Manuscript accepted on : 19-08-2020
Published online on: 21-08-2020
Plagiarism Check: Yes
Reviewed by: Shreeram Joglekar
Second Review by: Arpita Roy ![]()
![]()
Vandana Kashyap1 , Bijaya Ketan Sarangi2, Manoj Kumar Yadav3
, Bijaya Ketan Sarangi2, Manoj Kumar Yadav3  and Dinesh Yadav1*
and Dinesh Yadav1*
1Department of Biotechnology, D.D.U Gorakhpur University, Gorakhpur, Uttar Pradesh- 273 009, India
2Environmental Biotechnology and Genomics Division, National Environmental Engineering research Institute- CSIR, Nehru Marg, Nagpur-440 020, India
3College of Agriculture, Department of Biotechnology, S.V.P university of Agriculture and Technology, Meerut 250 110, India
Corresponding Author E-mail : dinesh_yad@rediffmail.com
DOI : http://dx.doi.org/10.13005/bbra/2847
ABSTRACT: The inherent regeneration ability among selected varieties of pigeonpea using decapitated embryonal axis and stem-node explants in the presence of different growth regulators were assessed for multiple shoot bud induction. Among three different hormones namely BAP, Kinetin and TDZ tested for in vitro regeneration at different concentration for decapitated embryonal axis explants, BAP was found to be comparatively better as evident from number of buds per explants. IPA-242 variety was found to be the best for direct organogenesis resulting in the formation of 10 buds when subjected to MS Medium supplemented with 2.0 mgL-1 of BAP. Under treatment with different concentration of TDZ, Pusa-9 revealed 10 shoot buds with 0.15 mgL-1 of TDZ. The overall response of these varieties at different concentration of kinetin was very poor. With stem-node explants under variable concentration of BAP, IPA-3088 performed best showing 17 buds per explants. Similarly Pusa-9 and IPA 3088 revealed maximum regeneration ability forming 10 and 8 buds under TDZ and kinetin respectively. NAA was found to be effective growth regulator for rooting of shoots regenerated both from decapitated embryonal axis and stem-node explants.
KEYWORDS: Decapitated Embryonal Axis, Explant; Multiple Shoot Bud Induction; Pigeonpea (Cajanus cajan L. Millsp.); Regeneration; Stem-Node
Download this article as:| Copy the following to cite this article: Kashyap V, Sarangi B. K, Yadav M. K, Yadav D. Genotype-Dependent in Vitro Regeneration Assessment from Decapitated Embryonal Axis and Stem-Node Explants among Selected Pigeonpea Varieties. Biosci Biotech Res Asia 2020;17(3). |
| Copy the following to cite this URL: Kashyap V, Sarangi B. K, Yadav M. K, Yadav D. Genotype-Dependent in Vitro Regeneration Assessment from Decapitated Embryonal Axis and Stem-Node Explants among Selected Pigeonpea Varieties. Biosci Biotech Res Asia 2020;17(3). Available from: https://bit.ly/3giTwHN |
Introduction
Pigeonpea (Cajanu scajan L) is economically and nutritionally important legume of tropical and subtropical regions serving as a major source of proteins1,2 (Saxena et al., 2010; Sekhon et al., 2017). Sequencing of pigeonpea genome3,4 (Singh et al., 2012; Varshney et al., 2012) has provided an opportunity for developing appropriate strategies for overcoming the limitations of enhancing crop productivity owing to its narrow genetic base and adverse effect of biotic and abiotic stresses. Conventional plant breeding, molecular breeding, genomics assisted breeding and tissue culture based technologies together could be used to enhance the productivity of pigeonpea5-8 (Pazhamala et al., 2015; ChandaVenkata et al., 2018; Pratap et al., 2018; Bohra et al., 2020).
Transgenic technologies have immense potential for legume improvement but limited successes have been reported owing to the fact that highly efficient regeneration protocols are lacking 9,7 (Chandra and Pental 2003; Pratap et al., 2018). Studies on developing regeneration methods and genetic transformation using different genotypes of pigeonpea are recently reviewed10 (Krishna et al., 2010).
In pigeonpea, direct organogenesis has been preferred over somatic embryogenesis as method of in-vitro regeneration and is often genotype-specific. Efforts have been made to use diverse explants for direct organogenesis using different genotypes. Leaf explants have been reported for organogenesis11-18 (Eapen and George 1993; Kumar et al., 1983; George and Eapen 1994; Eapen et al., 1998; Tyagi et al., 2001; Yadav and Padmaja, 2003; Villiers et al., 2008; Kashyap et al., 2011). Cotyledonary nodes have been preferred as explants with several genotypes of pigeonpea for direct organogenesis19-23 (Franklin et al., 1998; Geetha et al., 1998; Singh et al., 2003; Shiva Prakash et al., 1994; Nalluri and Karri, 2019).
Direct organogenesis using different explants like cotyledons24-26,13,27,20,28 (Mehta and Mohan Ram, 1980; Kumar et al., 1984; Sarangi and Gleba, 1991; George and Eapen 1994; Naidu et al., 1995; Geetha et al., 1998; Chandra et al., 2003), hypocotyls29,30,20 (Shama Rao and Narayanaswamy, 1975;Cheema and Bawa, 1991; Geetha et al., 1998;), epicotyls25,13,27,20 (Kumar et al., 1984; George and Eapen 1994; Naidu et al., 1995; Geetha et al., 1998), apical meristem30,19,31 (Cheema and Bawa, 1991; Franklin et al., 1998; Parekh et al., 2014), leaf petiole32,23 (Srinivasan et al., 2004; Nalluri and Karri, 2019), distal cotyledonary segments33 (Mohan and Krishnamurthy, 1998), root13,15 (George and Eapen 1994; Tyagi et al., 2001) and seed29,13,20 (Shama Rao and Narayanaswamy, 1975; George and Eapen 1994; Naidu et al., 1995) have also been reported.
Expanding the range of genotypes amenable to the requisite tissue culture processes for complete plant regeneration provides opportunity for developing efficient genetic transformation systems for transgenic production. In order to achieve this goal, in vitro process development, including refinement of the existing regeneration processes, is a task of primarily importance. The existing regeneration protocols are optimized for few selected varieties. Therefore, screening of different varieties could reveal the variability in the inherent regeneration ability, which could be further targeted for developing appropriate regeneration and transformation protocols. Thus, the present study was an attempt to investigate the variability in regeneration ability of selected eleven varieties of pigeonpea exclusively for decapitated embryonal axis and stem node explants for direct organogenesis.
Materials and Methods
Seeds of Pigeonpea Varieties
The pigeonpea varieties IPA-2013, IPA-3088, Pusa-9, IPA-34, IPA-204, IPA-242, T-7, IPA-61, IPA-337, IPA-341 and IPA-98-3 of ICAR-Indian Institute of Pulses Research, Kanpur, India was used in the present study as reported earlier18,34 (Kashyap et al., 2011; Kashyap et al., 2014).
Preparation of Explants
Prior to culture, the pigeonpea seeds were sterilized using 1% cetrimide solution, 70% ethanol and 0.2% HgCl2 as reported earlier18,34 (Kashyap et al., 2011; Kashyap et al., 2014). Murashige and Skoog (MS) medium35 (Murashige and Skoog 1962) was used for culture and temperature of 25±20C with 16 hours light and 8 hour dark interval was maintained in tissue culture lab. For preparation of stem-node explants, 10 days germinated seedlings was used while for decapitated embryonal axis explants 2 days sprouted seed were used and after removing seed coat, epicotyl and hypocotyls regions were dissected carefully and about 2mm in length was taken as explants. The MS media with different concentration of three growth regulators i.e. BAP, kinetin and TDZ were used for multiple shoot bud induction while for rooting NAA, IAA and IBA were used. A total of 10 explants were used for each type of treatment for all the varieties. The statistical analyses was carried out by ANOVA test and treatment means were compared.
Results and Discussion
More than 50 genotypes of pigeonpea have been used for developing regeneration protocols, some of which were used for genetic transformation and production of transgenics10 (Krishna et al., 2010). Important factors which influence organogenesis includes selection of genotypes/cultivars, explants tissue, media composition and growth regulators. In an attempt to develop reliable in-vitro regeneration protocol by direct organogenesis amenable to genetic transformation, selected eleven Indian varieties of pigeonpea were studied using embryonal axis and stem node tissue explants under variable concentration of common growth regulators.
Regeneration using Decapitated Embryonal Axis Explants
The direct organogenesis using decapitated embryonal axis with different concentration of BAP ranging from 0.5-4.0 mgL-1 revealed variability resulting from 3 to 10 shoot buds among different varieties. The variety IPA-242 with 10 buds was found to be most amenable for in-vitro regeneration via direct organogenesis when cultured on MS media with BAP at 2.0 mgL-1. The variability in the formation of shoot buds among these varieties with growth regulator BAP is shown in Table-1 and Figure-1a.
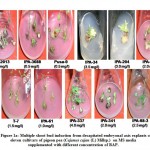 |
Figure 1a: Multiple shoot bud induction from decapitated embryonal axis explants of eleven cultivars of pigeon pea (Cajanus cajan (L) Millsp.) |
Table 1: Number of Shoots formed per explants among eleven cultivars of pigeonpea under the influence of different concentration of BAP (0.5-4.0 mgL-1) during in vitro multiple shoot bud induction and regeneration by decapitated embryonal axis explants. Data recorded after 4 weeks of culture with an average of 10 replicates. Means followed by the same letter are not significantly different by ANOVA test while different letters denoted as a,b,c differ significantly at p=0.05.
| Conc. of BAP
(mgL-1) |
0.5 | 1.0 | 1.5 | 2.0 | 2.5 | 3.0 | 3.5 | 4.0 |
| Cultivars | Number of shoots (Mean±S.D.)
|
|||||||
| IPA-2013 | 1.0±0.0a
|
3.1±0.3b | 3.2±0.5b | 3.2±0.4b | 3.4±0.4b | 3.4±0.4b | 3.9±0.7ab | 1.5±0.3a |
| IPA-3088 | 5.0±0.0 ab
|
3.9±0.5a | 2.8±0.4a | 2.0±0.0 | 1.0±0.0 | 1.0±0.0 | 4.2±0.7b | 1.0±0.0 |
| Pusa-9 | 2.7±0.4ab
|
1.0±0.0a | 0.0±0.0a | 0.0±0.0a | 0.0±0.0a | 2.1±0.3b | 0.0±0.0a | 0.0±0.0a |
| IPA-34 | 1.1±1.0b
|
0.0±0.0a | 0.4±0.5b | 1.2±1.1b | 0.0±0.0a | 1.8±0.9ab | 0.9±1.1b | 0.0±0.0a |
| IPA-204 | 3.3±0.7b
|
4.1±1.3b | 2.7±1.1b | 4.5±1.4b | 1.0±0.8a | 5.6±2.5ab | 1.1±1.1a | 3.1±1.7b |
| IPA-242 | 3.7±1.2b
|
4.1±1.3b | 5.6±3.9b | 6.3±3.1b | 1.9±0.7b | 3.9±1.4b | 1.8±0.6b | 3.8±2.3b |
| T-7 | 3.0±0.0a
|
2.8±1.0b | 4.7±1.0ab | 2.0±0.6b | 0.8±0.0a | 1.0±0.5a | 1.3±0.9a | 1.5±1.3a |
| IPA-61 | 1.6±0.4b
|
2.6±1.2ab | 1.9±0.7b | 1.4±0.4b | 1.0±0.0b | 0.5±0.3a | 0.5±0.3a | 1.0±0.0b |
| IPA-337 | 1.3±0.4a
|
1.3±0.4a | 1.9±0.8a | 1.8±0.7a | 2.0±0.0a | 2.3±0.6a | 2.5±0.5c | 4.3±1.1ac |
| IPA-341 | 2.7±0.6b
|
2.7±1.0b | 4.1±0.9b | 4.3±2.1b | 2.8±1.0b | 2.5±1.0b | 2.4±1.1b | 1.6±0.6b |
| IPA-98-3 | 1.0±0.0
|
2.1±0.7b | 2.1±0.7b | 2.6±0.4b | 3.0±0.0ab | 2.1±1.5b | 2.1±1.8b | 1.4±0.4a |
Direct organogenesis using mature and immature embryo axes using BAP growth regulator either individually or in combination with NAA and kinetin has been reported earlier for different genotypes like BDN-2, CO5, ICPL 161, ICPL 87N-290-21, PT 22, SA1, T-21,T-Visakha-1, VBN1 and VBN226,14,27,19 (Sarangi and Gleba, 1991; George and Eapen, 1994; Naidu et al., 1995; Franklin et al., 2000).
The response of kinetin was comparatively poor than BAP for inducing multiple shoot bud formation with a maximum of only 3 shoot buds. The varieties IPA-337, IPA-2013 and IPA-204 showed better regeneration ability with 0.5, 1.5 and 3.5 mgL-1 kinetin. The best response of variable concentration of kinetin among these varieties for multiple shoot bud formation is shown in Figure-1b. Shoot buds ranging from 2 to 10 were observed with TDZ at concentration from 0.05 to 0.40 mgL-1. Pusa-9 and IPA-61 varieties revealed 10 and 7 shoot buds respectively at 0.15 mgL-1 of TDZ. In case of other varieties namely IPA-204, IPA-242 and T-7 4-6 shoot buds were observed at 0.1 mgL-1 of TDZ growth regulator. The most suitable concentration of TDZ for direct organogenesis among these varieties is shown in Figure-1c. Comparative assessment of these varieties for multiple shoot buds formation at variable concentration of BAP, kinetin and TDZ is depicted in Figure-2.
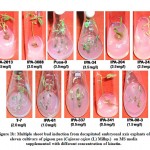 |
Figure 1b: Multiple shoot bud induction from decapitated embryonal axis explants of eleven cultivars of pigeon pea (Cajanus cajan (L) Millsp.) |
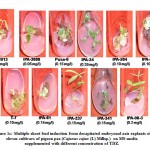 |
Figure 1c: Multiple shoot bud induction from decapitated embryonal axis explants of eleven cultivars of pigeon pea (Cajanus cajan (L) Millsp.) |
 |
Figure 2: Pictorial representation for the response of different growth regulators (a) BAP (b) kinetin (c) TDZ individually and (d) comparative effect of growth |
Overall, BAP seems to be promising as compared to kinetin and TDZ for direct organogenesis. Plantlet regenerated from decapitated embryonal axes under BAP and IAA has been reported36 (Rathore and Chand, 1999). Similarly genotype response of two cultivars namely UPAS-120 and Bahar under the influence of different growth regulators has also been reported37 (Yadav and Chand, 2001). A reliable regeneration protocol from decapitated mature embryo axes using genotype T-15-15 has been reported using combination of BAP and IAA growth regulators38 (Mohan and Krishnamurthy, 2003). Similar study of organogenesis with pigeopea variety JKR105 revealed greater regeneration of shoot buds in the presence of BAP39 (Krishna et al., 2011). Recently, efficient shoot regeneration of pigeonpea genotype Durga NTL-30 has been reported using embryonic axis using combination of zeatin and kinetin growth regulators along with silver nitrate40 (Raut et al., 2015).
Rooting Response in Decapitated Embryonal Axis Derived Plantlets
The rooting of shoot buds from decapitated embryonal axis was attempted with full strength MS basal medium along with NAA, IAA and IBA growth regulator at 0.1, 0.2 and 0.3 mgL-1 as reported earlier18,34 (Kashyap et al., 2011; Kashyap et al., 2014). In most of the cases 0.1 mgL-1 of NAA was found to be effective resulting in 80-100% rooting (Table-2).
Table 2: Rooting responses of in- vitro regenerated shoots from decapitated embryonal axis explants under different concentrations of NAA. Data was recorded after 4 weeks of culture with 10 replicates for each treatment and experiment was repeated twice.
| Cultivars | NAA (0.1 mgL-1) | NAA (0.2 mgL-1) | NAA0.3 (mgL-1) | |||
| % of rooting | Number of primary roots Mean±S.D. | % of rooting | Number of primary roots Mean±S.D | % of rooting | Number of primary roots Mean±S.D | |
| IPA-2013 | 100 | 6.2±0.4 | 100 | 2.0±0.0 | 70 | 1.4±0.9 |
| IPA-3088 | 80 | 6.4±3.2 | 50 | 1.4±0.4 | 50 | 1.6±1.9 |
| Pusa-9 | 80 | 7.2±2.2 | 50 | 4.0±1.8 | 70 | 3.6±3.2 |
| IPA-34 | 100 | 2.8±1.2 | 40 | 1.4±0.4 | 80 | 1.4±0.48 |
| IPA-204 | 100 | 3.0±0.8 | 60 | 2.0±0.8 | 40 | NR |
| IPA-242 | 100 | 11.4±1.7 | 80 | 11.8±1.5 | 80 | 2.4±0.4 |
| T-7 | 80 | 3.2±0.97 | 40 | 2.8±1.6 | 40 | 4.4±1.9 |
| IPA-61 | 80 | 3.2±1.6 | 40 | 5.2±1.6 | 100 | 4.4±1.0 |
| IPA-337 | 80 | 6.2±1.4 | 60 | 2.0±0.8 | 40 | 1.2±0.4 |
| IPA-341 | 80 | 5.4±0.6 | 50 | 2.6±1.8 | 40 | 2.0±0.9 |
| IPA-98-3 | 90 | 6.0±1.1 | 60 | 1.8±0.7 | 50 | 1.0±0.0 |
The number of primary roots formed was highest in IPA-242 subjected to 0.2 mg/l of NAA, though the percentage of rooting was only 80%, while 0.1 mgL-1 of NAA resulted in 100% rooting with more or less similar number of primary roots formed. The root formation observed with shoot buds of IPA-2013, IPA- 3088, Pusa-9 and IPA-242 is shown in Figure-3.
 |
Figure 3: Rooting response of in-vitro regenerated shootlets derived from decapitated embryonal axis explants of selected cultivars of pigeon pea viz. |
The rooting response in the presence of IAA was comparatively poor than NAA with overall 60-100% rooting in only few varieties. It was also observed that 0.3 mg L-1 of IAA was comparatively better for rooting. The percentage of root formation and number of primary roots was found to be best for IPA-34. Only few of the varieties responded to rooting in the presence of IBA, though overall 50-100% rooting frequency was attained. Pusa-9 with 100% rooting and with a maximum number of primary roots was achieved with 0.2 mgL-1 of IBA, while in case of other varieties IBA at 0.1 mgL-1 showed better response.
In-Vitro Regeneration using Stem-Node Explants
An in vitro grown plant of 10 day old was used for stem node explants preparation. For each treatment 10 explants were used with all varieties and explants were vertically inoculated in respective media for multiple shoot bud formation. Effect of BAP, kinetin and TDZ growth regulators at different concentration for direct organogenesis among these varieties were assessed. Variability in regeneration ability among different varieties was observed with BAP at variable concentration ranging from 0.5 to 4.0 mgL-1 and shoot buds formed were recorded as shown in Table-3. IPA-3088 revealed 17 shoot buds while in case of IPA-341 a minimum of 5 buds were observed. Further, it was also observed that BAP at 4 mgL-1 gave better regeneration ability for IPA-3088 revealing the fact that higher concentration of BAP is comparatively better for direct organogenesis as reported earlier10 (Krishna et al., 2010) (Figure-4a).
Table 3: Number of Shoots formed per explants among eleven cultivars of pigeonpea under the influence of different concentration of BAP (0.5-4.0 mgL-1) during in vitro multiple shoot bud induction and regeneration by stem-node explants. Data recorded after 4 weeks of culture with an average of 10 replicates. Means followed by the same letter are not significantly different by ANOVA test while different letters denoted as a,b,c differ significantly at p=0.05.
| Con. of BAP
(mgL-1) |
0.5 | 1.0 | 1.5 | 2.0 | 2.5 | 3.0 | 3.5 | 4.0 |
| Cultivars | Number of Shoots (Mean±S.D.) | |||||||
| IPA-2013 | 2.9±0.7a | 6.0±1.1ab | 5.5±1.1b | 5.2±1.2b | 5.0±0.8b | 5.0±1.5b | 5.7±0.7b | 5.4±0.4b |
| IPA-3088 | 5.8±0.8a | 5.0±2.1c | 9.3±1.6b | 9.7±2.7b | 7.9±1.3b | 8.6±2.3b | 11.3±3.1b | 11.4±3.1abc |
| Pusa-9 | 7.2±0.9ab | 3.4±0.4b | 5.4±0.6a | 3.6±1.1a | 4.8±0.4a | 2.6±0.4a | 4.4±0.4a | 2.2±0.4a |
| IPA-34 | 4.0±0.6ab | 2.5±0.5a | 4.0±0.7a | 2.4±0.4a | 3.4±0.4b | 3.4±0.9b | 3.9±0.8b | 3.0±0.0b |
| IPA-204 | 3.6±0.4a | 3.6±0.8a | 4.7±0.4a | 5.2±0.6a | 5.1±0.3a | 5.2±0.4a | 4.6±0.6a | 6.6±0.4a |
| IPA-242 | 3.1±0.3b | 2.2±0.4a | 3.5±0.6b | 2.3±0.4b | 4.0±1.1ab | 3.6±0.4b | 3.1±0.53b | 2.5±0.5b |
| T-7 | 2.0±0.0a | 3.1±0.3c | 4.2±0.8b | 3.5±0.5a | 4.5±0.6b | 3.7±0.9b | 5.1±0.3abc | 4.0±0.6a |
| IPA-61 | 2.2±0.4a | 3.0±0.6a | 3.9±0.7a | 4.4±0.4b | 3.7±0.4a | 3.4±0.4a | 3.3±0.4a | 5.4±0.6ab |
| IPA-337 | 2.0±0.0a | 2.0±0.0a | 2.0±0.0a | 2.0±0.0a | 2.9±0.3a | 2.4±0.0b | 3.6±0.4b | 4.4±1.6ab |
| IPA-341 | 1.9±0.4a | 2.0±0.0a | 2.0±0.0a | 3.6±0.9ab | 3.5±0.8b | 3.3±0.4b | 3.2±0.4b | 3.0±0.0b |
| IPA-98-3 | 2.0±0.0a | 4.1±1.0ab | 3.8±0.6b | 2.9±0.5b | 3.3±0.7b | 3.4±0.6b | 3.4±0.8b | 3.0±0.0b |
 |
Figure 4a: Multiple shoot bud induction from stem-node explants of eleven cultivars of pigeon pea (Cajanus cajan (L) Millsp.) |
With variable concentration of kinetin, IPA 3088 formed 8 shoot buds and was found to be best among these varieties. In general, lower concentration of kinetin showed better resposnse for direct organogenesis, though IPA-2013 and IPA-34 were exceptions revealing better response at higher concentration of kinetin. As compared to BAP, lower percentage of multiple shooting was observed with kinetin. Mulitple shoot bud formation among these varieties under the best responsive concentration of kinetin is shown in Figure-4b. Similary genotype based variability among these varieties for in vitro regneration was also observed under the influence of different concentration of TDZ growth regulator. Pusa-9 was found to be most suitable for direct organogenesis among these varieties with 10 shoot buds when treated with TDZ (0.25 mgL-1). It was also observed that TDZ in the range of 0.25 to 0.30 mgL-1 revealed better response for direct organogenesis from stem-node explants in most of the varieties. The lower concentration of TDZ was exceptionally better for IPA-3088 and IPA-61 while TDZ at 0.40 mgL-1 was found to be most effective for shoot bud induction in IPA-242 and IPA-337 (Figure-4c).
 |
Figure 4b: Multiple shoot bud induction from stem-node explants of eleven cultivars of pigeon pea (Cajanus cajan (L) Millsp.) |
 |
Figure 4c: Multiple shoot bud induction from stem-node explants of eleven cultivars of pigeon pea (Cajanus cajan (L) Millsp.) |
Comparative assessment of growth regulators revealed BAP to be most promising compared to TDZ and kinetin. Substantial variability in regeneration potential for direct organogenesis with different concentration of these growth regulators was observed with pigeopea varieties as shown in Figure 5.
 |
Figure 5: Pictorial representation for the response of different growth regulators (a) BAP (b) kinetin (c) TDZ individually and (d) |
Rooting Response in Stem-Node Derived Plantlets
As attempted for root formation from shoot buds derived with leaf and plumule junction explants18, 34 (Kashyap et al., 2011; Kashyap et al., 2014) of these varieties, growth regulators namely NAA, IAA and IBA at 0.1, 0.2 and 0.3 mg/l was also used with stem-node explants. The root formation was found to be better with 0.1 mgL-1 NAA resulting with a maximum number of primary roots as shown in Figure-6. The root formation in terms of percentage ranged from 50 to 80% and comparative response of these varieties for root formation from shoots derived from stem-node explants is shown in Table-4. IPA-2013 was found to be the best variety for root formation though the best variety showing direct organogenesis with stem-node explants IPA-3088 also revealed good root formation when treated with 0.1 mgL-1 of NAA.
Table 4: Rooting responses of in- vitro regenerated shoots; stem-node explants under different concentrations of NAA. Data was recorded after 4 weeks of culture with 10 replicates for each treatment and experiment was repeated twice.
| Cultivars | NAA 0.1 mgL-1 | NAA 0.2 mgL-1 | NAA0.3 mgL-1 | |||
| % of rooting | Number of primary roots Mean±S.D. | % of rooting | Number of primary roots Mean±S.D | % of rooting | Number of primary roots Mean±S.D | |
| IPA-2013 | 80 | 9.5±4.8 | 80 | 8.1±4.0 | 70 | 3.4±2.2 |
| IPA-3088 | 80 | 8.2±4.1 | 50 | 1.0±1.0 | 50 | 1.0±1.0 |
| Pusa-9 | 80 | 5.4±0.9 | 50 | 2.8±2.5 | NR | NR |
| IPA-34 | 50 | 6.6±6.6 | 50 | 0.8±0.9 | NR | NR |
| IPA-204 | NR | NR | NR | NR | NR | NR |
| IPA-242 | 50 | 0.5±0.5 | 80 | 3.2±1.6 | 50 | 1.5±1.5 |
| T-7 | NR | NR | 80 | 1.6±0.8 | NR | NR |
| IPA-61 | 50 | 2.1±2.1 | 80 | 3.3±1.7 | 60 | 1.8±1.5 |
| IPA-337 | NR | NR | 80 | 4.1±2.1 | NR | NR |
| IPA-341 | 50 | 1.0±1.0 | 80 | 3.2±1.6 | NR | NR |
| IPA-98-3 | NR | NR | 80 | 2.8±1.6 | NR | NR |
 |
Figure 6: Rooting response of in-vitro regenerated shootlets derived from stem-node explants of selected cultivars of pigeon pea in MS media supplemented with 0.1 mgL-1 NAA. |
Similarly variable response was observed for the plantlets derived from stem-node explants of these varieties for rooting under the influence of different concentration of IAA namely 0.1, 0.2 and 0.3 mgL-1. The concentration of IAA at 0.1 mgL-1 revealed 50-70% rooting with IPA-2013 being most responsive for root formation. As compared to NAA and IAA, the response of rooting was poor with IBA. In most of the cultivars there were no response to variable concentration of IBA for rooting.
There are only few reports of multiple shoot bud induction using stem node explants.30 Cheema and Bawa, 1991 has reported multiple shoot bud induction using stem node along with the apical meristem in the MS media supplemented with kinetin ranging from 0.1 to 9.0 mgL-1. The lower concentration in the range of 0.5 to 3.0 mgL-1 revealed healthy shoots while higher concentration resulted in the formation of clusters.
Comprehensive analysis of more than 50 cultivars/genotypes of pigeonpea for in vitro regeneration with diverse explants revealed variability both for organogenesis and somatic embryogenesis10 (Krishna et al., 2010) and hence there is great potential for screening of genotypes to develop efficient regeneration protocols for transgenic development.
Hardening and Acclimatization of Plantlets Derived from Decapitated Embryonal Axis and Stem-Node Explants
Genotype dependent variability was also observed during acclimatization of plantlets derived from both decapitated embryonal axis and stem-node explant sources. The percentage acclimatization of multiple shoot buds derived from decapitated embryonal axis explants with proper rooting in soil ranged from 55 to 80% with cultivar IPA-242 showing maximum percentage of acclimatization while cultivars IPA-3088, IPA-204 and IPA-61 showed 75% acclimatization. In case of stem-node explants derived plantlets, the percentage acclimatization of multiple shoot buds with proper rooting in soil ranged from 25 to 70% with cultivar IPA-2013, IPA-3088 and IPA-61 showing 70 and 65% acclimatization. The overall percentage of acclimatization during hardening observed among these varieties derived from different explants is shown in Table-5.
Table 5: Percentage acclimatization of well rooted plantlets derived from embryonal axis and stem-node explants of different pigeonpea varieties.
| Pigeonpea varieties | Decapitated embryonal axis explants (%) | Stem-node explants (%) |
| IPA-2013 | 60 | 70 |
| IPA-3088 | 75 | 65 |
| Pusa-9 | 65 | 25 |
| IPA-34 | 60 | 25 |
| IPA-204 | 75 | 20 |
| IPA-242 | 80 | 20 |
| T-7 | 60 | 25 |
| IPA-61 | 75 | 65 |
| IPA-337 | 60 | 50 |
| IPA-341 | 55 | 55 |
| IPA-98-3 | 60 | 25 |
Conclusion
Plantlet regeneration via organogenesis has been preferred over somatic embryogenesis for developing appropriate regeneration protocols amenable for genetic transformation in pigeonpea. Among several factors considered for developing suitable regeneration protocol by direct organogenesis, selection of genotypes/cultivars has been considered as the major factor and hence genotype-dependent response needs to be investigated. Other factors influencing regeneration are explants tissue, media composition and growth regulators. Varied concentration of growth regulators namely cytokinins, auxins, gibberellins and abscisic acid either individually or in combination has been studied for organogenesis-mediated regeneration in different pigeonpea genotypes. The selected Indian pigeonpea varieties in the present study revealed genotype dependent response for direct in vitro organogenesis in the presence of varied concentration of growth regulators exclusively for decapitated embryonal axes and stem-node explants. The varieties IPA-242 and IPA-3088 showed best response for in vitro regeneration using decapitated embryonal axes and stem-node explants respectively. The growth regulator BAP was found to be effective as compared to kinetin and TDZ for direct organogenesis irrespective of explant sources. Further, comparatively higher concentration of BAP (0.5-4.0 mgL-1), lower concentration of kinetin (0.5-4.0 mgL-1) and medium concentration of TDZ (0.05-0.40 mgL–1) was found to be effective for multiple shoot bud induction. The rooting response of plantlets derived from these explants source among these varieties was found to be better with growth regulator NAA as compared to IAA and IBA and effective rooting was observed with 0.1 mgL-1 of NAA.
Acknowledgements
Authors highly acknowledge Director, ICAR-IIPR Kanpur for providing seeds of pigeonpea. The authors sincerely acknowledge the support of Director, CSIR-NEERI, Nagpur for providing Plant Tissue Culture Lab facility to carry out some part of the work in NEERI, Nagpur. Authors are also grateful to Department of Biotechnology, DDU Gorakhpur University for their support to carry out this research work.
Conflict of Interest
There is no conflict of interest.
References
- Saxena KB, Kumar RV and Gowda CLL. Vegetable pigeonpea-a review. J Food Legumes. 2010; 23:91-98.
- Sekhon J, Grewal SK, Singh I and Kaur J. Evaluation of nutritional quality and antioxidant potential of pigeonpea genotypes. J Food Sci Technol. 2017; 54(11):3598-3611.
CrossRef - Singh NK, Gupta DK, Jayaswal PK, Mahato AK, Dutta S et al. The first draft of pigeonpea genome sequence. J Plant Biochem Biotechnol. 2012; 21(1): 98-112.
CrossRef - Varshney et al. Draft genome sequence of pigeonpea (Cajanus cajan), an orphan legume crop of resource poor farmers. Nature Biotechnology. 2012; 30(1): 83-89.
CrossRef - Pazhamala L, Saxena RK, Singh VK, Sameerkumar CV, Sinha P et al. Genomics-assisted breeding for boosting crop improvement in pigeonpea (Cajanus cajan). Front Plant Sci. 2015; 6:50.
CrossRef - Chandra Venkata SK, Nadigatla Veera Prabha Ram GR, Saxena RK, Saxena K, Upadhyaya HD et al. Pigeonpea improvement: An amalgam of breeding and genomics research. Plant Breeding.2019; 138:445-454.
CrossRef - Pratap A, Prajapati U, Singh CM, Gupta G, Rathore M et al. Potential, constraints and applications of in vitro methods in improving grain legumes. Plant Breeding. 2018; 137:235-249.
CrossRef - Bohra A, Saxena KB, Varshney RK and Saxena RK. Genomics-assisted breeding for pigeonpea improvement. Theoretical and Applied Genetics.2020;133:1721-1737.
CrossRef - Chandra A and Pental D. Regeneration and genetic transformation of grain legumes: An overview. Curr Sci.2003;84(3):381-387.
- Krishna G, Reddy PS, Ramteke PW and Bhattacharya PS. Progress in tissue culture and genetic transformation research in pigeonpea [Cajanus cajan(L.) Millsp.]. Plant Cell Rep. 2010; 29:1079-1095.
CrossRef - Eapen S and George L. Plant regeneration from leaf disc of peanut and pigeonpea: Influence of benzyladenine, indole acetic acid and indoleacetic acid-amino-acid conjugates. Plant Cell Tiss Org Cult.1993; 5:223-227.
CrossRef - Kumar AS, Reddy TP and Reddy GM. Plant regeneration from different callus cultures of pigeopea (Cajanus cajan ). Plant Sci Lett.1983; 32:271-278.
CrossRef - George L and Eapen S. Organogenesis and embryogenesis from diverse explants in pigeonpea (Cajanus cajan). Plant Cell Rep.1994; 13:417-420.
CrossRef - Eapen S, Tivarekar S and George L. Thidiazuron-induced shoot regeneration in pigeonpea (Cajanus cajan). Plant Cell Tiss Org Cult.1998; 53:217-220.
CrossRef - Tyagi AP, Comai L and Byers B. Comparison of plant regeneration from root, shoot and leaf explants in pigeonpea (Cajanus cajan) cultivars. SABRAO J. 2001; 33:59-71.
- Yadav PBS and Padmaja V. Shoot organogenesis and plantlet regeneration from leaf segments of pigeonpea. Plant cell Tiss Org Cult.2003; 73:197-200.
CrossRef - Villiers SD, Emongor Q, Njeri R, Gwata E, Hoisington D et al. Evaluation of the shoot regeneration response in tissue culture of pigeonpea (Cajanus cajan (L.) Millsp.) varieties adapted to eastern and southern Africa. Afr J Biotechnol. 2008; 7:587-590.
- Kashyap V, Sarangi BK, Yadav MK and Yadav D. Assessment of in vitro multiple shoot bud induction from leaf explants among eleven Indian cultivars of pigeonpea (Cajanus cajan (L) Millsp.). Biotechnology. 2011; 10(6):534-539.
CrossRef - Franklin G, Jeyachandran R, Melchias G and Ignacimuthu S. Multiple shoot induction and regeneration of pigeonpea (Cajanus cajan(L.) Millsp.) cv. Vamban from apical and axillary meristem. Curr Sci. 1998; 74:936-937.
- Geetha N, Venkatachalam P, Prakash V and Lakshmi Sita G. High frequency induction of multiple shots and plant regeneration from seedling explants of pigeonpea (Cajanus cajan). Curr Sci.1998; 75:1036-1041.
- Singh ND, Sahoo L, Neera BS and Jaiwal PK. The effect of TDZ on organogenesis and somatic embryogenesis in pigeonpea (Cajanus cajan Millsp). Plant Sci.2003; 164:341-247.
CrossRef - Shiva Prakash N, Pental D and Bhalla-Sarin N. Regeneration of pigeonpea (Cajanus cajan) from cotyledonary node via multiple shoot formation. Plant Cell Rep.1994; 13:623-627.
CrossRef - Nalluri N and Karri VR. In vitro regeneration of ICP 8863 pigeonpea (Cajanus cajan (L) Millsp) variety using leaf petiole and cotyledonary node explants and assessment of their genetic stability by RAPD analysis. Indian Journal of Science and Technology.2019;12(9).
CrossRef - Mehta U and Mohan Ram HY. Regeneration of plantlets from the cotyledons of Cajanus cajan Ind J Exp Biol.1980; 18:800-802.
- Kumar AS, Reddy TP and Reddy GM. Multiple shots from cultured explants of pigeonpea and Atylasia SABRAO J.1984; 16:101-105.
- Sarangi BK and Gleba YY. Direct multiple regeneration in Cajanus cajan (L.) Millsp. Acta Hortic. 289;149-150.
CrossRef - Naidu RB, Kulkarni DD and Krishnamurthy KV. Genotype-dependent morphogenic potentiality of various explants of a food legume, the pigeonpea (Cajanus cajan). In Vitro Cell Dev Biol Plant. 1995; 31:26-30.
CrossRef - Chandra A, Gupta V, Burma P and Pental D. Patterns of morphogenesis from cotyledon explants of pigeonpea. In Vitro Cell Dev Biol Plant.2003; 39:514-519.
CrossRef - Shama Rao HK and Narayanaswamy S. Effect of gamma irradiation on cell proliferation and regeneration in explanted tissue of pigeonpea, Cajanus cajan (L.) Millsp. Radiat Bot.1975; 15:301-305.
CrossRef - Cheema HK and Bawa J. Clonal multiplication via multiple shoots in some legumes (Vigna unguiculata and Cajanus cajan). Acta Hortic. 1991; 289:93-94.
CrossRef - Parekh MJ, Mahatma MK and Kapadia CV. In vitro regeneration of pigeonpea (Cajaus cajan(L.) Millisp) genotype GT-102 using apical meristem. Journal of Cell & Tissue Research. 2014; 14(1): 4099-4103.
- Srinivasan T, Verma VK and Kirti PB. Efficient shoot regeneration in pigeonpea, Cajanus cajan (L) Millsp. Using seedling petioles. Curr Sci. 2004; 86:30-32.
- Mohan ML and Krishnamurthy KV. Plant regeneration in pigeonpea (Cajanus cajan (L.) Millsp.) by organogenesis. Plant Cell Rep. 1998; 17:705-710.
CrossRef - Kashyap V, Sarangi BK, Yadav MK and Yadav D. In vitro multiple shoot bud induction and regeneration from plumule junction explants of pigeonpea (Cajanus cajan (L) Millsp.) cultivars. African Journal of Biotechnology. 2014; 13(41): 4061-4069.
CrossRef - Murashige T and Skoog F. A revised medium for rapid growth and bioassay with tobacco tissue cultures. Physiol Plant. 1962; 15: 473–497.
CrossRef - Rathore RS and Chand L. Plantlet regeneration from decapitated embryonic axes of pigeonpea Cajanus cajan (L.) Millsp.). Indian J Exp Biol.1999; 37(5):496-498.
- Yadav V and Chand L. Plantlet regeneration from decapitated embryonic axes of pigeonpea varieties. Indian Journal of Plant Physiology.2001; 6(2):208-211.
- Mohan ML and Krishnamurthy KV. Plant regeneration from decapitated mature embryo axis and Agrobacterium mediated genetic transformation of pigeonpea. Biologia Plantarum.2003; 46(4): 519-527.
CrossRef - Krishna G, Reddy PS, Ramteke PW, Rambabu P, Sohrab SS, Rana D and Bhattacharya P. In vitro regeneration through organogenesis and somatic embryogenesis in pigeonpea[Cajanus cajan (L.) Millsp.] cv. JKR105. Physiol Mol Biol Plant2011; 17(4):375-385.
CrossRef - Raut RV, Dhande GA, Rajput JC and Ingale AG. Rapid and highly competent shot regeneration of pigeonpea (Cajanus cajan) using variable explants by in vitro culture system. Journal of Pharmacognosy and Phytochemistry. 2015; 4(4):01-05.

This work is licensed under a Creative Commons Attribution 4.0 International License.





