Manuscript accepted on : 06 February 2017
Published online on: --
Plagiarism Check: Yes
Sunny Bindra1, Rashmi Sharma1, Azhar Khan1 and Saurabh Kulshrestha1
Faculty of Applied Sciences and Biotechnology Shoolini University of Biotechnology and Management Sciences, Bajhol, Solan, Himachal Pradesh, India.
Corresponding Author E-mail: saurabh_kul2000@yahoo.co.in
DOI : http://dx.doi.org/10.13005/bbra/2443
ABSTRACT: Today the most quandary situation in the world is to fulfil growing energy demand without impeding environmental balance. Rising population growth has led to the increase in world energy demand. However, the fossil fuel reserves are deteriorating at a faster rate. So, biofuels derived from biological materials through contemporary biological processes serve as an alternative choice to replace the existing demand of fossil fuels. These biofuels have basic renewable origin which set them apart from the fossil fuels. There are three categories; first, second and third generation biofuels depending upon the source crop and technology involved in energy generation. Of the three generations, first generation derived from food crops, second generation from non-food crops and third generation biofuels consist of microalgae-derived biofuels. This article reviews the various energy sources used during different generations of biofuels along with microalgae derived biodiesel production. Various constraints and concerns are associated with first and second generation biofuels and it has been observed that, microalgae derived biofuels, can be developed as more reliable and sustainable energy source to fulfil the world energy demand in future. They have a number of advantages over their counterparts such as non-feedstock, high growth rate with higher biomass yield, possess oils with higher lipid content and possibility of utilizing waste as well as salt water for growth. In addition to energy source, microalgae can provide various types of economic by-products adding to their advantages. In the future, the technology as well as algal strains should be improved to increase the energy efficiency and create economic as well as environmental benefits so as to plan a road map for the industries to produce third-generation biofuels.
KEYWORDS: Biofuel; biodiesel; generations; microalgae; renewable
Download this article as:| Copy the following to cite this article: Bindra S, Sharma R, Khan A, Kulshrestha S. Renewable energy Sources in different Generations of Bio-fuels with special Emphasis on Microalgae Derived Biodiesel as Sustainable industrial fuel Model. Biosci Biotech Res Asia 2017;14(1). |
| Copy the following to cite this URL: Bindra S, Sharma R, Khan A, Kulshrestha S. Renewable energy Sources in different Generations of Bio-fuels with special Emphasis on Microalgae Derived Biodiesel as Sustainable industrial fuel Model. Biosci Biotech Res Asia 2017;14(1). Available from: https://www.biotech-asia.org/?p=21749 |
Introduction
Industrial revolution and urbanization has increased the demand for fossil fuels and according to the world energy reserves it is estimated that coal will last another 218 years, oils 41 years, and natural gas 63 years.1 Fossil fuels are the major energy sources that are being used in the world but their over utilization has led to the severe environmental issues such as air pollution etc. They are the largest contributors of green house gases (GHG’s) emission in the atmosphere.2 The petro-diesel burning causes increase in GHG’s emissions such as carbon monoxide, hydrocarbons and smoke like pollutants causing climate change and health problems. They are non-renewable sources of energy as they are derived from prehistoric biological matter and won’t be available once they are consumed. Their sources are limited and are depleting at a faster rate. So there is a need for alternative renewable energy sources of ecofriendly bio-fuels.
A biofuel is a fuel produced through contemporary biological processes, such as agriculture and anaerobic digestion, rather than geological processes involved in the formation of fossil fuels. They are derived from biological material, mainly from microorganisms, plants, animals and wastes. All biofuels have the basic renewable origin based upon the photosynthetic conversion of solar energy to chemical energy, thus setting them apart from the fossil fuels which are derived from geothermal processes. Depending on the origin and production technology involved, biofuels are categorized as the first, second and third generation biofuels.3 Fourth generation biofuels have been also evolved which make use of novel synthetic biological tools but still emerging at the basic research level. On a step towards sustainable bioenergy production, solar energy based biofuel production strategies are of pivotal importance. Despite a large advancement in photovoltaic technologies for conversion of solar energy to electricity, the biofuels are still of prime importance today, particularly in the transport sector. In view of this, biofuels have emerged as the largest renewable fuel produced and consumed in the world due to growing demand to replace the fossil, thus reducing GHG’s emissions and palliating the climate change. Scientific breakthroughs in biofuel production technology are playing important role towards sustainable biofuel production, increasing quantity and quality of biofuels and carbon–neutral economy.4
First generation biofuels also called “conventional biofuels” are derived from various crop plants as energy-containing molecules like sugars, starch, vegetable oils and cellulose. As they are produced mostly from food feedstocks thus have a negative impact on food security and provide only limited biofuel yields. Most common first generation biofuels are bioethanol, biodiesel, biofuel gasoline, bioethers, biogas and solid biofuels such as wood, sawdust, charcoal and non-food energy crops. Efforts are required to generate advanced biofuels by identifying and producing effective non-food energy crops thus bringing down the cost as well as improving the performance and quality of biofuels for different transport sectors.3 The second generation biofuels known as advanced biofuels, are produced from biomass including lignocellulosic, non-food materials such as straw, bagasse, forest residues and purpose grown energy crops. First generation biofuels are synthesized from sugars and vegetable oils which are found in arable crops and can be extracted easily using conventional technology. Whereas, second generation biofuels are derived from lignocellulosic biomass of woody crops and agricultural waste, which makes it harder to extract the required fuel and series of physical and chemical treatments are required to convert lignocellulosic biomass to liquid biofuels.5 The third generation biofuels are based on algal biomass production. Extensive research is being carried out to improve production of these fuels and their separation during bio-oil production to remove non fuel residues as well as to reduce the production cost4.
Among various bio-fuels used, bio-diesel may act as good choice because automobiles are the major contributors of pollution and global warming. It is defined as the mono-alkyl esters of long chain fatty acids derived from the biological sources such as edible/non-edible oils, agricultural crops, animal fats, and microalgae. It is non-toxic, renewable, biodegradable, environmental friendly, portable, readily available and eco-friendly fuel.6 In addition to this, it has higher cetane number, good lubricity, lower particulates and lesser sulphur content. Due to which it enhances engine’s life and ignition quality, can’t harm aquatic life during shipping as it is biodegradable. Biodiesel doesn’t corrode the engine due to absence of sulphur in it.7 It gives complete combustion due to higher oxygen content by which it produces lesser emissions of pollutants and GHG’s emissions. Other fuels also accumulate carbon deposits on the fuel injectors which weaken the engine performance and leads to mechanical damage. But, the main hurdle behind its production and use is the cost that mainly depends upon on 60-75% of the feedstock’s cost. So, there is a need of using cheap feedstock’s that are required for biodiesel production to make it cost effective and economical.8 Microalgae derived biodiesel is one of the best alternative renewable fuels produced by using cheap substrate. Microalgae derived biodiesel is the best third generation bio-fuel for its high oil content, stress adaptability (pH, temperature, salinity, toxicants) and not competing with food crops.9 The photosynthetic microalgae require mainly light, carbon dioxide, and some nutrients (nitrogen, phosphorus, and potassium) for its growth, and produce large amount of lipids and carbohydrates, which can be further processed into various biofuels and other valuable co-products.10 Moreover, the low content of hemicelluloses and negligible amount of lignin in algal biomass leads to increased hydrolysis efficiency during fermentation process.11 It can use waste discharges/effluents from industries like processing plants, dairy industries, rice straw etc as growth mediums. In return it can also used in treatment of waste water as it consumed nutrients from it for its growth thus it can reduce water pollution. It can also control air pollution produced after burning of rice straw (residue left after harvesting of crop) in open fields. Microalgae can use this residue (rice straw) for its growth. Other than biofuels, algae can be used in other fields of bio-remediation, human nutrition, animal feed, health products, biofertilizer, and by-products obtained from it having commercial market importance.12,13,14,15,16,17 Therefore, the present review provides an account of various renewable energy sources used in different generations of biofuels with emphasis on exploring the scope of algae for production of different biofuels and evaluation of its potential as an alternative feedstock.
Renewable energy Sources in different Generations of Biofuel
First Generation biofuels
First generation biofuels are derived directly from food crops and the abstracted oil is either used as biodiesel or used to produce bioethanol through fermentation. The three main types of first generation biofuels which are used commercially are bioethanol, biodiesel, and biogas. The production process of these biofuels is an ‘established technology’ from which large quantities have been produced worldwide (www.task39.org). Bioethanol is produced through fermentation of starch present in crops such as wheat, barley, corn, potato or sugar present in sugarcane, sugarbeet etc. Bioethanol can serve as a feedstock for ethyl tertiary butyl ether (ETBE) which easily blends with gasoline. Biodiesel is produced by transesterification of oil from crops such as soybean, sunflower, rapeseed, coconut, used cooking oil or animal fat. It can serve as a full substitute for diesel. Biogas is a fuel that can be used for cooking purpose as well as in gasoline vehicles with slight modifications. It can be produced by anaerobic digestion of organic matter in the form of manure and other digestible crop residues. First generation biofuels signify a step towards energy independence, reducing the load on fossil fuels to fulfil energy demands. These biofuels also support agricultural industries and rural population through increased demand for feed crops. At present, these first generation biofuels are produced from commodities that are used for food and the demand is in increasing trend, so it difficult to use the agricultural food crop for biofuel production (www.task39.org).
Constraints and Concerns for first Generation Biofuels
However, first generation biofuels have a number of associated economical and environmental issues. There is much debate over their actual benefits in terms of reducing green house gases and CO2 emission, as some biofuels can produce negative net energy gains and release more carbon in their production than their growth. Besides this the most litigious issue with first generation biofuels is “fuel vs food”. Since the majority of first generation biofuels are produced directly from food crops, the rise in demand for biofuels has contributed to increase in world prices for food and animal feeds over the last couple of years.18
They also have a negative impact on biodiversity and competition for water in some regions. In addition, biomass production from first generation bio fuels requires a large land area to grow. They only provide a little benefit over fossil fuels in regards to greenhouse gases emission, since they require large amounts of energy to grow, assemble, and process. Moreover, biodiesel most commonly derived from recycled oils from restaurants, as opposed to virgin oils, so the supply is limited (agsci.oregonstate.edu/sites/agsci…edu/…/generations-of-biofuels).
The overall concerns for many, but not all, of the 1st generation biofuels are that they:
Contribute to higher food prices due to competition with food crops.
Are expensive options for energy security taking into account total production costs excluding government grants and subsidies.
Do not meet their claimed environmental benefits because the biomass feedstock may not always be produced sustainably.
Are accelerating deforestation.
Potentially have a negative impact on biodiversity.Compete for scarce water resources in some regions.
Additional uncertainty has also recently been raised about GHG savings if indirect land use change is taken into account. Certification of biofuels and their feedstocks is being examined, and could help to ensure biofuels production meets sustainability criteria, although some uncertainty over indirect land use impacts is likely to remain. Additional concerns over the impact of biofuels on biodiversity and scarce water resources in some countries also need further evaluation.
Second Generation biofuels
Many of the issues associated with 1st generation biofuels can be overcome by the production of biofuels manufactured from non-food crop feedstocks, agricultural and forest residues. Second generation biofuels are produced from plant biomass not competing with food production. In the context of biofuel production, the term ‘plant biomass’ largely refers to lignocellulosic material which constitute the majority of the cheap and plentiful non-food materials available from plants.19,20 The ligno-cellulosic biomass is produced from specialist energy feedcrops grown on arable land, still concerns remain over competing land use. Although energy yields are likely to be higher than if crops are grown for production of 1st-generation biofuels on the same land. In addition, poorer quality land could possibly be utilised.
New scientific breakthroughs are required in developing the new genome-based breeding programmes and the biomass processing techniques for production of second generation sustainable biofuels. Second generation biofuels include;
(i) Specific bioenergy crops i.e. the plants that are specifically grown for bioenergy production on marginal land (area not suitable for food production) and or (ii) inedible parts of common crops and forest trees that should be efficiently processed for bioenergy production by improvement of the current technologies.
Lignocellulosic feedstocks for second Generation biofuels
Lignocellulosic biomass is an abundant and renewable feedstock, though only a small portion of it could be utilised in practice. This include cereal straw, wheat and rice husk, corn cobs, sugarcane bagasse, wood pieces and forest harvest residues. The technical potential from available annual supplies has been estimated in energy terms at over 100 EJ per year, with costs in the range of USD 2-3/GJ annually.21
Chemical and structural composition of plant cell wall has hindered the process of cellulosic and hydrocarbon-based biofuels production. Moreover, the development of commercial-scale cellulosic biofuel facilities has been much slower than expected during the past decade(s). Scientists are effortlessly trying to discover naturally occurring microbes that can easily break down lignin, present in plant cells, to give easier access to cellulose. Cellulose is a naturally occurring polymer structure within plant cell walls which keeps the cells together. To be used, cellulose must be hydrolysed to sugar, which can then be converted into ethanol or other liquid fuels, like biodiesel and butanol. Currently, the biochemical conversion of cellulosic biomass requires a set of enzymes for sugar liberation. So, this tightly packed cellulosic structure is required to be unravel into soluble sugars using a few enzymes. This could be achieved by engineering microbes those could simultaneously break down lignin, cellulose and hemicelluloses without requiring expensive enzymes, moreover enzyme kinetics can be improved by developing suitable model system. Biofuel production from cellulosic biomass could be improved by changing the composition of the cell wall by genetic engineering technique. The increasing research in the field of chemistry, biochemistry and molecular biology of plant cell walls is paving the way towards this direction.22 The production of biofuels from lignocellulosic feedstocks can be achieved through two types of processes (Figure 1).
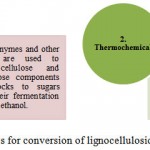 |
Figure 1: Processes for conversion of lignocellulosic biomass into biofuel
|
Biochemical conversion of cellulose is brought about by microbes such as bacteria and fungi. Unfortunately, there are only a small number of bacteria which can brought about this conversion including those present in the stomach lining of ruminants and certain fungi. These microbes contain enzymes which can hydrolyze lignin, cellulose and hemicelluloses. Thermochemical conversion involves pyrolysis/gasification and Fischer-Tropsch synthesis to produce distillate fuels, unlike biochemical conversion, does not depend on the bacterial and enzymatic processes and hence cellulosic nature of foodstocks is less of a concern. However, feedstock properties (organic matter, moisture content etc.) are more important in thermochemical conversion.
Constraints and concerns for second Generation biofuels
Second generation biofuels are superior to most of the first generation biofuels in terms of competition for land use requirement, food, fibre, water, energy balance and greenhouse gases emission reduction. However, they do not produce co-products such as animal feed.3 Over all these advantages, second generation biofuels still face a number of disadvantages:
High production cost: Reduction in the cost of biomass feedstocks, transportation, and their conversion processes will be required to lower the cost.
Logistics and supply chain challenges: Current harvesting, storage and transport systems are inadequate for processing and distributing biomass at the scale needed to support significant production of large volumes. The lack of experience in operating large scale plants demanding large volumes of biomass creates the problem of requiring expensive infrastructure expansions where current handling and storage facilities are inadequate.
Development of second generation biofuels requires changes in agriculture and forestry sectors. Significant changes in policy and business practices are required and these will take time to achieve.
The development of 2nd generation biofuels is still at an early stage so there may be misunderstanding of environmental/energy tradeoffs. There is a risk that without extensive evaluation, they could result in negative unexpected consequences for GHG emission, land use, biodiversity and environment.
Third generation biofuels
The third generation biofuels are based on improvements in the production of biomass. They depend upon specially engineered energy crops such as algae as its energy source.23 Algae are a diverse group of prokaryotic and eukaryotic organisms ranging from unicellular genera such as Chlorella and diatoms to multicellular forms such as the giant kelp, a large brown alga that may grow up to 50 m in length24. They live in varied ecological niches such as freshwater, marine, hyper-saline and brackish water, with a range of temperatures, pH and osmotic pressure.25 The diversity and range of cellular lipids and inherent ability to modify lipid metabolism in response to changing environment can be credited to the ability of algae to survive or proliferate over a wide range of environmental conditions.26,27 Under optimal growth conditions, algae through process of transesterification, convert fatty acids into glycerol-based membrane lipids, constituting 5-15% of their dry cell weight.
The algae can be converted into various types of renewable biofuels including bioethanol, biodiesel, biogas, biohydrogen, and further into bio-oil and syngas through process of liquefaction and gasification, respectively.28 Biofuel production involves conversion of algal biomass into energy sources and the conversion processes can be divided into three categories; biochemical, chemical, and thermochemical.
Microalgae based biodiesel production
Microalgae normally grow in water bodies or ponds and are very small in sizes usually measured in micrometers. Biodiesel is a mixture of monoalkyl esters of long chain fatty acids. It can be obtained from different lipid feedstocks and biomass. The extraction of lipids from microalgae dates back to the period of World War-II.29 Microalgae contain more lipids than macroalgae and higher oil yield in microalgal biomass as compared to oil seed crops makes them more economic alternative for biodiesel production.30 The microalgae such as Ankistrodesmus fusiformis, Ankistrodesmus falcatus and Chlamydocapsa bacillus containing high levels of polyunsaturated fatty acid methyl esters are generally preferred for biodiesel production.31 The oil productivity expressed as mass of oil produced per unit volume of the microalgal broth per day, depends upon the growth rate and biomass content of the algal species. The biomass can multiply vigorously during exponential phase with a doubling time of approximate 24 h and oil content of is generally very high, which exceed up to 80% by weight of its dry biomass. About 8,000–15,000 gallons of biodiesel can be produced from algal biomass per acre per year, thus reflecting the potentiality of algal biomass for biodiesel production.23 The short harvesting cycle is the key feature of microalgae for biofuel production unlike other coventional crops that can be harvested only once or twice in a year.32 This has shifted the focus on algal biomass for its application in biofuel area. The main advantages of algal biomass for biofuel production are summarized in Figure 2.
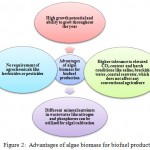 |
Figure 2: Advantages of algae biomass for biofuel production
|
Despite all the advantages, there are several disadvantages associated with algae as a feedstock for biofuel such as the higher cultivation cost as compared to conventional crops, high energy input techniques such as centrifugation, flocculation, filteration and sedimentation, are required for algae harvesting.33,34
Process of biodiesel production from algae
Process of biodiesel production from algal biomass involves cultivation of microalgae, drying of algal biomass, extraction of oil from algal biomass and transesterification (Figure 3).
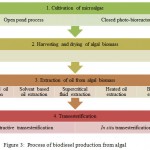 |
Figure 3: Process of biodiesel production from algal
|
Cultivation of Microalgae
Microalgae are photoautotrophs, require sunlight to grow and proliferate. They can be cultivated in conventional open ponds or closed bioreactors besides this heterotrophic method can be used in which algae are provided with sugar as a carbon source and grown in the absence of sunlight35. A generalized set of conditions required to culture microalgae are given in Table 1.
Table 1: Conditions required to culture microalgae
| Parameters | Range | Optimum conditions |
| Temperature (°C) | 16-27 | 18-24 |
| Salinity (gL-1) | 12-40 | 20-24 |
| Light intensity (lux) | 1,000-10,000 | 2,500-5,000 |
| Photoperiod (light: dark, hours) | 16:8 (minimum); 24:0 (maximum) | |
| pH | 7-9 | 8.2-8.7 |
Source: http://www.fao.org/docrep/003/w3732e/w3732e06
Light, temperature, pH, nutrient concentration and CO2 flow rate determine the photosynthesis activity and growth rate of microalgae.36 Therefore designing an optimum cultivation system and integrating it with efficient downstream processing is required for optimum biodiesel production.
open Pond System
Open pond system includes algal cultivation in natural waters such as lakes, lagoons, natural as well as artificial ponds and containers. Cultivation of algae in open ponds has been widely studied.37,38 The main advantage of open ponds is that they are much easier to construct and operate than closed systems.39 However, there are many disadvantages such as poor light utilization by algal cells, evaporative loss of water, loss of CO2 in the atmosphere, and larger land area requirement. Furthermore, they can be contaminated by predators and other unwanted species. Due to poor stirring process in open cultivation system, the mass transfer rates are low resulting in low algal biomass productivity.32
Open ponds in which the algae are cultivated have variety of shapes and sizes but the most commonly used ponds are “raceway ponds”. In these ponds, the algae, water and nutrients circulate around a racetrack (https://www.e-education.psu.edu/egee439/node/695). The pond is provided with a paddlewheel to drive the water flow continuously inside the pond. The algae are kept suspended in the water, and are circulated back to the surface on a regular frequency. Due to continuous light requirement by algae the ponds are usually kept shallow. The ponds are operated in a continuous manner, with nutrients and CO2 being continuously fed and algae-containing water is removed at the other end.40
The open pond is typically inoculated with the desired algal strain which initiates the growth and soon dominates in the pond. Cultivating extremophiles in the open pond system tolerating variatable pH and osmotic pressure can encourage sustainable and consistent growth of a single species. For example, Spirulina can survive and efficiently grows at a very high pH (9 to 12), therefore emerged as a dominant species in soda lakes.41 Its spiral shape makes it easy to harvest. Another example is Dunaliella salina, a unicellular green alga grown predominantly in Australia. The strain is capable of growing in water with high solute potential due to its high intracellular glycerol content, which provides protection against osmotic pressure. Dunaliella salina is rich in caretenoids which protect it against the intense light in the shallow salt pan ponds.42
Closed photo-bioreactor
A photo-bioreactor is closed vessel which provides a controlled environment and enables high productivity of algal biomass. Here, all the growth requirements of algae are fed into the system and the system is controlled as per the requirement. This system enable better control of culture conditions such as light exposure, water and CO2 supply, temperature, culture density, pH, etc. Most commonly used closed photo-bioreactors are tubular reactors and plate or bubble column reactors.43,44 To achieve economic feasibility in the process, it is important to design a reactor which meets with all demands of algal growth along with a cheap design construct.39 In this regard, optimization of photo-bioreactor design is very important to achieve maximum growth and yield of algae.45,46,47 This also reduces the production and downstream processing costs. So, it is important to gain knowledge about bioprocess engineering to control systems for process parameters like temperature, pH, light, cell density, nutrient concentration, CO2 level, mixing rate which eventually determine the growth of microalgae.32
The closed bioreactor system consists of a mixing vessel having inlet for water, nutrients, algae and a CO2 valve (http://www.algaeproductionsystems.com/equipment.html). The mixture is directed to photo-bioreactor which is used to promote the growth of algae with controlled process parameters including light. The photo-bioreactor tubes are made of acrylic and provide fixed light and dark intervals for algal growth. There is a built-in cleaning system which automatically cleans the tubes without hampering the production process. The algal biomass completes many flows through in between mixing vessel and photo-bioreactor and when algae are ready for harvesting, they are processed through connecting filtering system. The algae which are ready for further downstream processing are collected and the rest passes back to the vessel and the cycle continues.48
Closed photo-bioreactors save water, energy and chemicals, thus making them the matter of choice for biofuel production. Cultivation of algae is in controlled conditions, therefore they support up to fivefold higher biomass productivity with respect to vessel volume and subsequently have a smaller “footprint” on a yield basis.49 They provide better protection from outside contamination. An enclosed bioreactor will enhance commercial algal biomass productivity by keeping algae genetics pure and reducing the possibility of predatory and parasitic infestation.50 Despite many advantages, there are some disadvantages associated with closed photo-bioreactor such as, high capital cost and difficulty in sterilization has restricted their application to grow algae for specific pharmaceutical products.48
Harvesting and drying of algal biomass
Harvesting of algal biomass and drying is important prior to extraction of oil from algal biomass. The term ‘harvesting’ refers to concentrating the dilute microalgal culture suspension to a paste containing 5-30% of total suspended solids (TSS). This paste can be concentrated in a one or two-step harvesting process. Concentration of algal culture suspension significantly influences downstream processes, including drying. More concentrated product decreases the cost of extraction, purification and effective unit cost of biomass. Macroalgae can be harvested simply using nets, whereas, microalgae can be harvested using conventional processes, such as filtration, flocculation, centrifugation, sedimentation, and ultrasonic separation.51,52,53,54,55 Microalgae are a type of colloidal particle structures with negative charge and the intensity of charge depends upon species, ionic strength, and pH of the cultivation media. Therefore, electrostatic repulsion between the algal cells and cell interaction with the surrounding water provide stability to the algal suspension56. These electrostatic forces maintain the culture growth as they aid in keeping the cells in water column in a way so that they do not settle to the bottom of the pond.
Drying of algal biomass is carried to increase the shelf-life and avoid spoilage during post-harvest operations.57 The most commonly used methods for drying algal biomass include sun drying, spray-drying, drum-drying, freeze drying or lyophilisation.58,59,60 Sun-drying is generally not recommended due to high moisture content in biomass.47 However, success have been observed in drying high moisture containing Spirulina and Scenedesmus using solar drying process.61 The drying temperature during lipid extraction from algal biomass affects the concentration of triglycerides and ultimately lipid yield is affected.62
Extraction of oil from algal biomass
Microalgae produce a cell wall containing lipids and fatty acids, which differ them from higher plants and animals. Therefore, oil from algae cannot be extracted by the more conventional methods such as pressing de-husked seeds. Lipids in algae are stored inside the cell or in the cell membrane63 and thickness of the cell wall prevents simple expulsion of oil from the cell. Extraction of these oils requires specific cell disruption methods such as mechanical extraction, solvent extraction, supercritical fluid extraction, heated oil extraction and biological extraction as described in Table 2 in detail.
Table 2: Methods of oil extraction from algal biomass
| Sr. No. | Method | Process | Reference |
| 1. | Mechanical extraction | Mechanical treatments, such as ultra sonication and homogenization may be used to disrupt the cells which lead to high oil recovery. Sometimes presses or expellers are used to extract microalgal oil. The algal biomass is dried prior to extraction and cells are broken down with a press or ultrasonication to trickle out the oil. | Popoola and Yangomodou91 ;Topare et al. 92; Munir et al. 57 |
| 2. | Solvent extraction | A number of organic solvents such as hexane, cyclo-hexane, chloroform, acetone, and benzene have been evaluated alone or in combination to extract and purify oil from dried algal feedstock. Out of various organic solvents, hexane is found to be most effective due to its low toxicity and cost. Solvent based extraction recovers almost all the oil leaving only a trace amout (0.5-0.7%) in the biomass. Therefore, solvent based extraction method has been found to be more suitable than the mechanical extraction of oil. The hydrophobic solvents react on algae cells and release the oil, which is later recovered from the aqueous medium. | Nagel and Lemke93; Fajardo et al.94; Lee et al.95 ; Afify et al.96; Shalaby,97; Ryckebosch et al.98 |
| 3. | Supercritical fluid extraction | Supercritical CO2 has fluid as well as gas properties, thus allowing it to penetrate the biomass and act as an organic solvent to extract the oil. Moreover it does not required to be separated from the final product thus save the expenses required in the process.
|
Dufreche et al.99; Couto et al.100 |
| 4. | Heated oil extraction | Algae wet paste is contacted from a gravitational thickener with heated oil and then removal of water after centrifugation. Centrifugation is followed by oil extraction in a three phase centrifuge which could separate oil, water, and solids. A fraction of the oil is returned to the heater and then to extraction, the remaining is carried forward for biofuel production.
|
Oswald,101 |
| 5. | Biological extraction | Low technology based and low-cost method for harvesting algae followed by lipid extraction. Here, enzymes degrading algal cell wall are used for oil extraction. Besides this, the organisms feeding on microalgae such as brine shrimp are used. The organisms concentrating the algae are later harvested, crushed and homogenized to recover the algal oil. | Brune and Beecher,102 |
Transesterification
Transesterification is a process of conversion of organic oil to biodiesel.64 It involves reactions between triglycerides or fatty acids and alcohol. Different alcohols such as methanol, ethanol, propanol, butanol, and amyl alcohols can be used in transesterification. However, methanol and ethanol are more commonly used in commercialised process due to their low cost and physical and chemical advantages.65
The biodiesel transesterification reaction is summarised as:

An inorganic catalyst such as potassium hydroxide or lipase enzyme is used during the process. Glycerol is formed as a by-product which is denser than biodiesel and should be continuously removed during the process to shift the equilibrium in forward reaction. The excess of methanol and other co-solvents cause engine failure thus they are separated from the methyl esters through evaporation and the final biodiesel is recovered by rinsing with water and pH neutralized.23,67,57
Previous study described homogeneous, heterogeneous and enzymatic catalysis for transesterification of waste cooking oil to produce biodiesel.68 Similarly, acid base catalysed tranesterification was also studied during biodiesel production.69 Kim and co-workers described biodiesel production through acid/alkali catalysed transesterification process.70 A high biodiesel conversion (55.07%) based on lipid by weight was observed in NaOH catalysed tranesterification compared to H2SO4 catalysed transesterification where 48.41% biodiesel conversion was observed from algal lipids.
Besides acid and alkali catalysed tranesterification, enzyme catalysis is also used due to substrate specificity, verstality, regioselectivity and high catalytic rate of enzymes.71 Lipases are most widely used biocatalysts in tranesterification but in many cases it is not possible to hydrolyze the ester bond at secondary position by some lipases, whereas other group of enzymes can hydrolyze both primary and secondary esters. A lipase gene lipB68 was cloned and expressed in Escherichia coli BL21 and further used for enzyme catalysed transesterification in biodiesel production. The strain yielded 92% of biodiesel production after 12 h at substantially low temperature which can reduce the net energy requirement.72 The process of transesterification can be carried out in two ways:
Extractive transesterification
In situ transesterification
Extractive transesterification
It this process, the oil extraction is carried out after harvesting of algal biomass. The overall process consists of multiple steps such as algae harvesting and drying, oils extraction, transesterification, and biodiesel refining.73
In situ transesterification
This method of biodiesel production skips the oil extraction step. Here the alcohol acts as extraction solvent as well as esterification reagent, which by enhancing porosity of cell membrane helps to remove the oil from algal cells. The commercial biodiesel production process involves use of organic solvents for oil extraction, which adversely affects the environment. Thus, eliminating oil extraction step can serve as an impressive solution to reduce the chemical and energy requirement during biodiesel production process.74
Different feedstock’s reported for microalgae cultivation
Chemical synthesized Fog’s medium has been used for microalgae cultivation which is maintained at 28°C75 but, it is not economical for mass scale cultivation of microalgae for biodiesel production. So algae can be cultured on other waste resources such as Botryococcus braunii cultured in effluent obtained from seafood processing plant,76 industrial sludge waste more specifically dairy waste water has been investigated and reported for maximum growth of Chlorella pyrenoidosa.75 Dairy industry is one of the major industries among food and agricultural sector which is a major contributor of water pollution. Its waste water contains toxic elements with milk solids, liquid effluents and slurries including organic and inorganic chemicals. It needs prior treatment before dispose off to the environment or fresh water resources like rivers causing health hazards and destroy natural flora and fauna. This huge amount of waste water generated globally from dairy industries can act as growth medium for microalgae cultivation as it contains the entire nutrients needed for its growth and in return algae can aid in treatment of waste water.77 Similarly, rice straw has been reported for the cultivation of microalgae Chlorella pyrenoidosa. This residue is left behind after harvesting of grain from rice crop and poses a problem for dispose off due to which majority of farmer’s burn this huge amount of rice straw residue in open fields.78 Burning of it produces GHGs, pollutants and particulate matters. In India alone the total agricultural residue production was 347 million tons, of which wheat and rice straw made more than 200 million tons.79 Alternatively, these agricultural residues can be treated with cellulose degrading methods to hydrolyze the cellulose into glucose which can be used as a carbon source in the growth medium of alga which is used for achieving maximum yield of microalgae.80
Other applications of microalgae
Carbon dioxide emission is the key factor responsible for global warming and climate change which is mainly produced by combustion of fossil fuels of approximate 2 × 102 tons/year. The 7% of total world CO2 emissions are from flue gases of power plants. Industrial emissions contribute around 15% CO2. Since, CO2 is required during the photosynthesis hence, CO2 emitted in the industrial flue gases can be utilized by microalgae in biodiesel production. It was reported that algae Monoruphidium minutum can utilized CO2 from flue gases.81
It has been reported that various products and byproducts have been synthesized and extracted from microalgae such as glycerin, carotenoids, protein supplements, anti-fouling properties, plasminogen activating factor with clionasterol isolated from Spirulina sp., health-food, omega-3-fatty acids, nannochloropsis, Vitamin A, anti-oxidant astaxanthin, fucosterol, therapeutics such as anti-diabetics, anti-oxidants, hepatoprotective, anti-hyperlipidemic, anti-fungal, anti-histaminic, anti-cholinergic, anti-adipogenic, anti-photodamaging, anti-osteoporotic and blood cholesterol reducing compounds.82 Spirulina is consumed as food by which it stimulates immune system, prevents cancer and viral infection. It also increases beneficial intestinal bacteria such as lactic acid bacteria. In addition to this, it contains 55-70% protein content of total dry weight. Extracts from Chlorella sp. can decrease blood sugar level, increase hemoglobin content, ethionine intoxification, hypocholesterolemic and hepatoprotective. It also increases the count of cytokines and splenocytes to stimulate immune responses in mammals.83
Phenols are the major toxic pollutants of fresh water resources produced by industrial waste and are harmful to aerobic as well as anerobic bacteria. So, they are unsuitable for sewage treatment. Therefore, two microalgae species; Ankistrodesmus braunii and Scenedesmus quadricauda were selected for phenols degradation (Pinto et al., 2002). Biofouling is the major challenge to marine ecosystem which is caused by microorganisms (microorganisms, fungi, etc). It causes huge loses to anthropogenic structures84 and to protect these structures, antifouling agents such as paints containing tributyltin (TBT), copper and organic biocides are used.85 The international maritime organization (IMO) has banned TBT-coatings due to toxic effects and harm to the environment. Cyanobacteria are able to produce antibiotic substances with antifouling properties.86 Azo dyes are the major group of dyes used in industries such as textiles, paint, ink and plastic industries. These dyes remain in waste waters after processing and are harmful to aquatic life and mutagenic to humans.87 These dyes can also be biologically degraded by algae.88
Olive oil waste is becoming major problem to the environment due to its chemical composition and high organic load which makes it resistant to degradation. It has an acidic pH of in-between 4 and 6, high chemical oxygen demand of up to 200 gL-1 and phytotoxic components that limits the growth of microbe. It also inhibits the vegetative growth and germination of plants. Olive oil industries produce 2.7 million tones of olive oil worldwide annually and its production has increased to about 30% in the past few years.89 It has been observed that toxic phenolic compound load from olive oil waste is thousand times more than domestic sewage toxic load. Microalgae Chlorella, Ankistrodesmus and Scenedesmus species are capable of degrading and removing this waste load from olive oil and paper industries.90
Conclusion and Future Perspectives
With the tremendous increase in demand and consumption of non-renewable fossil fuels in past few decades, there is a challenge to find alternative renewable green energy sources, which can replace fossil fuels. Now a day, different green energy sources are being used as biofuels which have economic, environmental and social negative consequences. Among these, microalgal derived biodiesel seems to be a sustainable solution as microalgae could be used to treat the wastewater and reduce the CO2 emissions from industries. Due to presence of various advantages in algal biofuels such as non food biomass material, low land requirement for growth, high lipid content giving high productivity and remove competition of ‘food vs feed’. Therefore, it has been considered as the most suited option to replace the liquid petroleum fuel. There are a few disadvantages associated with the process such as low biomass production, and high energy inputs required during biomass harvesting thus increasing the overall cost of production. For an economic process development, high biomass production, and efficient harvesting methods are required with low energy input. Moreover, improvement in photobioreactor design, bioprocess engineering and optimization of production process will further reduce the cost of biofuel production. For high biodiesel yield, genetic engineering of algal strains to produce high biomass with high oil productivity will determine the future of algal biology.
References
- Agarwal A. K. Biofuels (alcohols and biodiesel) applications as fuels for internal combustion engines. Prog Energ Comb Sci. 2007;33:233-271.
CrossRef - E.I. A. Environmental Impact Assessment World Energy outlook Paris, International Energy Agency. 2006. http://tonto.eia.doe.gov/oog/info.gdu/gasdiesel.asp.
- The current status of biofuels in the European Union, their environmental impacts and future prospects. EASAC Policy Report. 2012;19. http://www.easac.eu.
- Eva-Mari A. From first generation biofuels to advanced solar biofuels. Ambio. 2016;45(1):24-31.
- Ramirez J., Rainey T., Brown R. A review of hydrothermal liquefaction bio-crude properties and prospects for upgrading to transportation fuels. Energies. 2015;8:6765-6794.
CrossRef - Sharif A. B. M. H., Nasrulhaq A. B., Majid H. A. M., Chandran S., Zuliana R. Biodiesel production from waste cooking oil as environmental benefits and recycling process a review. Asia Biofuel Confer Book. 2007. Singapore.
- Keskin A., Gürü M., Altiparmak D., Aydind K. Using of cotton oil soapstock biodiesel diesel fuel blends as an alternative diesel fuel. Renew Energy. 2008;33(4):553-557.
CrossRef - Banerjee A., Chakraborty R. Parametric sensitivity in transesterification of waste cooking oil for biodiesel production: a review. Resourc Conserv Recycl. 2009;53:490-497.
CrossRef - Illman A. M., Scragg A. H., Shales S. W. Increase in Chlorella strains calorific values when grown in low nitrogen medium. Enzyme Microbe Technol. 2000;27(8):631-635.
CrossRef - Nigam P. S., Singh A. Production of liquid biofuels from renewable resources. Pro Energy Combust Sci. 2011;37: 52-68.
CrossRef - Saqib A., Tabbssum M. R., Rashid U., Ibrahim M., Gill S. S., Mehmood M. A. Marine macroalgae Ulva: a potential feed-stock for bioethanol and biogas production. Asian J Agri Biol. 2013;1:155-163.
- Thomas D. N. Seaweeds (Smithsonian Institution Press Washington DC in association with the Natural History Museum, London). 2002.
- Tamer E., Amin M. A., Ossama E. T., Bo M., Benoit G. Biological treatment of industrial wastes in a photobioreactor. Water Sci Technol. 2006;53:117-25.
CrossRef - Raja R., Hemaiswarya S., Kumar N. A., Sridhar S., Rengasamy R. A perspective on the biotechnological potential of microalgae. Crit Rev Microbiol. 2008;34:77-88.
CrossRef - Omar, H.H. Algal decolorization and degradation of Monoazo and Diazo dyes. Pak J Biol Sci.,2008; 11: 1310-6.
CrossRef - Barre S. L., Potin P., Leblanc C., Delage L. The halogenated metabolism of brown algae (Phaeophyta) its biological importance and its environmental significance. Mar Drugs. 2010;8:988-1010.
CrossRef - Choi W., Han J., Lee C., Song C., Kim J., Seo Y. Bioethanol production from Ulva pertusakjell man by high-temperature liquefaction. Chem Biochem Eng. 2012;26:15-21.
- UN Report Sustaiable bioenergy a framework for decision makers. 2007.
- Gomez A., Rodrigues M., Montanes C., Dopazo C., Fueyo N. The technical potential of first generation bio fuels obtained from energy crops in Spain. Biomass Bioenerg. 2011;35:2143-2155.
CrossRef - Zabaniotou A. A., Kantarelis E. K., Theodoropoulos D. C. Sunflower shells utilization for energetic purposes in an integrated approach of energy crops: Laboratory study pyrolysis and kinetics. Biores. 2008;99:3174-3181.
CrossRef - International Energy Agency: World Energy outlook 2008. (OECD/IEA, Paris). Available from http://www.iea.org/textbase/nppdf/free/2008/weo2008.pdf. 2008:569.
- Furtado A., Lupo J. S., Hoang N. V., Healey A., Singh S., Simmons B. A., Henry R. J. Modifying plants for biofuel and biomaterial production. Plant Biotech J. 2014;12:1246-48.
CrossRef - Chisti Y. Biodiesel from microalgae. Biotech Advance., 2007; 25: 294-306.
CrossRef - Li Y., Wang B., Wu N., Lan C. Q. Effects of nitrogen sources on cell growth and lipid production of Neochloris oleoabundans. Applied Microbiol Biotechnol. 2008;81:629-636.
CrossRef - Falkowski P. G., Raven J. A. edited by Siegenthaler PA and Murata N. Aquatic Photosynthesis. (Blackwell Science, Malden, MA). 1997:375.
- Wada H., Murata N. Membrane lipids in cyanobacteria. In: Lipids in photosynthesis: structure, function and genetics, (Kluwer Academic Publishers, Dordrecht, The Netherlands). 1998:65.
- Guschina I. A., Harwood J. L. Lipids and lipid metabolism in eukaryotic algae. Prog Lipid Res. 2006;45(2):160-186.
CrossRef - Kraan S. Mass cultivation of carbohydrate rich microalgae, a possible solution for sustainable biofuel production. Mitig Adapt Strateg Glob Change. 2013;18:27-46.
CrossRef - Cohen Z., Norman H. A., Heimer Y. M. Microalgae as a source of x-3 fatty acids. World Rev Nutr Diet. 1995;77:1-31.
CrossRef - Lee K., Eisterhold M. L., Rindi F., Palanisami S., Nam P. K. Isolation and screening of microalgae from natural habitats in the Midwestern United States of America for biomass and biodiesel sources. J Nat Sci Biol Med. 2014;5(2):333-339.
CrossRef - Nascimento I. A., Marques S. S. I., Cabanelas I. T. D., Pereira S. A., Druzian J. I., de Souza C. O. Screening microalgae strains for biodiesel production: lipid productivity and estimation of fuel quality based on fatty acids profiles as selective criteria. Bioenerg Res. 2013;6:1-13.
CrossRef - Schenk P. M., Thomas-Hall S. R., Stephens E., Marx U. C., Hankamer B., Mussgnug J. H., Kruse O., Posten C. Second generation biofuels: high-efficiency microalgae for biodiesel production. Bioenerg Res. 2008;1(1):20-43.
CrossRef - Demirbas A. Use of algae as biofuel sources. Energy Convers Manag. 2010;51:2738-2749.
CrossRef - Ho S. H., Chen C. Y., Lee D. J., Chang J. S. Perspectives on microalgal CO2-emission mitigation systems-a review. Biotechnol Adv. 2011;29(2):189-198.
CrossRef - Blinova L., Bartosova A., Gerulova K. Cultivation of microalgae Chlorella vulgaris for biodiesel production. Research Paper Faculty of Material Science and Technology Slovak University of Technology. 2015;23:87-95.
- Harun R., Singh M., Forde G. M., Danquah M. K. Bio process engineering of microalgae to produce a variety of consumer products. Renew Sustain Energ Rev. 2010;14:1037-1047.
CrossRef - Becker E. W. Microalgae biotechnology and microbiology. Cambridge University Press, Cambridge). 1994:293.
- Yang C., Hua Q., Shimizu K. Energetics and carbon metabolism during growth of microalgal cells under photoautotrophic, mixotrophic and cyclic light-autotrophic/dark-heterotrophic conditions. Biochem Eng J. 2000;6:87-102.
CrossRef - Weissman J. C., Tillet D. M., Goebel R. P. Design and operation of an outdoor microalgae test facility. Report SERI/STR-232-3569. (Solar Energy Research Institute, Cole Boulevard, Golden, Colorado). 1989:232.
- Cultivation of algae in open ponds, http://www.oilgae.com/algae/cult/op/op.html. (2016 a) Accessed 05-09-2016.
- Belkin S., Boussiba S. Resistance of Spirulina platensisto Ammonia at High pH Values. Plant Cell Physiol. 1991;32:953-958.
CrossRef - Borowitzka M. A., Hallegraeff G. M. edited by McCarthy P. M., Orchard A. E. Economic importance of algae. In: Algae of Australia: introduction, (ABRS, Canberra). 2007:594-622.
- Weissman J. C., Tillett D. M. Design and operation of an outdoor microalgae test facility. In: Aquatic Species Program Annual Report, edited by Bollmeier W. S., Sprague S., SERI/SP2321-3579. (Solar Energy Research Institute, Cole Boulevard, Golden, Colorado). 1992.
- Pulz O., Scheibenbogen K., Grob W. edited by Rehm H. J., Reed G., Puhler A., Stadler P., (Wiley-VCH, Weinheim). Biotechnology with cyano bacteria and microalgae. In. Biotechnology. 2001;10:107-136.
- Haag A. L. Algae bloom again. 2007;447:520-521.
- Hankamer B., Lehr F., Rupprecht J., Mussgnug J. H., Posten C., Kruse O. Photosynthetic biomass and H2 production by green algae from bioengineering to bioreactor scale-up. Physiol Plant. 2007;131(1):10-21.
CrossRef - Mata T. M., Martins A., Caetano N. S. Microalgae for biodiesel production and other applications a review. Ren Sust Energy Rev. 2010;14(1):217-232.
CrossRef - Cultivation of algae in photo bioreactor, http://www.oilgae.com/algae/cult/pbr/pbr.html. (2016b) Accessed 05-09-2016.
- Barbosa M., Jansen M., Ham N., Tramper J., Wijffels R. H. Microalgae cultivation in air-lift reactors. Biotechnol Bioeng. 2001;82:170-179.
CrossRef - Li X., Xu H., Wu Q. Large-scale biodiesel production from microalga Chlorella protothecoides through heterotrophic cultivation in bioreactors. Biotechnol Bioeng. 2007;98(4):764-71.
CrossRef - Rossignol N., Vandanjon L., Jaoue P., Quemeneur F. Membrane technology for the continuous separation of microalgae/culture medium: compared performances of cross-flow microfiltration and ultrafiltration. Aquac Eng. 1999;20:191-208.
CrossRef - Bosma R. V.,Spronsen W. A., Tramper J., Wijffels R. H. Ultrasound a new s separation technique to harvest microalgae. J Appl Phycol. 2003;15:143-153.
CrossRef - Heasman M., Diemar J. O., Connor W., Shushames T., Foulkes L., Nell J. Development of extended shelf-life microalgae concentrate diets harvested by centrifugation for bivalve molluscs-a summary. Aquac Res. 2008;31(89):637-659.
CrossRef - Liu J., Zhu Y., Tao Y., Zhang Y., Li A., Li T. Freshwater microalgae harvested via flocculation induced by pH decrease. Biotechnol Biofuels. 2013;6(1):98. DOI: 1186/1754-6834-6-98.
- Prochazkova G., Safarik I., Branyik T. Harvesting microalgae with microwave synthesized magnetic microparticles. Bioresour Technol. 2013;130:472-477.
CrossRef - Taylor G., Baird D. J., Soares A. M. V. M. Surface binding of contaminants by algae consequences for lethal toxicity and feeding to. Daphnia magna Environ Toxicol Chem. 1998;17:412-419.
CrossRef - Munir N., Sharif N., Naz S., Saleem F., Manzoor F. Harvesting and processing of microalgae biomass fractions for biodiesel production (a review). Sci Tech Dev. 2013;32(3):235-243.
- Leach G., Oliveira G., Morais R. Spray-drying of Dunaliella salinato produce a b-carotene rich powder. J Ind Microbiol Biotechnol. 1998;20:82-85.
CrossRef - Richmond A. Handbook of microalgal culture: biotechnology and applied phycology. (Blackwell Publishing Ltd, Osney Mead, Oxford). 2004:588.
- Williams P. J. L. B., Laurens L. M. L. Microalgae as biodiesel and biomass feedstocks: review and analysis of the biochemistry, energetics and economics. Energy Environ Sci. 2010;3(5):554-590.
CrossRef - Prakash J., Pushparaj B., Carlozzi P., Torzillo G., Montaini E., Materassi R. Microalgal biomass drying by a simple solar device. Int J Solar Energy. 2007;18:303-311.
CrossRef - Singh J., Gu S. Commercialization potential of microalgae for biofuels production. Renew Sustain Energ Rev. 2010; 14:2596-2610.
CrossRef - Darzins A. l., Pienkos P., Edye L. Current status and potential for algal biofuels production(PDF). IEA Bioenergy Task. 2010. Report T39-T2.www. Task 39.org.
- Demirbas A. Progress and recent trends in biofuels. Prog Energy Combus Sci. 2007;33(1):1-18.
CrossRef - Surendhiran D., Vijay M. Microalgal biodiesel-acomprehensive review on the potential and alternative biofuel. Res J Chem Sci. 2010;2(2):71-82.
- Stergiou P. Y., Foukis A., Filippou M., Koukouritaki M., Parapouli M., Theodorou L. G. Advances in lipase-catalyzed esterification reactions. Biotechnol Adv. 2013;31(8):1846-59.
CrossRef - Xu H., Miao X. L., Wu Q. Y. High quality biodiesel production from a microalga Chlorella protothecoides by heterotrophic growth in fermenters. J Biotechnol. 2006;126(4):499-507.
CrossRef - Lam M. K., Lee K. T. Mixed methanol-ethanol technology to produce greener biodiesel from waste cooking oil a breakthrough for SO42_/SnO2–SiO2. Fuel Process Technol. 2011;92(11):1639-1645.
CrossRef - Dennis Y. C., Xuan W. L., Leung M. K. H. A review on biodiesel production using catalyzed transesterification. Appl Energy. 2010;87(4):1083-1095.
CrossRef - Kim G. V., Choi W. Y., Kang D. H., Lee S. Y., Lee H. Y. Enhancement of biodiesel production from marine alga, Scenedesmus through in situ transesterification process associated with acidic catalyst. BioMed Res Int. 2014;2014:11.
- Knezevic Z. D., Siler-Marinkovic S. S., Mojovic L.V. Immobilized lipases as practical catalysts. Acta Period Technol. 2004;35:151-164.
CrossRef - Luo Y., Zheng Y., Jiang Z., Ma Y., Wei D. A novel psychrophilic lipase from Pseudomonas fluorescenswith unique property in chiral resolution and biodiesel production via transesterification. Appl Microbiol Biotechnol. 2006;73(2):349-355.
CrossRef - Hidalgo P., Toro C., Ciudad G., Navia R. Advances in direct transesterification microalgal biomass for biodiesel production. Rev Environ Sci Biotechnol. 2013;12:179-199.
CrossRef - Patil P. D., Gude V. G., Mannarswamy A., Cooke P., Nirmalakhandan N., Lammers P. Comparison of direct transesterification of algal biomass under supercritical methanol and microwave irradiation conditions. 2012;97:822-831.
CrossRef - Kothari R., Pathak V. V., Kumar V., Singh D. P. Experimental study for growth potential of unicellular algae Chlorella pyrenoidosa on dairy waste water integrated approach for treatment and biofuel production. Biores Technol. 2012;116:466-470.
CrossRef - Dumrattana P., Tansakul P. Effect of photoperiod on growth and hydrocarbon content of Botryococcus braunii cultured in effluent from seafood processing plant. Songkl J Sci Technol. 2006;28(1):99-105.
- Kushwaha J. P., Srivastava V. C., Mall I. D. An overview of various technologies for the treatment of dairy wastewaters. Crit Rev Food Sci Nut. 2011;51(5):442-452.
CrossRef - Gupta R. K., Naresh R. K., Hobbs P. R., Jiaguo Z., Ladha J. K. edited by Ladha J. K., Hill J. E., Duxbury J. M., Gupta R. K., Buresh R. J. Sustainability of post-green revolution agriculture the ricewheat cropping systems of the Indo-Gangetic plains and China. In: Improving the productivity and sustainability of rice-wheat systems:issues and impacts(ASA Special Publication 65, Madison, Wisconsin). 2003:1-25.
- Thakur T. C. Crop residue as animal feed addressing resource conservation issues in rice–wheat systems of South Asia, a resource book. Rice Wheat Consortium for Indo-Gangetic Plains (CIMMYT). 2003:305.
- Miao X., Wu Q. Biodiesel production from heterotrophic microalgal oil. Biores Technol. 2006;97:841-846.
CrossRef - Wang B., Li Y., Wu N., Lan C. Q. CO2 bio-mitigation using microalgae. Appl Microbiol Biotechnol. 2008;79:707-718.
CrossRef - Raja R., Hemaiswarya S., Kumar N. A., Sridhar S., Rengasamy R. A perspective on the biotechnological potential of microalgae. Crit Rev Microbiol. 2008;34(2):77-88.
CrossRef - Barrow C., Shahidi F. Marine nutraceuticals and functional foods., (CRC Press, Taylor & Francis Group). 2008;512.
- Yebra D. M., Kiil S., Dam-Johansen K. Anti fouling technology– past present and future steps towards efficient and environmentally friendly anti fouling coatings. Prog Organic Coat. 2004;50:75-104.
CrossRef - Turley P. A., Fenn R. J., Ritter J. C., Callow M. E. Pyrithiones as anti foulants environmental fate and loss of toxicity. 2005;21:31-40.
CrossRef - Zhong J. Imidazole, oxazole and thiazole alkaloids. Nat Prod Rep. 2006;23:464-496.
CrossRef - Bafana A., Krishnamurthi K., Devi S. S., Chakrabarti T. Biological decolorization of C.I. direct black 38 by gallinarum. J Hazard Mater. 2008;157(2):187-193.
CrossRef - Omar H. H. Algal decolorization and degradation of monoazo and diazo dyes. Pak J Biol Sci. 2008;11:1310-6.
CrossRef - FAO, Food and Agriculture Organization. Sustainable bio energy: A framework for decision makers united nations energy. 2007. http://www.fao.org/docrop/012/ai484e04.html.
- Niaounakis M., Halvadakis C. P. Olive-mill waste menagement literature review and patent survey. (Typothito-George Dardanos, Athens). 2004:960.
- Popoola T. O. S., Yangomodou O. D. Extraction properties and utilization potentials of cassava seed oil. 2006;5:38-41.
- Topare N., Rout S. J., Renge V. C., Khedkar S. V., Chavan Y. P., Bhagat S. L. Extraction of oil from algae by solvent extraction and oil expeller method. Int J Chem. 2011;9(4):1746-1750.
- Nagle N., Lemke P. Production of methyl-ester fuel from microalgae. Appl Biochem Biotechnol. 1990;24:355-361.
CrossRef - Fajardo A. R., Cerdan L. E., Medina A. R., Fernandez F. G. A., Moreno P. A. G., Grima E. M. Lipid extraction from the microalga Phaeodactylum tricornutum. Eur J Lipid Sci Technol. 2007;109(2):120-126.
CrossRef - Lee A., Lewis D., Ashman P. Microbial flocculation, a potentially low-cost harvesting technique for marine microalgae for the production of biodiesel. J Appl Phycol. 2009;21(5):559-667.
CrossRef - Afify A. M. M., Shanab S. M., Shalaby E. A. Enhancement of biodiesel production from different species of algae. Grasas y Aceites. 2010;61(4):416-422.
CrossRef - Shalaby E. A. Algal biomass and biodiesel production. In: Biodiesel-feedstocks and processing technologies, edited by Margarita S., (InTech; Rijeka). 2011:111-132.
- Ryckebosch E., Muylaert K., Foubert I. Optimization of an analytical procedure for extraction of lipids from microalgae. J Am Oil Chem Soc. 2012;89(2):189-198.
- Dufreche S., Hernandez R., French T., Sparks D., Zappi M., Alley E. Extraction of Lipids from Municipal Wastewater Plant Microorganisms for Production of Biodiesel. J Am Oil Chem Soc. 2007;84:181-187.
CrossRef - Couto R. M., Simões P. C., Reis A., Silva T. L. D., Martins V. H., Sánchez-Vicente Y. Super critical fluid extraction of lipids from the heterotrophic microalga Crypthecodinium cohnii. Eng Life Sci. 2010;10:158-164.
CrossRe - Oswald W. J. edited by Borowitzka M. A., Borowitzka L. J. Large-scale algal culture systems (engineering concepts). In: Micro-algal biotechnology., (Cambridge University Press, Cambridge). 1988:357.
- Brune D. E., Beecher L. E. Proceedings of the 29th annual symposium on biotechnology for fuels and chemicals, simhq.org/meetings/29symp/index.html. 2007.

This work is licensed under a Creative Commons Attribution 4.0 International License.





