Manuscript accepted on : 08-03-2022
Published online on: 24-03-2022
Plagiarism Check: Yes
Reviewed by: Dr. Salim Albukhaty
Second Review by: Dr. Ahmed Lafi, Dr. Abigail Ogbonna
Final Approval by: Dr. Fernando José Cebola Lidon
Sally A. Ali1*, Shimaa Zakarya2 and Shimaa Khaled2
1Botany and Microbiology Department, Faculty of Science, Helwan University, Cairo 11795, Egypt.
2Chemistry Department, Faculty of Science, Helwan University, Cairo 11795, Egypt.
Corresponding Author E-mail: Sally_ali@science.helwan.edu.eg
DOI : http://dx.doi.org/10.13005/bbra/2980
ABSTRACT:
The accumulation of low density polyethylene, used extensively in packaging for industrial and agricultural applications, in the ecosystem is a great threat. This study focuses on the isolation of micro-biota from the plastic polluted sites to screen and optimise their potential for low density polyethylene (LDPE) film biodegradation. Firstly, the plastic samples from soil dumping plastic debris and plastic polluted water were collected; then fungi and bacteria were isolated using potato dextrose agar media and nutrient agar media, respectively, while screening low density polyethylene film biodegradation performed on mineral salt media (MSM) using the isolated micro-biota. The measurement of the potential biodegradation was assessed by visual observation. The most microbial colonization for low density polyethylene films was identifying molecular which was then utilized for optimisation of the biodegradation processes with different parameters such as media type, inoculum size, shaking speed, different incubation temperature and pH at different incubation time. Then the weight loss in the LDPE films percentage was calculated measuring dry mycelium weight and bacterial absorbance. The results revealed that, among the isolated micro-biota fifteenth, the most colonization was Fusarium equiseti and Brevibacillus parabrevis depending on the scanning electron microsope (SEM) and Fourier transform infrared (FTIR) analysis, in addition to optimum media, inoculum size, shaking speed, incubation temperature, pH, MSM, 2 disks and 2 ml, 30˚ C and 35˚C, pH5 and pH7 for 30:20 days for F.equiseti and B.parabrevis, respectively. The overall results confirmed that F.equiseti and B.parabrevis from the plastic polluted sites play an essential role in low density polyethylene films biodegradation.
KEYWORDS: Biodegradation; Low density polyethylene films; Optimisation; Potential
Download this article as:| Copy the following to cite this article: Ali S. A, Zakarya S, Khaled S. Screening and Optimisation of the Biodegradation Potential for Low Density Polyethylene (LDPE) Films by Fusarium Equiseti and Brevibacillus Parabrevis. Biosci Biotech Res Asia 2022;19(1). |
| Copy the following to cite this URL: Ali S. A, Zakarya S, Khaled S. Screening and Optimisation of the Biodegradation Potential for Low Density Polyethylene (LDPE) Films by Fusarium Equiseti and Brevibacillus Parabrevis. Biosci Biotech Res Asia 2022;19(1). Available from: https://bit.ly/3urOZvJ |
Introduction
Plastic are polymeric compounds, nowadays synthetic plastic apply at many different fields due to their advantages such as durability and tenability1. The plastic waste disposal into the environment and land pollution problems has led to concern about plastics waste management 2.
The plastic waste accumulation, caused a serious threat to the environment. Furthermore, swallowing the waste plastic debris by animals affect animal health3. Because of the disadvantage of conventional plastic degradation methods, the biological biodegradation methods become more demanding to be apply4. Polyethylene is a synthetic plastic commercially produced, while chemically it is petroleum-derived terephthalate acid (TPA) and ethylene glycol. Annually low density polyethylene production reached 60%. Due to the absence of appropriate disposal methods, polyethylene wastes are usually burnt in open areas which cause environmental pollution1.
Biodegradation is eco-friendly process relies on living organisms to degrade the polymeric compounds5. There are different factors affecting the plastic biodegradation process such as surface area, organism type, polymer nature, temperature, pH, and the addition of nutrients. The degradation process carried out at different steps. Firstly, the plastic is converted to its simple form, and then are mineralized6. Recently, using of microorganisms to degrade the plastic wastes becomes more attractive rather than the chemical and physical disposal methods. The most commonly used methods for the evaluation of plastic biodegradation process are (SEM), and (FTIR) 7. Moreover, the mass loss of test specimens widely applied degradation tests to measure weight loss or determine residue polymer. The main distinguishing objectives of this study are: screening and optimization the biodegradation potential for low density polyethylene films using F.equiseti and B.parabrevis as novel microbial strains, and local eco-friendly methods.
Materials and Methods
Materials
Low density polyethylene (LDPE) used in this study was obtained in the form of LDPE films from plastic bags of market, Cairo, Egypt.
Media
Media for microbial isolation were added in g/L. Rose Bengal was added to inhibit bacterial growth; pH was adjusted to pH7 using either (0.1 M) NaOH and (0.1 M) HCL solution, media supplied by Mycology lab, Faculty of science, Helwan University.
Potato-Dextrose Agar8 (PDA)
Infused white potato 200
Glucose 20
Agar 20
Nutrient Agar 9
Peptone 5
Beef extract 3
NaCl 5
Methods
Sample collection
Plastic samples were collected from different plastic polluted sites as shown in Picture (1):
 |
Picture 1: Isolates Sites (www.google.com/maps). |
Soil dumping of plastic debris was obtained from Tura El-Asmanet Metro Station, Cairo, Egypt as shown in Picture (2E-2F). Biodegradable microorganisms from soil were collected from surface and 10 cm depth. Few grams of soil were transferred into the laboratory and stored until used at room temperature.
 |
Picture 2: Collection sites (2A-2D) Abbas Bridge and Scout Club, (2E-2F) Tura El-Asmanet Metro Station, Cairo, Egypt. |
Water polluted by plastic was obtained from below Abbas Bridge and Scout Club, Cairo, Egypt as shown in Pictures (2A-2D), (3).
Water samples were collected from surface and depth 10 cm through sterile glass bottle (Screw Cap bottles) and stored until used at 4˚C.
 |
Picture 3: Water samples were collected from the surface through sterile glass bottle (Screw Cap bottles) and stored until used at 4˚C. |
Isolation of micro-biota
Isolation from soil
The collected soil was sieved through mesh with a 2mm pore size sieve; then, two media for fungal and bacterial isolation were used by the isolation method (serial dilution10). One gram of soil was added in 9 ml of sterile distilled water, and this suspension vortex was left to settle down.
Next, a series of 10-fold dilution was used to isolate fungi. One ml of each dilution was cultured on potato dextrose agar (PDA); meanwhile, in case of bacteria, nutrient agar media was used.
Isolation from water
Water samples were prepared for fungal and bacterial isolation by serial dilution methods, and the plates were prepared through pouring plate techniques using PDA and Nutrient Agar media. Pure cultures were sub-cultured and then preserved until used.
Screening of the isolated micro-biota biodegradation potential for low density polyethylene films
Basal media were prepared as previously described by Esmaeili et al11 with a slight modification. Briefly, the synthetic mineral salt media (MSM) composition was as follows: (g/L: K2HPO4, 1.2; KH2PO4, 0.14; NaNO3, 2; MgSO47H2O,0.5; KCl, 0.5; FeSO47H2O, 0.01; MnSO4H2O, 0.001; CuSO45H2O, 0.001; ZnSO47H2O, 0.001; Agar, 15; pH 7.0). Then it was autoclaved and poured into petri-dishes. Suspension of microbial isolates was prepared as described previously by Ibrahim et al12 by suspending mycelium plug 8 mm diameter of fungal growth in test tubes (9% NaCl w/v). Bacteria were streaked into the same test tubes. (LDPE) films were cut into strips with dimensions of 1.5 x 1.5 cm weighed and cleaned (30 min in 70 % ethanol), air-dried for 15 min and added to petri-dishes supplemented 15 ml of mineral salt medium (MSM), and each test isolate (1 ml of suspension) was added over the (LDPE) films. Control (+ve) was (LDPE) films and (MSM) while the control (-ve) was 1 ml suspension of test isolates and (MSM). Then cultures were incubated at 30˚C for 21 days.
Identification of the most microbial colonization of LDPE films
Molecular identification of F.equiseti
The fungal strain was grown using Czapek`s yeast extract agar (CYA) for Penicillim species and V8 Juice for Alternaria species followed by incubation for 7 days at 28˚C 13. The growing culture was prepared for DNA extraction using Patho-gene-spin DNA/RNA extraction kit provided by the Intron Biotechnology Company, Korea.
The fungal DNA was then sent to SolGent Company, Daejeon, South Korea for polymerase chain reaction (PCR) and rRNA gene sequencing. PCR was performed using ITS1 (forward) and ITS4 (reverse) primers which were incorporated in the reaction mixture.
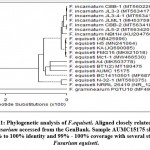
Primers have the following composition: ITS1 (5′ – TCC GTA GGT GAA CCT GCG G – 3′), and ITS4 (5′- TCC TCC GCT TAT TGA TAT GC -3′). The purified PCR product (amplicons) was sequenced with the same primers with the incorporation of ddNTPs in the reaction mixture14. The obtained sequences were analyzed using Basic Local Alignment Search Tool (BLAST) from the National Center of Biotechnology Information (NCBI) website. Phylogenetic analysis of sequences was implemented with the help of Meg Align (DNA Star) software version 5.05.
Molecular identification of B. parabrevis
Bacterial strain was cultured in sterile test tubes containing 10 ml of nutrient broth medium9. Culture was incubated at 28ºC for 48 hours.
Patho-gene-spin DNA/RNA extraction kit provided by the Intron Biotechnology Company, Korea was used. The extracted DNA samples were sent to SolGent Company, Daejeon, South Korea for polymerase chain reaction (PCR) and gene sequencing. PCR was performed using two universal primers namely 27F (5’-AGAGTTTGATCC TGGCTCAG-3’) and 1492R (5’-GGTTACCTTGTTACGACTT-3’).
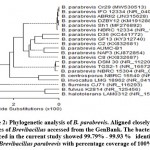
The purified PCR products (amplicons) were reconfirmed using a size nucleotide marker (100 base pairs) by electrophoresis on 1% agarose gel. Purified amplicons were sequenced in the sense and antisense directions using 27F and 1492R primers with the incorporation of dideoxynucleotides (dd NTPs) in the reaction mixture14. Sequences were further analyzed using Basic Local Alignment Search Tool (BLAST) from the National Center of Biotechnology Information (NCBI) website. Phylogenetic analysis of sequences was carried out using Meg Align (DNA Star) software version 5.05.
Measurement of biodegradation
Visual observation (microbial attack the plastic surface bio-film or hyphae penetration formation).
SEM in order to check for any changes in surface morphology.
III. FTIR analysis to detect the degradation on the basis of changes in the functional groups.
(SEM) analysis
The positive control and the treatment were prepared for (SEM) analysis (washing with70% ethanol). The samples were pasted onto the (SEM) sample stub using a carbon tape and the sample was coated with gold and analyzed under high-resolution scanning electron microscope (Quanta FEG 250, FEl, USA) at Desert Research Center, Cairo, Egypt.
(FTIR) analysis
Fourier transform infrared spectroscopic analysis was performed for the control and the treatment. The analysis was performed using Perkin-Elmer Spectrum version 10.5.4 at Central lab, Faculty of Science, Helwan University, Cairo, Egypt.
Weight loss measurement (determine of residue polymer)
The weight loss in LDPE film percentage calculated using the equation 1:
Weight loss in (LDPE) (%) = (Initial Weight – Final Weight) / Initial Weight) x 10015.
Where: Initial weight= before treatment.
Final weight= after treatment with F.equiseti and B.parabrevis.
Optimisation of the biodegradation potential of LDPE films by F.equiseti and B.parabrevis
The growth conditions effect such as media types, inoculum size, shaking speed, incubation temperature, pH at different incubation time were studied.
Effect of media types
There are two types of broth media used, namely, Czapek-Dox broth and mineral salt media (MSM). One disk (8 mm in diameter) of F.equiseti was inoculated into each of three sets of autoclaved flasks (250 ml) containing 50 ml of broth supplemented with sterilized LDPE films (1.5 cm x 1.5 cm), then it was incubated at 30˚C for 21 days. After incubation, the dry mycelium weight was determined through a fungal mycelium filtration by using vacuum filtration, then dried mycelia were weighed by using digital balance, and weight loss in the (LDPE) films percentage was also calculated. On the other hand, in the case of B.parabrevis, one ml (bacterial suspension) was inoculated into each of the three sets of autoclaved mineral salt media, separately supplemented with sterilized (LDPE) films (1.5 cm x 1.5 cm) as shown in Pictures (4A & 4B), then incubated at 35˚C for 21 days. After incubation, the bacterial growth was determined at 600 nm spectrophotometrically and weight loss in the LDPE film (%) was calculated. Positive control was media + plastic film (LDPE), while the negative control was media+ inoculum (B.parabrevis or F.equiseti).
Effect of inoculum size changes
Different inoculum size of F.equiseti (1, 2, 3 disks with 8 mm in diameter) and B. parabrevis (1, 2, 3 ml bacterial suspension) in three sets of 250 ml contained 50 ml (MSM) were supplemented with sterilized (LDPE) films (1.5 cm x 1.5 cm), then incubated at 30˚C and 35˚C for F.equiseti and B.parabrevis, respectively, for 21 days. After incubation, weight loss in (LDPE) films percentage was calculated.
Effect of shaking speed and static
Mineral salt media were supplemented with sterilized films and dispensed in three sets of 250 ml flasks containing 50 ml, and then the flasks were inoculated with two ml B.parabrevis. F. equiseti inoculated with two disks (8 mm in diameter) and incubated at both static and shaking speed (150 rpm) for 21 days at 30˚C and 35˚C for F. equiseti and B.parabrevis, respectively. After the end of the incubation, weight loss in the (LDPE) films percentage was calculated.
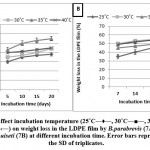
Effect of incubation temperature at different incubation times
Fifty ml of liquid (MSM) with one piece of LDPE film (1.5 cm x 1.5 cm) was prepared and filled into in 250 ml flasks, and it was incubated at different temperatures 25, 30, 35, and 40 ˚C in intervals of 5 days (i.e. 5, 10, 15, and 20 days) for B.parabrevis while in the case of F.equiseti, the intervals include 7, 14, 21, and 30 days. Optimal temperature was employed depending on the weight loss in (LDPE) films percentage.
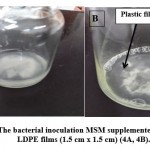 |
Picture 4: The bacterial inoculation MSM supplemented with sterilized LDPE films (1.5 cm x 1.5 cm) (4A, 4B). |
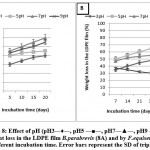
Effect of pH at different incubation times
The ability of B.parabrevis and F.equiseti to utilized (LDPE) as a sole source of carbon and energy, (MSM) with one piece of (LDPE) at different pH values (i.e. 3, 5, 7 & 9) was adjusted using phosphate and acetate buffers. Then the cultures were incubated at 30˚C and 35˚C for 21 days and 30 days for F.equiseti and B.parabrevis, respectively. The weight loss in (LDPE) films percentage was calculated after the incubation.
Results and discussions
Isolation of micro-biota
A total of fifteen, micro-biota were isolated from the soil dumping of plastic debris and water polluted of plastic. The isolates were labelled as 1S through 15 S. PDA was used for fungal isolation and nutrient agar for bacterial isolation as shown in Table (1). The first seven, micro-biota was isolated from the water polluted of plastic, and the other eight, micro-biota were isolated from the soil dumping of the plastic debris.
Table 1: Summary of the microbial species isolated from the plasticized polluted sites
| Microbial isolates | Source | Identification |
| 1S | Water | Bacteria sp1 |
| 2S | Water | Aspergillus sp1 |
| 3S | Water | Alternaria sp |
| 4S | Water | Trichoderma sp |
| 5S | Water | Cladosporium sp |
| 6S | Water | Aspergillus sp2 |
| 7S | Water | Bacteria sp2 |
| 8S | Soil | Bacteria sp3 |
| 9S | Soil | Fusarium sp1 |
| 10S | Soil | Aspergillus sp3 |
| 11S | Soil | Penicillum sp1 |
| 12S | Soil | Penicillum sp2 |
| 13S | Soil | Rhizopus sp |
| 14S | Soil | Apergillus sp4 |
|
15S |
Soil |
Fusarium sp2 |
Screening of the isolated micro-biota biodegradation potential for low density polyethylene films.
Screening micro-biota biodegradation potential was determined by visible observation through the growth observed above LDPE film as attack with the fungi as shown in Pictures (5A- 5C) and bacteria isolates. The results in Table (2) show that the fungal isolate named 9S and the bacterial isolate named 1S achieved a positive visual observation result when the growth of these two isolates was observed compared to other isolates after 21 days of incubation. These results agreement with Merina & Santosh16 that indicate, the formation and attachment of a biofilm on LDPE film. On the other hands, the microbial colonization on polymer surface is the first requirement for its biodegradation 17, 18.
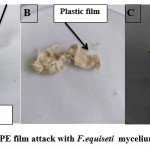 |
Picture 5: LDPE film attack with F.equiseti mycelium (5A, 5B, 5C). |
Table 2: Screening of the isolated micro-biota biodegradation potential for low density polyethylene films.
| Isolated Micro-biota | Visual observation |
| Bacteria sp1 | +ve |
| Aspergillus sp1 | -ve |
| Alternaria sp | -ve |
| Trichoderma sp | -ve |
| Cladosporium sp | -ve |
| Aspergillus sp2 | -ve |
| Bacteria sp2 | -ve |
| Bacteria sp3 | -ve |
| Fusarium sp1 | +ve |
| Aspergillus sp3 | -ve |
| Penicillum sp1 | -ve |
| Penicillum sp2 | -ve |
| Rhizopus sp | -ve |
| Apergillus sp4 | -ve |
| Fusarium sp2 | -ve |
Identification of the most microbial colonization of LDPE films shown in figures (1, 2).
Sample Fusarium sp1: Fusarium equiseti AUMC15175 (534 letters).
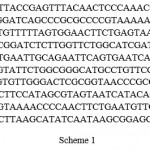 |
Scheme 1 |
Sample Bacteria sp1 Brevibacillus parabrevis AUMC-B1 (1405 letters)
 |
Scheme 2 |
Measurement of biodegradation
SEM analysis
Picture (6) shows SEM of LDPE film surface before and after 21 days of incubation with F.equiseti and B.parabrevis. Before, the sample had a smooth surface with no pits, cracks or any attached on the surface (Pic. 6A, 6B). However, after incubation with F.equiseti and B.parabrevis, surface with defects and changes was observed (Pic. 6C, 6D, 6E, 6F, 6G, 6H & 6I). For both F.equiseti and B.parabrevis, the different places on the surface of LDPE film colonized forming biofilm; this proved its strong adhering capabilities as well as LDPE utilization capacities.
These results are similar to those obtained by Merina and Santosh 16. In the case of F.equiseti, it was found the hyphal growth over LDPE film surface and hyphal penetration (Pic. 6C, 6D & 6E) while in the case of B.parabrevis, several cracks and cavities on the surface developed in addition to the biofilm formation were observed (Pic. 6F, 6G, 6H & 6I). Our results are agreed with11.
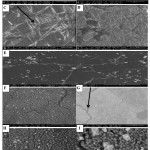 |
Picture 6: SEM analysis of (LDPE) film, control (6A, 6B), (LDPE) treatment with F.equiseti (6C, 6D, 6E), and LDPE treatment with B.parabrevis (6F, 6G, 6H, 6I). |
FTIR analysis
There was a variation in the intensity of bands after incubation with F.equiseti and B.parabrevis) compared with control as shown (Fig. 3A, 3B & 3C). For control spectrum, bands were assigned at 2915.03, 2848.13 cm-1 (both due to C-H stretch), 1471.99, 1463.03, 1368.22 cm-1 (C=C stretch), 908.56 cm-1 (C-H alkenes out of plane bend), and 729.99, 718.04 cm-1 (C-H bend-mono). Similar changes were observed in F.equiseti and B.parabrevis. The band at 1368.22 cm-1 corresponds to C=C and the band at 908.56 cm-1 corresponds to C-H alkenes that has been disappearing in the case of F.equiseti and B.parabrevis , this confirms the depolymerization activity of F.equiseti. These results are similar to those obtained by Merina and Santosh16. Moreover, the band appeared at 729.99 cm-1 for the control, and a shift in the band was observed at 730.13, 730.03 cm-1 in the sample treated by F.equiseti and B.parabrevis, respectively. Nupur et al18 carried out a similar study and observed that, the band appeared at control film and a shift in (LDPE) film, which was subjected to microbial treatment degradation of polyethylene by the fungal consortium.
 |
Figure 3: FTIR analysis, (3A) control (LDPE film only), (3B-3C) (LDPE) film treatment with F.equiseti and B.parabrevis, respectively. |
Optimisation of the biodegradation for LDPE films by F.equiseti and B.parabrevis
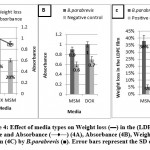
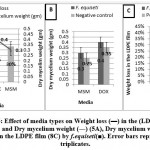
Effect of media types
The obtained results in Figures (4A- 4C & 5A-5C) show that the weight loss in the (LDPE) films % was achieved using (MSM) whereas the optimum absorbance for B.parabrevis and the optimum dry mycelium weight for F.equiseti were achieved using Dox’s media, compared to positive and negative control.
Therefore, (MSM) is chosen to optimize the weight loss in the (LDPE) films percentage. This observation may go back to MSM’s defect to carbon source while Dox’s media contain carbon source which retards (LDPE) film as a source of carbon.
Effect of inoculum size changes
The results in Figure (6A) reveal that the best inoculum size at which the highest weight loss in (LDPE) films percentage for B.parabrevis and F.equiseti was achieved was two ml and two disks, respectively.
In addition, there was not a significant difference between two and three ml/disks. Thus, two ml/disks were chosen for optimizing the weight loss in (LDPE) films.
Effect of shaking speed and static
The results in Figure (6B) show that the optimum weight loss in LDPE was achieved at static condition compared to shaking conditions after incubation for 21 days using MSM media with the following values: 40% and 45% for B.parabrevis and F.equiseti, respectively. These may be due to static conditions allowing the (LDPE) colonization by B.parabrevis and F.equiseti incontast shaking conditions.
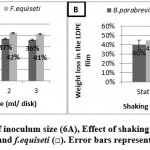 |
Figure 6: Effect of inoculum size (6A), Effect of shaking and static (6B) with B.parabrevis (■) and f.equiseti (□). Error bars represent the SD triplicates. |
Effect of incubation temperature at different incubation times
The results presented in Figures (7A, 7B) show that the optimum weight loss in (LDPE) film percentage was observed at 35˚C for B.parabrevis after incubating for 20 days while in the case of F.equiseti, it was at 30˚C for 30 days.
Effect of pH at different incubation times
The results in Figures (8A, 8B) demonstrate that there is no significant difference between pH5 and pH7 for F.equiseti. Hence, pH5 was chosen to optimize the weight loss in LDPE film percentage by F.equiseti while in the case of B.parabrevis, there is no significant difference between pH 7 and pH 9. Therefore, pH7 was chosen to optimize the weight loss in (LDPE) film percentage by B.parabrevis. Longer incubation times revealed no significant difference between the weight losses in (LDPE) film percentage.
Conclusion
The present study gives the evidence for biodegradation of (LDPE) films. Here fifteen, micro-biota were isolated from soil dumping of plastic debris and water polluted of plastic and identified at a genus level.
Then there was a screening for (LDPE) film biodegradation using (MSM). Two isolates achieved the highest surface colonization of (LDPE) films, identifying molecular to F.equiseti and B.parabrevis. Measurement of biodegradation using (SEM) and (FTIR) analysis show the attachment or adhering of F.equiseti and B.parabrevis to the surface of (LDPE) and difference in functional groups. Furthermore, optimization the weight loss in (LDPE) film percentage was performed through different growth parameters such as media, inoculum size, shaking, incubation temperature, pH at different incubation times, and the results showed that the highest weight loss in (LDPE) films percentage reached to 60% and 65% by B.parabrevis and F.equiseti, respectively.
Acknowledgment
This work has been supported by Mycology Lab, Faculty of Science, Helwan University to perform the isolation and the screening studies in addition to optimising processes.
Future prospects
Further study is required to get more supportive data for degradation mechanism.
Author’s contributions
Dr. Sally A. Ali is a major contributor in writing the manuscript. She performed the practical part of the manuscript which includes the experimental methods and data reported on results, and participated in writing the manuscript; also, she is the corresponding author. All authors read and approved the final manuscript.
Miss Shimaa Zakarya collected the samples.
Miss Shimaa Khaled revised the manuscript.
Conflict of interest
The authors declare that they have no conflict of interests.
Funding sources
This is a self-funded research work.
References
- Brandon, C., Knott, E. E., & Mark, D. Characterization and engineering of a two-enzyme system for plastics depolymerization. 2020;pnas.org/cgi/doi/10.1073/pnas.2006753117.
- Aamer, A., Fariha. H., & Abdul Hameed, S. A. Biological degradation of plastics: A comprehensive review. Biotechnology Advances. 2008; 26, 246-265.
- F, & Fasil. A. Degradation of plastic materials using microorganisms: A review. Public Health open J. 2019; 4 (2):57-63.
- Wei and Zimmermann. Biocatalysis as a green route for recycling the recalcitrant plastic polyethylene terephthalate. Microbe. Biotechnol. 2017; 10, 1320-1307.
- Gu, J. D., Ford, T. E., & Mitton, D. B. Microbial corrosion of metals. In: Review, editor. The Uhlig Corrosion Handbook. 2nd Edition. New York: Wiley. 2000a; pp. 915-27.
- Artham, T. & Doble, M. Biodegradation of aliphatic and Aromatic polycarbonates. Macromol Biosci. 2008; 8 (1), 14-24.
- Kikkawa, Y., Abe, H., Iwata, T., Inoue, Y., & Doi, Y. Crystal morphologies an enzymatic degradation of melt-crystallized thin films of random copolyesters of -3-hydroxybutyric acid with -3- hydroxyalkanoic acids. Poly Degrad Stab. 2002; 76, 467-78.
- Ricker, A. J. & Ricker, R. S. Introduction to research on plant diseases. John’s swift Co, New york and Indianapedis1936; p. 117.
- Zimbro, M. J., Power, D. A., Miller, S. M., Wilson G. E., MBA, B.S., & Johnson, J. Difco & BBL. Manual of Microbiological Culture Media (2nd Ed.). 2009; Becton, Dickinson and Company, Maryland, USA.
- Benson, H. J. Microbial applications (8th edition). NewYor. 2002; 2,926.
- Esmaeili, A., Ali, A., Alikhani, H., Shaban, F., & Esmaeili, E. Biodegradation of Low-density polyethylene (LDPE) by mixed culture of Lysinibacillus xylanilyticus and Aspergillus niger in soil. Plos one. 2013; plosone.org.8(9).71720.
- Ibrahim, N., Anwar, M., Khalid, M., & Ismail, M. Assessment of potential plastic-degrading fungi in Jordaian habitats. Turk Biol. 2011; 35, 551-557.
- Pitt, J. I. & Hocking, A. D. Fungi and Food Spoilage. Springer Nature Switzerland AG. Part of Springer Nature. 2009; (524 pages).
- White, T. J., Bruns, T., Lee, S., & Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A guide to Methods and Applications. 1990; 315-322. Academic Press: San Diego, U.S.A.
- Caruso, G. Plastic degrading microorganisms as a tool for bioremediation of plastic contamination in aquatic environments. J. Pollut.Eff.Cont.2015; 3,112.
- Merina, P. D., & Santosh, K. An approach to low-density polyethylene biodegradation by Bacillus amyloliquefaciens. Biotech. 2015; 5, 81-86.
- Yabannavar, A., & Bartha, R. Biodegradability of some food packaging materials in soil. Soil Biol Biochem. 1993; 25, 1469-1475.
- Nupur, O., Nehe, P., Surjit, S., Anil, B., Anamika, S., Pradip, K., Vivek, R., &Sutapa, B. Evaluation of HDPE and LDPE degradation by fungus, implemented by statistical optimization. Scientific Reports. 2016; 7, 39515.

This work is licensed under a Creative Commons Attribution 4.0 International License.