Manuscript accepted on : 01-03-2024
Published online on: 21-03-2024
Plagiarism Check: Yes
Reviewed by: Dr. Dito Anurogo
Second Review by: Dr Durgesh Ranjan Kar
Final Approval by: Dr. Ali Mohamed Elshafei
Veterinary Scientific-Research Institute of the Ministry of Agriculture, Baku, Azerbaijan.
Corresponding Author E-mail: azizova_aygun@inbox.ru
DOI : http://dx.doi.org/10.13005/bbra/3213
ABSTRACT: The primitive blood parasites - piroplasmids are pathogens transmitted to the agricultural animals by the ticks and caused the death and serious economic damage. In Azerbaijan, in the economic regions where the animal husbandry is developed, the systematic investigation and epizootology of these parasites in small ruminants, were researched by us for the first time. For this purpose, the research work was conducted in the Shirvan-Salyan economic region. For this purpose, researches were conducted on the taxonomic study of primitive blood parasites and their transmitters - Ixodidae ticks of small ruminants in the Shirvan-Salyan economic region. Out of 887 sick and suspected diseases sheep, 483 heads (54.5%), and 125 heads (27.4%) of 456 goats had positive results of peripheral blood smear samples attributed to primitive blood parasites. Babesia ovis and Anaplasma ovis parasites were detected in the erythrocytes of sick sheep, and the infection was mostly associated. A.ovis parasite was detected in sero samples of goats. The organs of 56 sheep and 31 goats that died of high fever - spleen, kidney, liver - were examined for primitive blood parasites. The results of the classical examination method in the diagnosis of A.ovis and B.ovis parasites were compared with the effectiveness of the polymerase chain reaction (PCR). 2,875 sheep and goats (1,786 sheep, 1,089 goats) were examined for ectoparasites in livestock farms, 63.8 percent of sheep and 53.6 percent of goats were intensively infected with ticks. Tick infestation was mostly covered in spring, summer and autumn with high intensity. Rhipicephalus bursa ticks were dominant in sheep and Hyalomma plumbeum ticks in goats. In order to determine transovarial and transstadial transmission of disease agents, smears made from internal organs (salivary gland, ovary, intestine) and eggs of ticks were studied for A.ovis and B.ovis parasites.
KEYWORDS: İxodid Ticks; Pathogen; Piroplasmids; Peripheral Blood Sample; Parasite; Transovarial; Transstadial Transmission
Download this article as:| Copy the following to cite this article: Azizova A. The Taxonomıc Research of the Prımıtıve Blood Parasıtes and Transmıttıng Ixodıdae Tıcks of the Small Ruminants in the Shırvan-Salyan Economıc Regıon of Azerbaıjan. Biotech Res Asia 2024;21(1). |
| Copy the following to cite this URL: Azizova A. The Taxonomıc Research of the Prımıtıve Blood Parasıtes and Transmıttıng Ixodıdae Tıcks of the Small Ruminants in the Shırvan-Salyan Economıc Regıon of Azerbaıjan. Biotech Res Asia 2024;21(1). Available from: https://bit.ly/3VuR8ps |
Introduction
The pasture ticks belonging to the Ixodidae family caused the invasion and infectious diseases in small ruminants, transmit many bacteria and viruses, as well as the protozoa to the animals and even humans. The Ixodidae ticks parasitized in the agricultural animals are met in all the continents of the world. The ticks are carriers and transmitters of encephalitis, tularemia, cochiella, rickettsia and piroplasmids 1,2,3(Fournier, 2000; Parola and Raul, 2001; Gunther and Haglund, 2005). It has been confirmed that the ticks are the transmitting conducted the zoonotic babesiosis and e “ et al.,eletanaplasmosis to the humans 4,5,6,7,8,9,10(Strizova et al., 2020; Young, 2019; Ros-Garcia, 2013; Müzeyyen Atas et al, 2016; Vahid Noaman and , 2021; Haluk Kılıc et al, 2010; Johan et al 2015). The world researchers have confirmed that the Hyalomma anatolicum, H.marginatum, H.dromedari, Rhipicephalus sanguineus, Rh.microplus, Rh.appendiculatus, Rh.decolaratus ticks are transmitters of many piroplasmids parasitized in small ruminants and noted that the Babesia ovis parasite transmitted the ticks to small ruminants, cause the disease manifested with severe clinical symptoms 11,12,13(Kivaria, 2006; Rochlin, 2019; Asael, 2016). In different geographical regions of the Republic of Azerbaijan, it was confirmed that in small ruminants, the ticks are the transmitters of the primitive blood parasites manifested in heavy form of the babesiosis and anaplasmosis and resulted with high death 14,15,16(Kasumov et al, 1980; Gadzhiev et al, 1988; Azizova, 2022). In Azerbaijan, there is no information about the recording of these diseases in humans.
The Shirvan-Salyan economic region (Shirvan, Bilasuvar, Hajigabul, Neftchala and Salyan) is one of the conditional economic regions of the Republic of Azerbaijan. The Shirvan, Bilasuvar, Hajigabul, Neftchala and Salyan regions included to the economic region are located on the same relief and have similar climate, vegetation and soil types. The territory of the economic region is mainly used as winter pastures of the animals moved from the mountainous regions. The transfer of the nomadic farms to these areas affects directly the exchange of parasites between the animals, as well as the epizootology of endo- and ectoparasites.
The species belonging to the Babesiidae family of the Piroplasmida suborder of the Haemosporidia (blood-spored) order of the Coccidiomorph class damage seriously the farm by causing a disease called piroplasmidosis in small ruminants. The causative agents of the piroplasmidosis – the primitive blood parasites, piroplasmids multiply firstly in the internal organs, and then in the peripheral blood of the animals. These diseases have seasonal character and the extensiveness of the invasion varies depending on the natural climatic conditions 17,18(Khayat, 2008; Christensen and Schnittger, 2009).
The piroplasmidosis have been studied by researchers from different countries and noted that in small ruminants, the Babesia species spread intensively, damage seriously the farms. In the researches conducted in Turkey, it has been shown that in sheep, the seropositivity rate of the Babesia parasite is variable, and the intensive period of the babesiosis disease is observed mostly in the June-July months 19,20(Aktash et al., 2001; Sevinj and Khuan, 2015). In Iran, Iraq, Egypt, Israel, Spain, India and Pakistan, in addition to the Babesia species, the Anaplasma ovis parasite belonging to the Anaplasma genus of the Anaplasmatacea family of the Rickettsiales order, is also noted the intensively spreading in small ruminants. The researchers have researched the blood samples of sheep and goats by classical and ELISA methods according to the Babesia and Anaplasma parasites and determined that the infection was high 21,22,23,24,25,26,27(Esmaeilnejad, 2014; Renneker, 2013; Rjeibi, 2014; Yeruham, 1995; Ferrer, 1998; Baby, 2001; Adil and Arif, 2022). Researches were conducted in the spring, summer and autumn seasons of 2022 in livestock farms of Neftchala, Bilasuvar, Salyan, Hajigabul, Shirvan districts of Shirvan-Salyan economic region. In order to detect primitive blood parasites in small ruminants of different age groups were clinically examined starting from the first months of disease onset (end of March, first decade of April).
Materials and Methods
Blood samples were taken from sick and suspected febrile animals – 887 sheep and 456 goats, after staining with Romanovski-Giemza dye, they were examined under a microscope, and the species composition of primitive blood parasites was determined 28,29(Giemsa, 1904; Kapustin, 1955).
6670 ticks were collected from goats and 10552 ticks from sheep, the gender and species composition of ticks was determined. In order to determine transovarial (during transovarial transmission of piroplasmidosis agents, parasitic piroplasmids are transmitted to the new generation through the eggs produced by the invasive female individual tick) and transstadial (parasitic piroplasmids in female imagos are not transferred to the ovaries and eggs), internal organs of ticks (salivary gland, ovary, intestine) and smears made from their eggs were studied for Anaplasma ovis and Babesia ovis parasites. The smears were stained with Romanowski-Giemza dye and then subjected to microscopy and the causative pathogens were identified 30(Filippova, 1984).
DNA extraction was performed using the G-spinTM Total DNA Extraction Kit (iNtRON Biotechnology, Korea) according to the manufacturer’s instructions. 246 serological samples (120 sheep, 126 goats) were PCR tested for Anaplasma ovis and Babesia ovis parasites using BioinGentech Veterinary PCR Kits (Concepcion, Chile) according to the manufacturer’s instructions. Cycling conditions were initial denaturation at 94°C for 2 min, 35 cycles (94°C 30 s, 57°C 30 s, 72°C 30 sec) and a final extension at 72°C for 5 min.
Results
In Shirvan-Salyan economic region, the peripheral blood smear samples of 483 (54.5%) from 887 sheep and 125 (27.4%) from 456 goats showed the positive results according to the primitive blood parasites. The Babesia ovis and Anaplasma ovis parasites were detected in the positive blood smear samples of sheep, and the causative agents were observed intensively and in some cases in an associative form in all the research regions. The infection of the erythrocytes by the Babesia ovis parasite was dominant in the associative invasion. The Babesia ovis parasite is 0.5-1.7 μm in oval shape and was followed one by one in the erythrocytes. The Anaplasma ovis parasite was detected in the positive samples belonging to goats. Anaplasma ovis was observed in one copy on the edge of the erythrocytes, sometimes 2 copies, in size of 0.3-1.5 μm (Figure 1).
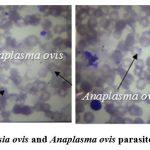 |
Figure 1: The Babesia ovis and Anaplasma ovis parasites in erythrocytes. |
483 (54.5%) of the serological samples belonging to 887 sheep, 125 (27.4%) of 456 goats gave positive results according to the piroplasmids. The organs of 56 sheep and 31 goats dead from the high temperature – spleen, kidney, liver were examined according to the parasites. The primitive blood parasites were followed in the spleens of dead animals. The Babesia ovis parasites were observed in the smear samples prepared from the spleen of 12 sheep, and Anaplasma ovis parasites in the samples belonging to 5 goats. (Table 1).
Table 1: The infection extensiveness of small ruminants with the piroplasmids in the serological and pathological samples.
| Animal | Blood smear samples | Organ (spleen) | ||
| Total number | Positive example (%) | Total number | Positive example (%) | |
| Sheep | 887 | 483 (54.5%) | 56 | 12 (21.4%) |
| Goat | 456 | 125 (27.4%) | 31 | 5 (16.1%) |
0.5-2.1 μm oval-shaped Babesia ovis parasite was observed in a smear sample prepared from the spleen of a dead sheep. Anaplasma ovis parasite with a size of 0.4-1.7 μm was observed in goat samples (Figure 2).
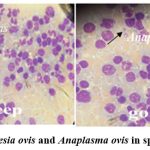 |
Figure 2: Babesia ovis and Anaplasma ovis in spleen sample |
In order to determine the dynamics of the infection on the age and sex groups of the animals, 246 (120 sheep, 126 goats) small ruminants were examined from both Romanovski-Giemza and PCR for comparison in the spring season. 75 out of the researched 120 sheep, were male and 35 were female. 15 out of the serological samples belonging to the male sheep and 10 samples belonging to the females were evaluated as positive. 76 out of the researched 126 goats, were male and 50 were female. 19 out of the serological samples belonging to the male goats and 15 samples belonging to the female goats were evaluated as positive. The analysis of the results showed that, in small ruminants the infection intensity and extensiveness of the male individuals with the piroplasmids is higher. The animals were selected and researched according to age groups up to 1 year, from 1 to 2 years and older than 2 years. The infection was followed in 12 (36.4%) out of 33 sheep under 1 year, 9 (24.3%) out of 37 sheep between 1 and 2 years old, and 4 (10.0%) out of 40 sheep over 2 years old. The infection was followed in 16 (25.8%) out of 62 goats under 1 year, 11 (35.5%) out of 31 between 1 and 2 years of age, 7 (21.2%) out of 33 over 2 years old. Due to the results of the examinations, it should be noted that the infection with the piroplasmids was different on the age groups. In sheep, in lambs up to 1 year old, the infection extensiveness was appreciated highly, between 1-2 years old, relatively low, and in older animals low. It was determined that, the older animals unfollowed any clinical signs are parasite carriers (Anaplasma ovis). And in animals of the younger age group, the piroplasmidosis was noted associatively with the other parasitic diseases and resulted in more with the deaths. And in goats, the main infection was followed in kids aged from 5-6 months to 1 year, the death was mainly in animals of this age group. Unlike sheep, the goats over 1 year of age were noted as parasite carriers, and the clinical signs have not been followed in these animals.
Table 2: The infection dynamics of the animals with the piroplasmidoses according to the age and sex (Romanovski-Giemza).
| Host | Demographic factor | Positive animals percentage | |
| Sheep (n = 120) | Gender | Male (75) | 15 (20,0%) |
| Female (35) | 10 (28,6%) | ||
| Age | Group I (33) | 12 (36,4%) | |
| Group II (37) | 9 (24,3%) | ||
| Group III (40) | 4 (10,0%) | ||
| Goat (n = 126) | Gender | Male (76) | 19 (25,0%) |
| Female (50) | 15 (30,0%) | ||
| Age | Group I (62) | 16 (25,8%) | |
| Group II (31) | 11 (35,5%) | ||
| Group III (33) | 7 (21,2%) | ||
The results of the classical examination method in the diagnosis of Anaplasma ovis and Babesia ovis parasites were compared with the effectiveness of the polymerase chain reaction (PCR). For this purpose, serological samples of 246 small ruminants (120 sheep, 126 goats) were subjected to PCR examination for Anaplasma ovis and Babesia ovis parasites. Positive results with Anaplasma ovis were 14.2% (35 heads), of which 20 (57.1%) were sheep and 15 (42.9%) were goats. Babesia ovis showed a negative result in PCR tests in goats. 25 out of 120 sheep serological samples (20.8%) were positive for Babesia ovis parasite. In sheep, the infection rate was higher among males (45,0%), animals of the age group I (55,0%). While in goats, the prevalence was 60,0% among males, 53,3% among age group I animals 31(Azizova, 2023).
The serious pathological changes were observed in the liver, lungs, spleen, heart and kidneys, during the autopsy examinations in the dead lambs and kids. The adipose tissue and mucous membranes are yellowish, the intestines are dystrophied, the hyperemia signs are noted in the lungs (Figure 3).
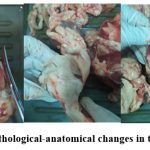 |
Figure 3: The pathological-anatomical changes in the animal carcass |
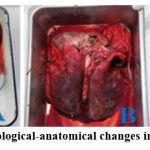 |
Figure 4. The pathological-anatomical changes in the perished lamb |
A – The hepatomegaly with necrosis in the liver, partially enlarged kidney, dark-colored bile accumulated in the enlarged gallbladder, discolored spleen; B – The hyperemia signs in the lungs; C – The enlarged heart muscle, the bleedings in the epicardial and endocardial surfaces
The infection dynamics of the animals by the Babesia ovis and Anaplasma ovis parasites were researched in the research regions, by months starting from the spring season. In sheep, babesiosis began to followed one by one from the end of March, and the infection cases with the disease intensified in April. In June-July, the infection reached a high level. In sheep, the infection cases were not followed in August, and the infection cases were noted one by one from the first decade of September to November. In goats, Anaplasmosis began to noted from the end of April, and the infection has been intensified relatively in May. A high level of the infection was noted from the end of June to the end of July. Starting from August, goats were infected by the ticks, but disease cases were not followed (Figure 5).
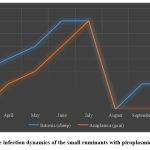 |
Figure 5: The infection dynamics of the small ruminants with piroplasmids for months |
2,875 sheep and goats (1,786 sheep, 1,089 goats) were examined for ectoparasites in livestock farms belonging to the Shirvan-Salyan economic region. During the examinations, 63.8% of sheep (1139 sheep) and 53.6% of goats (584 goats) were infected with ticks. The infection mainly covered spring, summer, partly autumn. Larval, imago, and larval stages of carrier ticks Hyalomma plumbeum, H. anatolicum, Rhipicephalus bursa and Boophilus calcaratus transmitted pathogenic piroplasmids to small animals.
Out of 6670 individual ticks collected from goats, 27.4% were Hyalomma plumbeum, 24.5% were H.anatolicum, 22.2% were Rhipicephalus bursa, 18.6% were Rh.turanicus, 7.3% were Boophilus calcaratus ticks. Out of 10,552 head ticks collected from sheep, 26.5% were Rhipicephalus bursa, 26.3% Hyalomma plumbeum, 23.6% Rh.turanicus and 23.6% H.anatolicum ticks. In the experiments, 3.5-4 kg weight loss was observed in tick-infected sheep over 2 years old, and 3 kg weight loss in goats. (Figure 6, Figure 7).
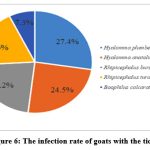 |
Figure 6: The infection rate of goats with the ticks |
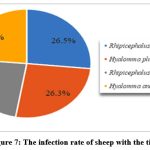 |
Figure 7: The infection rate of sheep with the ticks |
The intensive infection was by Hyalomma plumbeum ticks in goats and Rhipicephalus bursa ticks in sheep. In the experiments, the air temperature of 23-250C, humidity of 45-75% were noted as the active period of the ticks belonging to the genera of Hyalomma and Rhipicephalus. This period covered the period from April to September. In these months, the infection extensiveness was also high with the blood-parasitic diseases in the animals. In the epizootology of the ticks, macro-climatic factors such as temperature, humidity, precipitation, vegetation, and micro-climatic factors such as climate and altitude play a special role. The season and the parasite-host relationship also play an important role in the epizootology of the ectoparasites. In recent years, the climate change – the increasing of the air temperature (20-230C) from April in the research regions causes the activation of the ticks from the early spring in the pastures. And in summer, the intermittent rains increase the humidity and creating the favorable conditions for the intensive spread of the ticks.
The transovarial and transtadial transmission of the parasite protozoa was determined in the smears prepared from the organs (salivary gland, ovary, intestine) and eggs of the ticks. A.ovis – parasite was detected in the salivary gland of the Hyalomma plumbeum tick; A.ovis – in the intestine of the Hyalomma anatolicum tick; B.ovis, A.ovis – in the eggs of the Rhipicephalus bursa tick; B.ovis, A.ovis – in the salivary gland of the Rhipicephalus turanicus tick. In the smears belonging to the Boophilus calcaratus tick, the parasites caused the invasion diseases in the small ruminants were not detected (Table 3).
Table 3: The pathogen causatives in the organs of the ticks
| Tick species | Babesia ovis | Anaplasma ovis |
| Hyalomma plumbeum
(salivary gland) |
– |
+ |
| Hyalomma anatolicum
(intestine) |
– | + |
| Rhipicephalus bursa
(egg) |
+ | + |
| Rhipicephalus turanicus
(salivary gland) |
+ | + |
| Boophilus calcaratus
(ovary) |
– | – |
The Romanowski-Giemza staining of the serological samples is recommended as a more efficient method for the detecting the blood-parasitic diseases in large livestock farms. Because, while sensitivity to one causative agent is checked in molecular examinations, it is possible to detect the other causative agents at the same time in microscopic examinations. So 35 out of 246 PCR test samples gave a positive result (14.2%). And in microscopic examination, in 25 more samples, Babesia ovis, Theileria ovis, Th.recondita parasites were detected (though the Theileria species are detected in smear samples in Azerbaijani conditions, they do not cause the clinical disease). The results confirmed that 25 head animals were infected with the other piroplasmids. This shows that although the PCR tests are useful for identifying a parasite, they cannot determine the causative agents of the disease in the same way that microscopic examinations do. Our experiments confirmed that it is possible to detect the A.ovis and B.ovis parasites as well as the other piroplasmids and pathogens with this method. Also, the microscopic examinations are more efficient materially for the large farmer farms and more practical for the detecting several causative agents at the same time. Our experiments confirmed that the A.ovis parasite, which is characteristic for lowland landscapes, has spread intensively among sheep and goats in the mountainous and foothill regions of the republic in the recent years. And this informs an increase of the infection risk of anaplasma and babesia species with the zoonotic potential to humans.
Discussion
In the Shirvan-Salyan economic region, sheep bred in the sedentary livestock farms, are infected with 2 species (B.ovis, A.ovis) of the primitive blood parasites and goats with 1 species (A.ovis). 4 species of the Ixodidae ticks transmitted the disease causative agents are distributed intensively in sheep and 5 species in goats. In sheep, the primitive blood parasites – B.ovis and A.ovis cause the disease developed in an associative form. In small ruminants, the intensive infection with the primitive blood parasites causes the death in young animals that, it is related not only with the immune system of the animals, but also with their associative infection to other parasites. In sick sheep with the piroplasmids, from 6 months to 1 year old, at the same time, the infection with the dicroceliosis and respiratory tracts strongyliatosis in the associative form was noted. In the delayed form, the treatment was ineffective, the death was followed in animals. In goats of that age group, the infection was detected with the moniesiosis in an associated form with the anaplasmosis that it caused the death of kids. No deaths were followed in animals aged 1-2 years, the innovative treatment resulted with the recovery of the animals. And in animals over 2 years of age, the clinical signs were manifested weakly, no death was followed, the lossing of the weight was noted in animals infected intensively with the ticks. The productivity decreased by 3.5-4 kg in sheep infected with the ticks, during 6 months, and in goats by 3 kg. The animal skin was unsuitable for the leather production.
Similar studies were conducted in Nagorno-Shirvan, another economic region of the Republic of Azerbaijan. Babesia ovis, Anaplasma ovis, Theileria ovis, Th.recondita parasites in sheep, Anaplasma ovis parasites in goats were found in the serological samples of small ruminants. Transmissive ticks that transmit piroplasmids to small ruminants in this economic region were species belonging to the genera Ixodes, Boophilus, Rhipicephalus, Hyalomma and Haemaphysalis 32(Azizova, 2022).
The results obtained from the researches show that in the ecological zones located in different landscapes and with different climatic conditions, the infection of small ruminants with the different species of the piroplasmids and ticks is noted.
In the Iranian territories close to the Shirvan-Salyan economic region of Azerbaijan, it is noted that piroplasmids – Babesia ovis, B. motasi and B.crassa parasites cause disease in small ruminants. It is shown that Rh.bursa, Rh.sanguineus and Rh.turanicus ticks are potential carriers and conductors of parasites. Although these ticks are intensively observed in small ruminants in Azerbaijan, B.motasi and B.crassa parasites have not been observed 33(Gholamreza, 2022).
Conclusion
In Azerbaijan, the vaccine against the anaplasmosis and babesiosis diseases of small ruminants is not applied. Therefore, the treatment scheme against the parasites is used when the diseases are detected. Imidocarb dipropionate and Diminazine aceturate are mainly used in the etiotropic treatment of the anaplasmosis, and the parasitropic preparations which contains the Diminazine aceturate, are used in the treatment of the babesiosis. The prophylaxis is preferred as the main measure in disease prevention. For this purpose, starting from the last 10 days of March, the bathing measures are carried out for to free small ruminants from the disease-carrying ticks. As a result of scientific studies, it is recommended that preventive measures are more important for veterinarians and farmers to protect animals from the risks of infection with piroplasmidoses. Bathing measures prevent serious economic damage to farmers by protecting animals from harm
Acknowledgments
The author would like to thank the management of the Veterinary Research Institute and the Faculty of Medicine of the Istanbul Cultural University of the Republic of Turkey, Professor Dr. Ugur Uslu, for their assistance.
Conflict of Interest
There are no conflict of interest.
Funding Sources
There is no funding source.
References
- Fournier, P. Evidence of the Rickettsia helvetica infection in humans of the eastern France / P.Fournier, F.Grunnenberger, B.Zholhac [et al.] // – 2000. V.6, №4, 389-392.
CrossRef - Parola, P., Raul, D. The ticks and tick-borne bacterial diseases of humans: a new infectious threat // – 2001, 32, – 897-928.
CrossRef - Gunther, G., Haglund, M. Tick-borne encephalopathies: epidemiology, diagnosis, treatment and prevention [review]. CNS drugs // – 2005, 19, Issue 12, – p. 1009-1032.
CrossRef - Strizova, Z., Havlova, K., Patek, O., Smrz, D., Bartunkova, J. The first human case of babesiosis mimicking Reiter′s syndrome. Folia Parasitol. 2020, vol.67: 031.
CrossRef - Young, K. Zoonotic Babesia: A scoping review of the global evidence / K.Young, T.Corrin, B.Wilhelm [et al.] // PLoS One, – 2019, 14(12), e0226781.
CrossRef - Ros-Garcia A., Barandika J.F., Garcia-Perez A.L., Juste R.A., Hurtado A. Assessment of exposure to piroplasms in sheep grazing in communal mountain pastures by using a multiplex DNA bead-based suspension array. Parasit Vectors. 2013;6(1):277.
CrossRef - Müzeyyen Atas, Nazir Dumanlı, Kürşat Altay. Şanlıurfa yöresinde koyun ve keçilerde Anaplasma phagocytophilum’un moleküler yöntemlerle araştırılması. Manas J Agr Vet Life Sci, 2016, 6 (2), 1-8
- Vahid Noaman, Alireza Sazmand. Anaplasma ovis infection in sheep from Iran: molecular prevalence, associated risk factors, and spatial clustering. December 2021 Tropical Animal Health and Production 54(1):6. DOI:1007/s11250-021-03007-4
CrossRef - Haluk Kılıç, Şaban Gürcan, Hakan Kunduracılar, Muzaffer Eskiocak . Kene ısırığı öyküsü olan kişilerde anaplazmoz seropozitifliği. Trakya Üniversitesi Tıp Fakültesi Dergisi. 2010, 27(1), 79-82
- Johan S., Bakken , Stephen Dumler. Human Granulocytic Anaplasmosis. Infect Dis Clin North Am. 2015 Jun; 29(2): 341–355. doi: 10.1016/j.idc.2015.02.007
CrossRef - Kivaria, F. Estimated direct economic costs associated with tick-borne diseases on cattle in Tanzania // Trop Anim Health Prod., –2006, 38, – p. 291-312
CrossRef - Rochlin, I. Modeling the Asian Longhorned tick (Acari: Ixodidae) suitable habitat in North America // Med Entomol., – 2019, – 56, – p. 384-391.
CrossRef - Asael, R. Transmission of Babesia ovisby different Rhipicephalus bursa developmental stages and infected blood injection. Ticksand Tick-borne Diseases / Asael, W.Ricardo, L.Benjamin [et al.] // – 2016. Volume 7, Issue 1, – p. 13-19.
CrossRef - Kasumov, N.A. Comparative study of the course of anaplasmosis in fine-wool and coarse-wool sheep and the development of control measures in the Azerbaijan SSR// Abstract, Stavropol, 1980.
- Gadzhiev, A.T., Mustafaeva Z.A., Dubovchenko T.A. Ectoparasites and bloodsuckers of vertebrate animals of Azerbaijan // (Catalog). Baku: Elm, 1988, 234 p.
- Azizova, A.A. The associative invasions – the helminths, primary intestinal parasites, piroplasmids of the small ruminants in Azerbaijan // 7th International New York Conference on Evolving Trends in Interdisciplinary Research & Practices, Manhattan, New York city, – 1-3 October, – 2022, – 97-103.
- Khayat, A. “Ch. 36: Babesia Species”. The Neurological Manifestations of Pediatric Infectious Diseases and Immunodeficiency Syndromes / A.Khayat, Leslie, R.Neil [et al.] // – 2008. – p. 343-346.
CrossRef - Christensen, M., Schnittger, L. Piroplasmids and ticks: a long-lasting intimate relationship // Frontiers in Bioscience, – January, – 2009, 14, – p. 3064-3073.
CrossRef - Aktash, M., Duzgun, A., Babur, J. The seroprevalence of Babesia ovis in sheep in the Malatya region // Turk J Vet Anim Sci, – 25, – p. 241-243.
- Sevinj, F., Khuan, Kh. Major tick-borne parasitic diseases of animals: A frame of references in Turkey // Eurasian J Vet Sci, – 31 (3), – p. 132-142.
CrossRef - Esmaeilnejad, B. PCR-based detection of Babesia ovis in Rhipicephalus bursa and small ruminants / B.Esmaeilnejad, M.Tavassoli, S.Asri-Rezaei [et al.] // – Journal of Parasitology Research: Issue 2014 (2014/12).88-93
CrossRef - Renneker, S. Coinfection of sheep with Anaplasma, Theileria and Babesia species in the Kurdistan Region, Iraq / S.Renneker, J.Abdo, M.Bakheit [et al.] // – – p. 113-118.
CrossRef - Rjeibi, M. Prevalence of piroplasms in small ruminants in North-West Tunisia and the first genetic characterisation of Babesia ovis in Africa / Rjeibi, M.Gharbi, M.Mhadhbi [et al.] // Parasite, volume 21, 2014, article number 23, number of pages 9.
CrossRef - Yeruham, I. A study of an enzootic focus of sheep babesiosis (Babesia ovis, Babes, 1892) / I.Yeruham, A.Hadani, F.Galker [et al.] // Vet Parasitol., – 60, – p. 349-354.
CrossRef - Ferrer, D., Castella, J., Gutierrez, J. Seroprevalence of Babesia ovis in sheep in Catalonia, northeastern Spain // Vet Parasitol., – 79, – p. 275-281.
CrossRef - Baby, P. A subacute case of concurrent babesiosis and anaplasmosis in a she-goat / Baby, P.David, P.Ravindran [et al.] // Indian Vet J., – 2001. 78 (5), – p. 424-425.
- Adil, Kh., Arif, A. Tick-borne haemoparasitic diseases in small ruminants in Pakistan: Current knowledge and future perspectives // Saudi J. Biol. Sci., – April, –2022, 29(4), – 2014-2025.
CrossRef - Giemsa, G.A. simplification and perfection of methylene blue-eosin staining method to achieve Romanowsky-Noch chromatin staining // Central. bl. f. Bakt. etc. magazine, – – Vol. 37, – p. 308-311
- Kapustin, V.F. Atlas of the blood parasites of the animals and ixodid ticks, Ed. 2nd, add. and processed / V.F.Kapustin. Selkhozgiz, – – 213 p.
- Filippova, N.A. The taxonomic composition of the ticks of the Ixodidae (Acarina, parazitiformis) family in the fauna of the USSR and the perspectives of its study // Parasitol. collection., – V. 32, – p. 61-78.
- Azizova, A.A. The molecular detection of the Anaplasma ovis pathogens of the serological samples in small ruminants and ixodid ticks in Azerbaijan // Bulletin of Science and Practice Scientific Journal. 2023, Volume 9, İssue 12, p.175-185
CrossRef - Azizova, A.A. Parasitic fauna of small horned animals in Shirvan-Salyan economic regions of Azerbaijan // Scientific work International scientific journal. Materials of the IX International Scientific Research Conference, – 4 June, – 2022, – 57-61.
- Gholamreza, Razmi. A review onBabesia and tick vectors in animals in Iran. Iranian Journal of Veterinary Science and Technology. 2022; Vol.14, İssue 2, Serial number 27, p.1-10.

This work is licensed under a Creative Commons Attribution 4.0 International License.





