Manuscript accepted on : 23-11-2023
Published online on: 18-01-2024
Plagiarism Check: Yes
Reviewed by: Dr. Hiren B. Soni
Second Review by: Dr. Rajendra Prasad Mondal
Final Approval by: Dr. Fernando José Cebola Lidon
P. Kamalakkannan1 , Mohd Younis1,2*
, Mohd Younis1,2* , Sevgi Gezici3
, Sevgi Gezici3 , Som Kailash4
, Som Kailash4 and Javaid Iqbal4
and Javaid Iqbal4
1Department of Human Genetics and Molecular Biology, Bharathair University, Coimbatore, Tamil Nadu, India.
2Department of Zoology and Institute of Human Genetics, University of Jammu, Jammu, Jammu and Kashmir, India.
3Department of Medical Biology, Faculty of Medicine, Gaziantep University, Gaziantep, Turkey.
4Department of Zoology and Applied Aquaculture, Barkatullah University, Bhopal, India.
Corresponding Author E-mail: younisgenetic@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/3215
ABSTRACT: One of India's oldest and fastest-growing sectors is tannery production. The tanneries produce various types of pollutants in the environment depending upon the procedure that has been used. The present study investigated the physicochemical parameters of tannery effluents and its impact on the aquatic environment. Tannery effluent contains a variety of hazardous compounds, including chromium, calcium, sodium, potassium, chloride, sulphate, electrical conductivity, colour, odour, pH, temperature, TSS and TDS. All physicochemical parameters was found higher [chromium 1.17-1.52 (1.327±0.132), calcium 800 (713.83±50.33), sodium 1805 (1634.83±75.06), potassium 38-112 (78.44±9.05), chloride 2330-4210 (3334.22±241.92), sulphate 830-1008 (952.17±15.06), EC 1148-2905 (2378.61±124.27), temperature 29.3-38.0 (31.21±1.45), TSS 710-1623, (1199.39±137.99), TDS 7049-8500 (7669.17±141.99), BOD 1060–1664 (1347.17±73.68), COD 3025-4982 (4029.83±163.56), TH 2200-3417 (2794.50±136.49) and only pH levels were lower 4.4-8.8 (7.01±0.491) in tannery effluent]. The high levels of heavy metals were analyzed that might become a major source of pollution which affect the aquatic environment. The management of tannery effluent's physicochemical parameters and its impact on the aquatic environment involves a combination of regulatory, technological, and educational approaches. It requires a multi-faceted effort to mitigate environmental harm while supporting the tanning industry's sustainable development.
KEYWORDS: Aquatic Environment; Heavy metals; Physicochemical parameters; Tannery effluents
Download this article as:| Copy the following to cite this article: Kamalakkannan P, Younis M, Gezici S, Kailash S, Iqbal J. Characterization and Impact of Physicochemical Parameters of Tannery Effluent on the Aquatic Environment. Biotech Res Asia 2024;21(1). |
| Copy the following to cite this URL: Kamalakkannan P, Younis M, Gezici S, Kailash S, Iqbal J. Characterization and Impact of Physicochemical Parameters of Tannery Effluent on the Aquatic Environment. Biotech Res Asia 2024;21(1). Available from: https://bit.ly/3vM7DT8 |
Introduction
The tannery industry has shown remarkable extension and increasing pollutants to the aquatic environment during the last three decades. Total hardness (TH), biological oxygen demand (BOD), chemical oxygen demand (COD), total dissolved solids (TDS), total suspended solids (TSS), calcium, sodium, potassium, chloride, sulphate, and chromium are some of the component present in tannery effluent. The majority of underdeveloped nations do not treat the tannery effluents before they are dumped into the water supply. 1,2 The survival of aquatic species that breathe through their gills will be harmed by high BOD levels, while high COD levels indicate that the wastewater is hazardous and contains organic material that is resistant to biological processes. Eutrophication may result from the high levels of electrical conductivity (EC) and nitrogen, which are hazardous to aquatic species3,4.
One of the main new environmental issues in the production and disposal of chromium-contaminated sludge is the tanning sector. Nearly three thousand tonnes of chromium are emitted into the environment each year from tanneries in India, with a level of 3000-5000 mg/L in the aqueous effluent5. While the toxicity of a substance to small animals, fish, or other wildlife can be assessed by simply subjecting a small number of them to varying doses of contaminants in a laboratory setting. The most popular method is to expose animals that can be produced for commercial purposes and made widely available to pollutants that are of concern to humans. The indestructibility of heavy metals during bioremediation, unlike organic contaminants, and their negative consequences as pollutants are two of the most significant factors.
As industrialization grows, untreated garbage is dumped into aquatic environments, contaminating natural water with different metals and cause harmful impacts6. Since heavy metals are not biodegradable, understanding how they are absorbed, distributed, and remain in biological tissues is essential7. The most popular tanning agent is chromium. Because chromium is harmful, it must be determined in environmental sample because it is used to tan about 90% of all leather manufactured. Even though it is thought to be necessary for mammals to maintain metabolism, its biological, geochemical, and toxicological properties differ greatly from one another8,9. The tanning process creates an extremely offensive-smelling waste that also contains a significant amount of salts, including chromium, sulphur dioxide, ammonia chlorides, and other salts. The wastewater has been dumped to open land or into waterways, heavily contaminating them with hazardous compounds and suspended and dissolved pollutants.
The aquatic organisms may take up pollutants from sediments, water, suspended particle and food items. In addition to fish infections, other factors that contribute to fish mortality include deteriorating water quality, industrial effluent, metallic contaminants, and pesticide residues. Fishery experts are of the opinion that the continuous discharge of persistent industrial pollutants is likely to cause serious damage to fishes in view of their toxic effect on respiration, growth, reproduction, spawning behaviour, egg mortality and fish survival10,11. Channa punctatus, a freshwater fish, was chosen for the inquiry primarily because it is a crucial biological indicator of freshwater quality and is impacted by environmental deterioration on a global scale12,13.
The significance of present research lies in addressing these gaps and contributing to a more comprehensive and up-to-date understanding of the issue. Researchers can provide a more accurate assessment of the environmental and public health risks associated with tannery effluents, as well as the efficacy of treatment technologies and the impact of regulatory measures. This knowledge can inform policy decisions, support sustainable practices in the tanning industry, and protect both aquatic ecosystems and human communities from the harmful effects of tannery effluents.
Material and Methods
Study Area: In the present study, the selected area was Sempatu in Tiruchirappalli district, which is situated between 10o10’ and 11o 20’ North and 78o 10’and 79o 0′ East in the centre part of the Tamil Nadu. There are about fifteen tanneries situated in this area among them the NM tannery is selected for the present research work. Untreated sewage and effluent from the tannery business contaminate the water
Collection of Samples: Samples were taken straight from the collection tank in a plastic container, transported to the lab with due care, and held at 20oC for additional examination in order to determine the contamination status of tannery effluent water. A total of 18 months were spent collecting the water samples see in Figure 1.
Physicochemical Parameters: The samples’ physicochemical characteristics, including things like colour, odour, water temperature, pH, EC, TSS, TDS, BOD, COD, TH, calcium, sodium, potassium, sulphate, chloride and chromium. Colour: The colour of the site’s effluent water samples was visually assessed and noted. Odour: The odour of effluent water samples from the site was detected by smelling directly from water cans. Water temperature: Using a field thermometer, the temperature was determined at the time of sample collection. pH: The pH meter (Model Eltek Mx 61-A) was calibrated using different standard (pH 4.0, 7.0 and 9.2) solutions, thereafter the pH of samples were recorded. EC: It was measured using an EC metre from Elico, India; EC is a numerical indication of a sample’s capacity to conduct an electric current in soil and water.
TSS and TDS: The TSS and TDS were calculated by using standard procedure.
TSS or TDS = [(A-B) X 1000] / Sample volume (mL)
Whereas: A=Weight of filter and residue (g), B=Weight of crucible (g) and 1000 is constant.
BOD: The relative oxygen requirement of wastewater is assessed using standardised laboratory procedures in the BOD test. In this study, we adopted Winkler’s method14. COD: The COD is a measurement of the amount of organic material in the sample that is capable of being oxidised by a potent chemical oxidant14. TH: Using American Public Health Association Colour Scale techniques, the total hardness was determined and monitored14. Calcium, sodium, potassium, and sulphate: Using a flame photometer (Model Chemito-1000) and the Jackson method, the calcium, sodium, potassium, and sulphate of the samples were determined15. Chloride and chromium: The chloride and chromium contents of the effluent were estimated by argentometric method14,16,17.
Results
Physico-chemical Parameters: Table 1 shows the information that was gathered regarding the physicochemical quality of the tannery effluent water samples. The visually observed colour of the effluent water samples from the site are shown in Table 1. The odour of tannery effluent is found to possess sewage smell throughout the period of study (Table 1). The pH of aqueous solution represents the concentration of H+ ions. During the study period, the effluent’s pH fluctuated from 4.4-8.8, mean value 7.01±0.491 (Table 1 and Figure 1). The pH range was found to be acidic to basic, not good for the aquatic life. The water temperature of tannery effluent was found to vary from 29.3-38.0oC, mean value 31.21 ± 1.45 (Table 1 and Figure 1). The minimum and highest recorded temperatures occurred in January and April, respectively. The EC of effluent water samples were found to vary from 1148-2905 micro/ mho/cm, mean value 2378.61±124.27 (Table 1 and Figure 1) during the study period. The values increased between August 2007 and June 2008, while the TSS in samples of tannery effluent water throughout the study period ranged from 710-1623 mg/L, mean value 1199.39±137.99 (Table 1 and Figure 1).
 |
Figure 1: show PH, Temp and EC values of tannery effluent. |
The TDS was high in all months, ranged from 7049-8500 mg/L, mean value 7669.17±141.99 (Table 1, Figure 2). The BOD value of samples ranged started 1060-1664 mg/L, mean value 1347.17±73.68 (Table 1 and Figure 2). High BOD values were recorded during the study period, which indicates the quality of tannery water was unfit for the use of people. The water samples’ COD values ranged from 3025-4982 mg/L, mean value 4029.83±163.56 (Table 1 and Figure 2). High COD values were recorded during the study period that indicates the bad quality of tannery water for any humans use. The TH of water samples were found to be ranging from 2200-3417 mg/L, mean value 2794.50±136.49 during entire period of research (Table 1 and Figure 2). Because of how hard it is, no home use is possible with the water. The total hardness of the samples was discovered to be extremely high during the post-monsoon period. Among the chemical properties especially the dissolved substances such as calcium and sodium was encountered in the water samples of the tannery effluent (Table 1). The calcium was found to be high from March to June 2008 (<800 mg/L) while it was (>800 mg/L), mean value 713.83±50.33 during the rest of the research time (Table 1 and Figure 2). Sodium was bring into being to be high (1200-1805 mg/L), mean vale 1634.83±75.06 in the month of June 2008 (Table 1 and Figure 2).
Among the chemical properties, especially the dissolved substances like potassium and chlorides were encountered in the water samples of the tannery effluent (Table 1). The potassium (Table 1 and Figure 2) was also high during May 2008 (112 mg/L) and low in the month March of 2007 (38 mg/L), mean value 78.44±9.05. The chloride range (Table 1 and Figure 2) was from 2330-4210 mg/L, mean value 3334.22±241.92 during the time of research, being high in the month of June 2008. Among the chemical properties especially the dissolved substances like sulphate and chromium was encountered in the water samples of the tannery effluent (Table 1). The sulphate content (Table 1 and Figure 2) varied from 830-1008 mg/L, mean value 952.17±15.06 respectively. The sulphate content was found to be high in January (1005 mg/L) and June (1008 mg/L) months respectively. The analysis of various metal content showed that the range of chromium was started 1.17-1.52 mg/L, mean value 1.327±0.132 (Table 1, Figure 2). The most significant behavioural pathology of Channa punctatus included restlessness, erratic darting movements occasionally, movement displays towards the aquarium’s bottom, and gradual loss of buoyancy that resulted in fish floating upside down and horizontally with their open mouths and gill opercula (Figure 2). It is found that the symptoms and mortality rate was increased with the increase in the dose and the pathological incidence was directly related to dose and duration of exposure. It indicate the loss of equilibrium due to Irregular and erratic movements that might be due to the damage in the brain associated with the maintenance of equilibrium. The symptoms were depending upon the nature of the toxicant and the dose dependent manner.
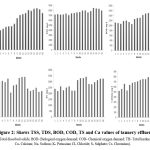 |
Figure 2: Shows TSS, TDS, BOD, COD, TS and Ca values of tannery effluent. |
 |
Table 1: Physico-chemical parameters of Tannery effluent collected from Sembattu area, Trichy, Tamilnadu. |
 |
Figure 3: Shows Na, K, CI, S and Cr values of the tannery effluent and Effect on Chenna punctatus |
Discussion
One of the most terrible ecological crises to which we are currently vulnerable is environmental pollution. India, which ranks seventh among the world’s industrialised developing nations, has a robust industrial infrastructure in a number of sectors, including chemicals, power, nuclear energy, petroleum, plastics, food, and pesticides18. The issue of environmental degradation is not limited to industrialised nations; it is now spreading to India19. The toxicants and other unfavourable chemicals eventually enter water bodies with rainwater and degrade the water media, creating an imbalance in the environment that causes ecosystems, particularly aquatic ones, to slowly deteriorate and result in unabated aquatic fauna mortality20. The results of the current research demonstrate that the tannery effluent had a brownish and blackish appearance and an unpleasant scent. A similar study found that the tannery wastewater leaves behind a dark colour and pungent smell21. In a similar vein, Jamal22 findings showed that a significant proportion of contaminants might transfer colour and odour to the receiving water. The wastewater was described as brownish and odourless in another study23.
Aquatic toxicity testing is now an essential step in the process of determining environmental danger and the evaluation of toxic compounds since the aquatic environment serves as the final sink for all contaminants. International organisations, such as the Environmental Protection Agency19, have advised understanding a variety of bioassays to evaluate the ecotoxic hazards to non-target creatures and their surroundings. Pollution’s toxic effects result from disruption of an organism’s normal morphological and physiological processes. Pathogen, pesticide, and heavy metal stress all cause changes in the biochemical components of tissues, and these changes follow a certain pattern. An organism’s metabolic activity shows how biochemical energy is used to combat toxic stress24. The use of fish as bio indicators for detecting pollution in the aquatic environment is common because fish are known to bio collect, concentrate, and biomagnify toxicants in their tissue25.
The tannery effluent’s pH was found to be lower range. The water temperature level was found to be higher range. Similarly, Rabah and Ibrahim26 reported a similar average pH of tannery effluent. This means in such level of pH the counts of microorganism high because most of them blooms well26. According to a study, Varadarajan and Sneha 27 The reported pH of the tannery effluent was 7.5. Therefore, The tannery effluent has a pH that is just barely alkaline. Fish do not thrive in the aquatic environment when tannery effluent with an alkaline pH is dumped into lakes, ponds, rivers, and other natural sources. The presence of carbonates, bicarbonates, hydroxides, borates, silicates, and phosphates in tannery effluent may be the cause of the substance’s alkaline nature28, 29.
In this investigation, the level of EC in the effluent was found higher range, whereas the acceptable limit is 1500, this shows that the tannery effluent had a larger discharge of chemicals in the form of cations and anions. This might be caused by the tannery effluent’s high levels of acid-base and salt22. The tannery effluent’s chelating capabilities are altered by the higher EC, which also affects how readily available free metals are to aquatic species30.
One important metric used to assess the effects of tannery effluent on water contamination is the estimation of BOD. Due to the significant amount of organic matter present, the effluent in the current study had found high levels of BOD. According to Noorjahan21 a high point of BOD was experimental in the tannery effluent. In another similar study high level of BOD was reported31.
To estimate organic matter the best method is COD test and total oxygen demand, determination by rapid test in the existence of organic matter. In current research, the level of COD was recorded higher range. The study of Noorjahan21 reported higher range of COD from the tannery effluent. The results of present study support the previous study32. Due to the high concentration of organic molecules in the effluent, which may not be affected by bacterial breakdown, the quantity of COD may have increased33.
In the current study, the level of TDS was recorded for tannery effluent higher range value. Similarly, Noorjahan21 reported the higher range of TDS value. Similar findings from a different study with high levels of physicochemical factors, including TDS, COD, TSS, chloride, sodium, nitrate, pH, the high colour intensity in tannery effluent34. TDS are mainly due to carbonates, bicarbonates, phosphates, sulphates, nitrogen, calcium, chlorides, iron and potassium35. The high levels of TSS and TDS may be caused by the presence of insoluble organic and insoluble inorganic materials in the tannery effluent36.
The level of TSS in the tannery effluent was measured in the current investigation found higher range, which exceeded the permissible levels less than two hundred. The tannery effluent becomes murky due to these TSS contaminants. The quality of the hides and skins treated in the tannery largely determines the composition of TSS found in tannery effluent29. When compared to the allowed limit of two hundred for effluent discharge, the TSS level in the effluent was found to be greater. High TSS concentrations harm aquatic species, limit the variety of life in the aquatic system, and hasten oxygen depletion37. A report claims that charges on the surface of small particles in the tannery effluent force the bigger TSS particles to remain suspended38.
The studies shows TH of tannery effluent level was found high range was observed, which exceeded the permissible limit of six hundred. In the investigation, the level of calcium was found higher in the effluent, although the permissible limit is 75-200. The level of sodium was recorded higher range in the effluent, however the acceptable limit two hundred. In the present research, the higher range of potassium was found in the effluent, whereas the permissible limit is less the 8.3. This is lower than the previous study23. In the previous research, it was discovered that the soil watered with tannery water had significant levels of exchangeable calcium, sodium, and potassium23.
In the current study, the level of chloride was observed higher range in tannery effluent, whereas the permissible limit is two hundred. Chlorides are added to tannery effluent, which slows the growth of aquatic organisms and, at high concentrations, can cause cell structure to break down. Rain-induced soil chloride loss causes it to re-enter the ecosystem and could end up in groundwater39.
The level of sulphate was found higher range in the effluent samples, whereas the permissible limit is two hundred. The level of sulphates were exceeded the limit. Sulphate is a component of tannery effluent, according to the earlier study, and it results from the use of sulphuric acid or goods with a high sulphate level39. Similar to this, Vijayanand40 reported that the association of organic matter, calcium, magnesium, carbonates, bicarbonates, sulphates, chlorides, and nitrates increases the hardness of water.
One of the most serious new environmental problems brought on by wastewater treatment by-products is the disposal of chromium in the tanning industry. Chromium, iron, nickel, cadmium, zinc, manganese, and copper are among the elements that are contaminating the aquatic environment at increasing quantities in the tannery effluent. In the present study, the level of chromium was found higher range, although the permissible limit is 0.05. According to Rabah and Ibrahim’s analysis, the tannery effluent contained (0.26) milligrammes of chromium26. High levels of chromium concentration are still hazardous to aquatic organisms and have been shown to change their food chains41.
In this study, Channa punctatus exposed to the tannery effluent at different concentrations showed severe and varied pathological symptoms depending upon the dose and nature of the toxicant. The most significant behavioural pathologies was restlessness, sporadic erratic darting movement, migrating to the aquarium’s bottom, rapid opercular motions, and gradually losing buoyancy, which led to fish floating upside down horizontally with their open mouth and gill opercula. Similar behavioural responses of Cyprinus carpio to industrial effluent were observed42,43. Irregular and erratic movements indicate the loss of equilibrium that might be due to the damage in the brain associated with the maintenance of equilibrium.
Conclusion
The current analysis has confirmed that the tannery effluent’s physicochemical characteristics exhibit high concentrations of EC, TSS, TDS, BOD, COD, TH, sodium, calcium, chlorides, sulphates and chromium that exceed the permissible levels. The physicochemical analysis showed that the morphological changes in the appearance of fish within tannery effluent water samples are because of variation in the salinity, odour and pH. Large volumes of mucous and excrement were discharged from the body, and behaviour changes like widely opened mouths, erratically moving operculums, and frequent up-and-down motion were noticed. The skin was also a poor shade. The current investigation thus showed the detrimental effects of tannery effluent on the aquatic ecosystem.
Acknowledgement
The authors thankfully acknowledge support from the NM tannery, Sembattu Pudukkottai, Trichy, Tamil Nadu to bring out the samples for do research. The authors would also like to articulate special appreciations to the Department of HGMB, BU, India.
Conflict of Interest
The authors declare that they have no conflict of interest.
Funding Sources
No funding source
Authors’ Contribution
PK drafted the original manuscript and sample collection; MY provided critical suggestions;
SK and JI edited the manuscript. All authors read and approved the final manuscript.
Data Availability Statement
Not applicable
Ethics Approval Statement
Not applicable
References
- Mondal NC, Saxena VK, Singh VS. Impact of pollution due to tanneries on groundwater regime. Current science. 2005; 25:1988-1994.
- Pathan TS, Thete PB, Shinde SE, Sonawane DL, Khillare YK. Histochemical changes in the liver of freshwater fish, Rasbora daniconius, exposed to paper mill effluent. Emirates Journal of Food and Agriculture. 2009; 21(2): 71-78.
- Wagner T, Erickson LE. Sustainable management of eutrophic lakes and reservoirs. Journal of Environmental Protection. 2017; 8(4): 436-463.
- Tadesse KB, Dinka MO. Eutrophic reservoir water suitability for irrigation in semi-arid region. Environment, Development and Sustainability. 2023; 1-1.
- Altaf MM, Masood F, Malik A. Impact of long-term application of treated tannery effluents on the emergence of resistance traits in Rhizobium sp. isolated from Trifolium alexandrinum. Turkish Journal of Biology. 2008; 32(1):1-8.
- Javed M. Studies on metals toxicity and physicochemistry of water in the stretch of river Ravi from Baloki headwork to Sidhnai barrage. Indus Journal of Biological Sciences. 2004;1(2):106-110.
- Lewis MA, Scott GI, Bearden DW, Quarles RL, Moore J, Strozier ED, Sivertsen SK, Dias AR, Sanders M. Fish tissue quality in near-coastal areas of the Gulf of Mexico receiving point source discharges. Science of the Total Environment. 2002; 284(1-3): 249-261.
- Stein K, Schwedt G. Speciation of chromium in the waste water from a tannery. Fresenius’ journal of analytical chemistry. 1994; 350: 38-43.
- Sperling M, Xu S, Welz B. Determination of chromium (III) and chromium (VI) in water using flow injection on-line preconcentration with selective adsorption on activated alumina and flame atomic absorption spectrometric detection. Analytical Chemistry. 1992; 64(24): 3101-3108.
- Mella B, Glanert AC, Gutterres M. Removal of chromium from tanning wastewater and its reuse. Process Safety and Environmental Protection. 2015; 95: 195-201.
- Holden AV. The effects of pesticides on life in fresh waters. Proceedings of the Royal Society of London. Series B. Biological Sciences. 1972; 180(1061): 383-394.
- Ahmad I, Ahmad M. Fresh water fish, Channa punctatus, as a model for pendimethalin genotoxicity testing: a new approach toward aquatic environmental contaminants. Environmental toxicology. 2016; 31(11): 1520-1529.
- Ahmed I, Zakiya A, Fazio F. Effects of aquatic heavy metal intoxication on the level of hematocrit and hemoglobin in fishes: a review. Frontiers in Environmental Science. 2022; 10: 919204.
- Rice EW, Bridgewater L, American Public Health Association, editors. Standard methods for the examination of water and wastewater. Washington, DC: American public health association. 2012; 1-40.
- Jackson TA. Accumulation of mercury by plankton and benthic invertebrates in riverine lakes of northern Manitoba (Canada): importance of regionally and seasonally varying environmental factors. Canadian Journal of Fisheries and Aquatic Sciences. 1988; 45(10): 1744-1757.
- Appiah-Brempong M, Essandoh HM, Asiedu NY, Dadzie SK, Momade FW. Artisanal tannery wastewater: quantity and characteristics. Heliyon. 2022; 8(1).
- Dziadel M, Ignatowicz K. Assessment of the Quality of Wastewater Generated During Production at a Tannery Plant. Journal of Ecological Engineering. 2022; 23(5).
- Konar SK. Laboratory studies on two organophosphorus insecticides, DDVP and phosphamidon, as selective toxicants. Transactions of the American Fisheries Society. 1969; 98(3): 430-437.
- Pandey S, Ahmad I, Parvez S, Bin-Hafeez B, Haque R, Raisuddin S. Effect of endosulfan on antioxidants of freshwater fish Channa punctatus Bloch: 1. Protection against lipid peroxidation in liver by copper preexposure. Archives of environmental contamination and toxicology. 2001; 41: 345-352.
- Nair CR. Changes in the acid and alkaline phosphatase activity during sub-lethal exposure of cyprinus carpio and oriochromis mossambicus to curacron. Asian Journal of Microbiology Biotechnology and Environmental Sciences. 2006; 8(4): 817.
- Noorjahan CM. Physicochemical characteristics, identification of fungi and biodegradation of industrial effluent. Journal of Earth and Environmental Sciences. 2014; 4(4): 32-39.
- Mohamed M.J. Sharief S.D., Dawood, D. Ilango B.K. Characterization of tannery effluent. Journal of Industrial Pollution Control. 2004; 20: 1-16.
- Smrithi A, Usha K. Isolation and characterization of chromium removing bacteria from tannery effluent disposal site. International Journal of AdvancedBiotechnology and Research. 2012; 3(3): 644-652.
- Jyothi B, Narayan G. Certain pesticide-induced carbohydrate metabolic disorders in the serum of freshwater fish Clarias batrachus (Linn.). Food and chemical toxicology. 1999; 37(4): 417-421.
- Corsi I, Mariottini M, Sensini C, Lancini L, Focardi S. Fish as bioindicators of brackish ecosystem health: integrating biomarker responses and target pollutant concentrations. Oceanologica Acta. 2003;26(1): 129-138.
- Rabah AB, Ibrahim ML. Physico-chemical and microbiological characterization of soils laden with tannery effluents in Sokoto, Nigeria. Nigerian Journal of Basic and Applied Sciences. 2010; 18(1): 65-71.
- Hemamalini Varadarajan SS. Biodiversity characterization of bacterial and fungal isolates from gold electroplating industry effluents. Journal of Applied & Environmental Microbiology. 2014; 2(5): 212-219.
- Saxena S, Shrivastava P. Ground water quality of a typical urban settlement: A case study of impact of town planning. Pollution Research. 2002; 21(2): 223-226.
- Islam BI, Musa AE, Ibrahim EH, Sharafa SA, Elfaki BM. Evaluation and characterization of tannery wastewater. Journal of Forest Products & Industries. 2014; 3(3): 141-150.
- Akan JC, Abdulrahman FI, Dimari GA, Ogugbuaja VO. Physicochemical determination of pollutants in wastewater and vegetable samples along the Jakara wastewater channelin Kano Metropolis, Kano State, Nigeria. European Journal of Scientific Research. 2008; 23(1): 122-133.
- Kulkarni RT. Source and characteristics of dairy wastes from a medium sized effluent on microorganisms, plant growth and their microbial change. Life Sciences Advanced. 1992; 3: 76-78.
- Alvarez-Bernal D, Contreras-Ramos SM, Trujillo-Tapia N, Olalde-Portugal V, Frías-Hernández JT, Dendooven L. Effects of tanneries wastewater on chemical and biological soil characteristics. Applied Soil Ecology. 2006; 33(3): 269-277.
- Nagarajan P, Ramachandramoorthy TR. Oil and grease removal from steel industry waste water by chemical treatment. Journal of Ecotoxicology and Environmental Monitoring. 2002; 12(3): 181-184.
- Sharma S, Malaviya P. Bioremediation of tannery wastewater by Aspergillus niger SPFSL2-a isolated from tannery sludge. Journal of Basic & Applied Sciences. 2013; 2: 88-93.
- Kannan K, Rajasekaran G, Raveen R. Bacterial analysis of soil samples collected in and around a sugar mill in Tamil Nadu. Journal of Ecobiology. 2009; 24(2): 191-195.
- Nagarajan P, Ramachandramoorthy T, Raja RE, Raj AP. Physico-chemical characteristics of water and soil at Senthanirpuram, Tiruchirappalli and their influence on germination of greengram and cowpea. Journal of ecotoxicology & environmental monitoring. 2005; 15(3): 229-234.
- Goel PK. Water pollution: causes, effects and control. New age international; 2006; 1-269.
- Deepa S, Valivittan K, Indira V, Tharadevi C. Characterization of tannery effluent, Thirumudivakkam, Chennai, Tamilnadu. Journal of Applied Biology and Biotechnology. 2011; 5: 265-270.
- Bosnic M, Buljan J, Daniels RP. Pollutants in tannery effluents. Regional program for pollution control in the tanning industry in South-East Asia S. RAS/92/120, United Nations Industrial Development organization; 2000; 1-567.
- Vijayanand S, Hemapriya J. Biosorption and detoxification of Cr (VI) by tannery effluent acclimatized halotolerant bacterial strain pv26. International Journal of Current Microbiology and Applied Sciences. 2014; 3(9): 971-982.
- Fent K. Ecotoxicological effects at contaminated sites. Toxicology. 2004; 205(3): 223-240.
- Ganeshwade RM, Rokade PB, Sonwane SR. Behavioral responses of Cyprinus carpio to industrial effluents. Journal of Environmental Biology. 2006; 27(1):159-170.
- Sujatha LB. Studies on the physiology haematology and histopathology in the Indian major carp catla catla hamilton as influenced by individual and synergistic toxic effects of a pesticide and two metallic compounds. University of Madras. 2006; 1-342.

This work is licensed under a Creative Commons Attribution 4.0 International License.





