Li C. Green biosynthesis of silver nanoparticles using leaves extract of Artemisia vulgaris and their potential biomedical applications. Coll Surf. 2017;158:408-15.
CrossRef - Al-Shmgani HS, Mohammed WH, Sulaiman GM, Saadoon AH. Biosynthesis of silver nanoparticles from Catharanthusroseus leaf extract and assessing their antioxidant, antimicrobial, and wound-healing activities. Artif Cell NanomedBiotechnol. 2017;45(6):1234-40.
CrossRef - Khanra K, Panja S, Choudhuri I, Chakraborty A, Bhattacharyya N. Antimicrobial and cytotoxicity effect of silver nanoparticle synthesized by Croton bonplandianumBaill. leaves. Nanomed J. 2016;3(1):15-22.
- Sathishkumar M, Sneha K, Yun YS. Immobilization of silver nanoparticles synthesized using Curcuma longa tuber powder and extract on cotton cloth for bactericidal activity. Biores Technol. 2010;101(20):7958-65.
CrossRef - Bar H, Bhui DK, Sahoo GP, Sarkar P, Pyne S, Misra A. Green synthesis of silver nanoparticles using seed extract of Jatrophacurcas. Coll Surf PhysicochemEng Asp. 2009;348(1-3):212-6.
CrossRef - Ajitha B, Reddy YA, Shameer S, Rajesh KM, Suneetha Y, Reddy PS. Lantana camara leaf extract mediated silver nanoparticles: antibacterial, green catalyst. J PhotochemPhotobiol. 2015;149:84-92.
CrossRef - Azizian-Shermeh O, Einali A, Ghasemi A. Rapid biologically one-step synthesis of stable bioactive silver nanoparticles using Osage orange (Maclurapomifera) leaf extract and their antimicrobial activities. Adv Powder Technol. 2017;28(12):3164-71.
CrossRef - Nouri A, Yaraki MT, Lajevardi A, Rezaei Z, Ghorbanpour M, Tanzifi M. Ultrasonic-assisted green synthesis of silver nanoparticles using Menthaaquatica leaf extract for enhanced antibacterial properties and catalytic activity. CollInterfSciCommun. 2020;35:100252.
CrossRef - Samad N, Farooq S, Khaliq S, Ahmed S, Alam M, Mustafa S, Raza U, Nadeem W. Biosynthesis of silver nanoparticles from Mukiamaderaspatana (L) and their biological activities. Pak J Pharm Sci. 2021;34:1837-1847.
- Miri A, Sarani M, Bazaz MR, Darroudi M. Plant-mediated biosynthesis of silver nanoparticles using Prosopisfarcta extract and its antibacterial properties. Spectrochim ActaMolBiomolSpectr. 2015;141:287-91.
CrossRef - Ahmed MJ, Murtaza G, Mehmood A, Bhatti TM. Green synthesis of silver nanoparticles using leaves extract of Skimmialaureola: characterization and antibacterial activity. Mater Lett. 2015;153:10-3.
CrossRef - Jinu U, Jayalakshmi N, SujimaAnbu A, Mahendran D, Sahi S, Venkatachalam P. Biofabrication of cubic phase silver nanoparticles loaded with phytochemicals from Solanumnigrum leaf extracts for potential antibacterial, antibiofilm and antioxidant activities against MDR human pathogens. J Clust Sci. 2017;28(1):489-505.
CrossRef - Elgorban AM, Al-Rahmah AN, Sayed SR, Hirad A, Mostafa AA, Bahkali AH. Antimicrobial activity and green synthesis of silver nanoparticles using Trichodermaviride. BiotechnolBiotechnol Equip. 2016;30(2):299-304.
CrossRef - Jyoti K, Baunthiyal M, Singh A. Characterization of silver nanoparticles synthesized using Urticadioica Linn. leaves and their synergistic effects with antibiotics. J Radiat Res Appl Sci. 2016;9(3):217-27.
CrossRef - Tehri N, Vashishth A, Gahlaut A, Hooda V. Biosynthesis, antimicrobial spectra and applications of silver nanoparticles: Current progress and future prospects. Inorg Nano Metal Chem. 2022 Jan 2;52(1):1-9.
- Telrandhe R, Bharti SK, Mahapatra DK. Anti-Proliferative Potentials of Silver Nanoparticles Synthesized from Natural Biomass. In: Applied Pharmaceutical Science and Microbiology 2020 (pp. 117-132). Apple Academic Press.
CrossRef - HabeebRahuman HB, Dhandapani R, Narayanan S, Palanivel V, Paramasivam R, Subbarayalu R, Thangavelu S, Muthupandian S. Medicinal plants mediated the green synthesis of silver nanoparticles and their biomedical applications. IET Nanobiotechnol. 2022;16(4):115-44.
CrossRef - Huq MA, Ashrafudoulla M, Rahman MM, Balusamy SR, Akter S. Green synthesis and potential antibacterial applications of bioactive silver nanoparticles: A review. Polymers. 2022;14(4):742.
CrossRef - Betihaa MA, Kheiralla ZM, Mansour AS, Emam AN, El-Henawya SB, Mohameda EA, Negm NA. A Review on Different Plants Extract Mediated Silver Nanoparticles: Preparation, Antimicrobials, and Antioxidant. Egypt J Chem. 2022;65(5):575-589.
- Khan F, Shariq M, Asif M, Siddiqui MA, Malan P, Ahmad F. Green Nanotechnology: Plant-Mediated Nanoparticle Synthesis and Application. Nanomaterials. 2022;12(4):673.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





