Manuscript accepted on : November 08, 2006
Published online on: --
Plagiarism Check: Yes
Anita Tilwari¹*, N.P. Shukla² and P. Uma Devi³
1School of Biotechnology, Rajiv Gandhi Proudyogiki Vishwavidyalaya and Peoples Group of Medical Sciences and Research Centre, Bhopal - 462 036 (India)
2M.P. Council of Science and Technology, Bhopal - 462 023 (India)
3Department of Research, Jawaharlal Nehru Cancer Hospital and Research Centre, Bhopal - 462 001 (India)
ABSTRACT: Tribulus terrestris is highly valued in Indian system of medicine and extensively used for tonic and rejuvenating properties. Therefore, this study was aimed to evaluate the effect of aqueous extract of tribulus terrestris on non specific immunity in rats. Adult healthy Wistar rats weighing 150 g-200 g were injected intraperitoneally with 0.5mg, 1mg or 2mg per kg body weight of the extract daily for 7 days. Parameters of the study included body weight, organ weight, peritoneal exudate cells (PEC) counts and phagocytic activity of PEC. Results indicate that all doses of aqueous extract of Tribulus terrestris significantly (p<0.001) increased counts of peritoneal macrophages and stimulated phagocytic index of macrophages.
KEYWORDS: Macrophages; Non-Specific Immunity; Peritoneal Cavity; Peritoneal Exudate Cells (PEC); Traditional System of Medicine
| Copy the following to cite this article: Tilwari A, Shukla N. P, Devi P. U. Augmentation of Non Specific Immunity in Rats by Aqueous Extract of Tribulus terrestris used in Traditional Systems of Medicines. Biosci Biotech Res Asia 2006;3(2a). |
| Copy the following to cite this URL: Tilwari A, Shukla N. P, Devi P. U. Augmentation of Non Specific Immunity in Rats by Aqueous Extract of Tribulus terrestris used in Traditional Systems of Medicines. Biosci Biotech Res Asia 2006;3(2a). Available from: https://bit.ly/2CKNHF8 |
Introduction
Macrophages play a critical role in the primary host defense against infection and also play a central role in inflammatory and host defense mechanism1 (Steller et al., 1995). Macrophages are ubiquitous mononuclear phagocytes in mammalian tissues and peritoneal macrophages are representative of other macrophage populations.² They are also readily available in large amounts in rats. Under physiological conditions, the peritoneal macrophages play an important role in the clearance of endotoxin.3 The sequence of functions carried out by macrophages in the course of their Phagocytic process (adherence to tissue, destruction of these agents by ROS production such as superoxide anion), represent the start of other biological activities that comprise the whole spectrum of immune response.4
Tribulus terrestris, commonly known as gokhru or puncture vine (F: Zygophyllaceae), is commonly used as a medicinal plant. It has been reported that Tribulus terrestris possess anti-inflammatory Fruits affects the liver, kidney, and cardiovascular system5 and are effective in urolithiasis, aging, antiseptic, inflammation and non-specific impotence.6 The fruits contain 5% of semidrying oil, peroxidase, traces of glycoside resin, proteins and inorganic matter,7 furastanol glycosides,8 Saponins isolated from the fruits are reported to have antihypertensive activity and inhibitory effect on breast cancer.9
Materials and Methods
Plant Material and Extract Preparation
Fruits of Tribulus terrestris was grounded to powder and extracted with Soxhlet apparatus using double distilled water for 48 hrs The extract was then concentrated on a rotatory evaporator below 400 C.The test extract was not soluble in water. Suspension in 0.1% sodium carboxy methylcellulose was used for administration to animals.
Test Animals
The experiments were performed on either sex of Swiss albino rats weighing 150-200g. The animals were household in standard conditions of temperature, humidity and light. Animals were provided with standard animal feed and tap water ad libitum.
Group Treatment
Group I Control
Received 0.1% carboxyl methyl cellulose (i.p.) for 7 days
Group II
Received 500 µg/kg b.w. of TE extract (i.p.) for 7 days.
Group III
Received 1 mg /kg b.w. of TE, (i.p.) for 7 days.
Group IV
Received and 2mg /kg b.w. of TE, i.p. for 7 days.
The animals were sacrificed by giving anesthesia by petroleum ether 24 hrs after the last dose for the study of various parameters.
Body Weight and Organ Weight
The body weight of the animals was recorded on day 1 (start of the treatment) and day 8th (before sacrifice). The weight of liver, kidney spleen and thymus was also recorded on day 8th after sacrificing the animals. The body weight gain (%) and relative organ weight (organ weight/100g of body weight) were calculated foe each animals.
Collection of Peritoneal Exudate Cells (PECs) and Cellularity Counts
After 24 hrs of last injection, peritoneal macrophage were obtained by a procedure described briefly, 15 ml of normal saline was injected intraperitoneally to the rats, the abdomen was messaged for 5 minutes and the peritoneal exudate cells (PECs), consisting of 60% lymphocytes and 40%macrophages were collected by syringe (5ml), were collected allowing recovery of 90-95 of the injected volume ‘Resting macrophages identified by morphology and neutral red staining, were counted and adjusted in RPMI-1640 with 10% FCS to 5 x 105 macrophage\ml. cellular disability was routinely measured before and after each experiment by the trypan blue exclusion test10. In all cases disability was higher that 95%. All incubations were performed at 370C in a humidified atmosphere of 5% CO2.
Macrophage Activation
The phagocytic activity of PEC was evaluated using the suspension assay method of Fujiki and Yan9 with some modifications as described by Ahmad et al.,11. Briefly, 0.1 ml aliquot of 10 x106 cells/ml density in RPMI-1640 medium was mixed with 0.1 ml medium containing 20 percent FCS and 100 x 10 6 cells /ml of heat treated (at 1000C for 1 hr) yeast (Saccharomyces cereviceae) cells. The mixture was incubated at 370C for 1 hr with occasional shaking. After incubation, 50 µl of this mixture was smeared on the glass slide, air dried and stained with Wright Giemsa stain. The slides were observed under a light microscope (Olympus BX 50, Japan) using oil immersion. At least 500 cells were counted. The phagocytic activity was expressed as phagocytic Index (PI). The PI was calculated using the following formula:
PI = AxB,
where A: the percentage of yeast ingesting phagocytes; and B: the number of yeast cells engulfed per phagocyte.
Statistical Analysis
The statistical analysis of data was carried out using one way ANOVA.
Results
Relative Organ and Body Weight
The aqueous extract of the Tribulus terrestris at doses (0.5mg, 1mg and 2mg/kg b.w.) for 7 days showed no significant changes in relative organ and body weights in experimental animals compared to control group.
Cellularity of Peritoneal Exudate Cells
The results of PEC counts are shown in Table -1. The extract elicited a significant (p<0.001) and dose dependent increase in macrophage counts as compared to control group.
Table 1: Effect of aqueous extract of Tribulus terrestris on the peritoneal exudate cells population in rats
| Treatment group (dose mg/kg) | PEC x 106 | |
| Control | 13.01 ± 1.96 | |
| Aqueous extract of Tribulus terrestris (0.5 mg/kg) | 45.09 ± 3.85* | |
| Aqueous extract of Tribulus terrestris (1 mg/kg) | 147.65 | ± 9.91* |
Datas were expressed as Mean ± SE of 6 animals per group, *p<0.001 compared to control group
Phagocytic Activity
The extract at doses (0.5mg, 1mg and 2 mg/kg) caused stimulation of phagocytes as evidenced by a significant (P<0.001) increase in phagocytic index (Fig. -1).
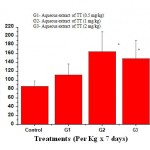 |
Figure 1: Effect of aqueous extract of Tribulus terrestris on Phagocytic Index (PI) of PEC in rat. |
Discussion
The aqueous extract of Tribulus terrestris showed stimulatory effect on macrophage function. The immune system, cells and effector molecules work in a close coordination and macrophages constitute an important cell type involved in the initiation of various immune reactions including neutralization of infectious agents or tumor cells.12-13 Activation of such reaction may help host to effectively neutralize the infection challenge. Peritoneal macrophages, as representative of macrophage population and other Phagocytic cells, play an essential role in the immune response of the host to inflammatory and infectious process such as endotoxic shock. Since the macrophages are involved in the pathogenesis, the study of the toxic effect of the reactive oxygen species produced by these cells is important for understanding the mechanism of toxins induced injury, as well as the possible therapeutics that can reduce this injury. Thus, the above study indicates its role as immunomodulator via macrophage activation.
Acknowledgements
This work was partially supported by grants from M.P. Council of Science and Technology, Bhopal, India.
References
- Steller, H. Science 267: 1445 (1995)
- Fernandez-Botran R, vetvicka V. CRC press: 244 (1995).
- Fujiki, K, Yano T. Fish Shellfish Immunol, 7: 417-27 (1997).
- Pramodkumar et al., J Sci Res Plants Med. 1: 9 (1980).
- Selected medicinal plants of India, CHEMEXCIL, Mumbai.
- Chopra, RN.: Indigenous drug in India. Academic publishers, Culcutta (1982).
- Tmova, M. et al., 1st Chem Biotechnology, Biol.Act. Nat. Prod., 3, 299 (1981), Chem, Abstr. 97: 156678v (1982).
- Jayaram S.; et al., Indian Drugs, 30(10): 498-500 (1993).
- Raisuddin S, Zaidi SIA , Singh KP, Ray PK, Drug chem. Toxicol, 15: 409-17 (1991).
- Mills G, Monticone V, Paetkau V., J Immunol., 117: 1325-30 (1976).
- Rosenstreich DL, Farrar JJ, Doughery S., 116: 131-9 (1976).
- Tagliabue A., Mantovani A, Kilgallen M, Herberman PB, Mc Coy JL, 122: 2363-70 (1979).

This work is licensed under a Creative Commons Attribution 4.0 International License.





