Manuscript accepted on : 02-01-2024
Published online on: 24-01-2024
Plagiarism Check: Yes
Reviewed by: Dr. Nagham Mahmood Aljamali
Second Review by: Dr. Marwa Fouad Hassan
Final Approval by: Dr. Hifzur R. Siddique
A Short Review - Biochemical Aspects and Advancements in Gastric Cancer
Rajeev Ramachandra Kolgi1 , Bhargavi G2
, Bhargavi G2 , Nataraju Angaswamy2
, Nataraju Angaswamy2 , M V. Srinivasulu3
, M V. Srinivasulu3 and S. Shankara Somashetty4*
and S. Shankara Somashetty4*
1Department of Chemistry and Biochemistry, Government Science College, Bengaluru, Karnataka , India.
2Department of Biochemistry, Karnataka State Open University, Mysuru-06. Karnataka, India.
3Department of Botany, Government Science College, Bengaluru-01. Karnataka, India
4Department of Microbiology, Government Science College, Bengaluru-01. Karnataka, India.
Corresponding Author E-mal: drshankarmicro@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/3203
ABSTRACT: Malignancy in the stomach is one of the silent causes of mortality due to a bad prognosis regardless of gender. It is the world's Fourth leading cause of death It is a disorder in which cancerous cells form in the stomach lining. The primary relationships begin between its carcinogenic route and Helicobacter pylori infection, following inflammation, and tissue regeneration. The review aims to evaluate biochemistry related to gastric cancer which focuses on cancer research including etiology, molecular basis, malignant transformation, tumor markers, prognosis, advancements in gastric (stomach) cancer and its therapeutics. The study of prognosis and advancements in gastric cancer helps a researcher, medical practitioner, or surgeon to develop safe, minimally invasive, and effective methods to prevent, screen, diagnose, and treat gastric cancer.
KEYWORDS: Adenocarcinoma; Chemotherapy; Etiology; Gastric Cancer; Helicobacter Pylori; Tumor Markers
Download this article as:| Copy the following to cite this article: Kolgi R. R, Bhargavi G, Angaswamy N, Srinivasulu M. V, Somashetty S. A Short Review - Biochemical Aspects and Advancements in Gastric Cancer. Biotech Res Asia 2024;21(1). |
| Copy the following to cite this URL: Kolgi R. R, Bhargavi G, Angaswamy N, Srinivasulu M. V, Somashetty S. A Short Review - Biochemical Aspects and Advancements in Gastric Cancer. Biotech Res Asia 2024;21(1). Available from: https://bit.ly/3S306He |
Introduction
Cancer is called for the swollen veins that surround the, affected area, which is taken from the Latin term. According to various research data the term cancer can be defined as a growth disorder marked by excessive cell proliferation with no obvious relationship to the biological challenges of the organ concerned 1.
Most of the malignancies originate generally from one aberrant cell. As a result of the aberrant proliferation of cells, there is the formation of tumors (neoplasm). It may be benign or malignant. In benign it is encapsulated and does not spread via blood or lymphatics. Malignant conquer local tissue and there is metastasis and destruction in the issue of origin 2.
Cancer that develops along the route of the gastrointestinal tract (GIT) in humans is termed GIT cancer which includes esophageal, stomach/Gastric cancer, and colorectal cancer (liver cancer, gall bladder, pancreas, colon, and anal/ rectal) 3.
The stomach is the second most significant portion or component of the human digestive system. Food that has been partially digested exits the stomach and travels through the small and large intestines. The stomach wall has 5 tissue layers namely Mucosa, submucosa, muscle, subserosa (connective tissue), and serosa are the different layers of skin.; from inner to outermost order.
Stomach cancer spreads to the mucosa! When it spreads through the outer layers over time, it reaches and affects lymph nodes, reducing immune system function. Tumors of the stomach arise from supportive connective tissue and are treated differently than gastric cancer 4,5.
The most prevalent types of early gastric carcinoma are highly distinguished histologically, having tubular and papillary architecture. It is difficult to distinguish between well-differentiated cancer and high-grade carcinoma with minimal mucosal tissue available for diagnosis. Hence it is necessary to properly identify early and advanced stomach cancer stages to treat patients effectively 6. Gastric cancer is the fourth greatest cause of cancer mortality globally, with a 5-year survival rate of 20%. While most stomach cancers are sporadic, genetic cancer risk syndromes account for 1%-3% of cases. Survival rate for cancer has increased as a result of modern treatment methods, although eastern and Asian countries as well as South American countries have higher rates of stomach cancer than other regions) 7.
Etiology
Chemical carcinogenic compound
A handful of the compounds are immediately carcinogenic, but the vast majority require previous alteration to become so. Some known chemical carcinogens are alkylating agents, aromatic hydrocarbons like benzopyrenes, and benzene 8, aromatic amines, azo dyes, nitroso compounds, and polycyclic aromatic hydrocarbons. Indirect carcinogens become the ultimate carcinogen by a series of enzymatic reactions involving Cytochrome 450. Conversion of benzo pyrene to mutagenic epoxides happens to be metabolic enzymes in the liver involving Cytochrome-450. Mutagenic epoxides are highly reactive with the DNA (deoxyribonucleic acids). Most of the cancers are multifactorial in origin. The reactive electrophiles produced by carcinogenic agents are responsible for severe DNA damage. Mutation is led by such chemical carcinogens, ultimately leading to cancer growth and development 9.
Microbial agent
Some cancers are persistently associated with various microbial infections. (Example- Helicobacter pylori infections among stomach cancer patients) The prevalence of stomach cancer is influenced by quite a few variables, such as the host’s genetic makeup, geographic location, nutrition, and lifestyle 10, 11. Nonetheless, Helicobacter pylori infection is essential for the onset of the condition l2. It’s interesting to note that the Epstein-Barr virus (EBV) is present in approximately 80% of Stomach malignancies’ using lymphoid stroma 13.
The transformed normal cell into something like a malignant cell entails a process in which genetic mutations involved in typical homeostatic mechanisms that regulate proliferation and cell death are mutated, which leads to the activation of genes boosting proliferation or protecting against cell death, known as oncogenes, and the inactivation of genes which would usually impede proliferation, widely recognized as tumor suppressor genes. Tumor antigens encoded by viruses were discovered and shown to be required for the establishment of the malignancy as well as the overall maintenance of the transformed cell phenotypes. DNA viruses such as adenovirus, human papillomavirus, polyomaviruses, and herpes virus alter gene expression (Table-2), inactivating tumor suppressor genes such as P53(Protooncogene 53) and Retinoblastoma protein, leading to cancer through both somatic and germline mutations in such transformed cells 14,15,16.
Gender and lifestyle reasons
Various lifestyle-related risk factors for stomach cancer include high salt and alcohol intake, unrestricted smoking, pernicious anemia, and a diet poor in fruits and vegetables; Alxiominal pain and weight loss are the main symptoms. Other symptoms include heartburn, stomach ache, heaviness, and vomiting. In contrast to men, women have a two times lower risk of developing stomach cancer because of dietary differences, use of drugs, and other lifestyle factors (figure I). Yet, due to an increase in smoking and unhealthy lifestyle choices among both men and women, as well as a progressive increase in aging, the prevalence of stomach cancer has recently become nearly equal across the sexes 17, 18.
To understand how prevalent gastric cancer is amongst men and women, age-based survival rates are crucial. In the example provided in Table. I, men with an age range of 60 to 80 years have an approximate 0.02% mortality rate, while women with combined risk of viral infections during the progression of gastric carcinoma have a slightly lower 0.01% rate. 90% of women who develop gastric cancer and breast cancer die from it. Males with neither combined risk factors nor stomach cancer have a mortality rate of 67%, compared to 83% for women 19. Germline mutations in CADHERIN are known to cause hereditary diffuse in gastric cancer which is confirmed through pedigree information 20.
 |
Figure 1: Symptoms, causes, diagnosis, and treatment amongst gastric cancer patients.
|
Genetic agent
A genetic alteration in a single cell that leads to unchecked cell division is the root cause of cancer monoclonal tumors. In recent years, a third group of genes that govern cell growth has emerged. In addition to oncogenes and antioncogenes, they are thought to have a role in carcinogenesis 21. Cancer-causing genes called oncogenes, were primarily discovered in viruses that cause tumors. These viral oncogenes were identified to mimic certain proto-oncogene genes expressed in host cells, which are liable for the development of regulatory proteins. Even though approximately 10-15% of tumors are caused by viruses, research on tumor viruses over the past century has made significant strides in oncology. The most obvious cancer-causing viruses include herpes family viruses, Human Immune deficiency (HIV) virus, and EBV; For EBV and other Herpes viruses, the connection to the emergence of neoplasms is well known 22.
Hereditary cancer is an important source of information regarding human cancer genes. The autosomal dominant genetic tendency imposes a high relative risk for one or more types of cancer. Retroviruses block apoptosis, causing irregularities in cell signal transduction pathways. Rous established in 1911 that avian sarcoma may well be transmitted from one animal to another through the administration of soluble components 23, 24.
Morphological and Biochemical Changes in Tumor Cells
The morphological and biochemical aspects during the development of tumors can be understood based on in vitro culture studies. Tumor cells are significantly rounder in form than normal ones. According to ‘Ewing’ there is often gradual structural differentiation in metastatic tumors. There is a loss of contact reverse metabolism, hence cancer enhances multilayer growth as a result there is a loss of anchorage dependence. Tumor cells synthesize fetal proteins like alfa fetoprotein generally these genes are suppressed in adult cells 25.
Nutrient metabolism varies in thriving cells. Rising energy requirements of proliferating cells increase glycolysis; yet, compared to normal cells, oxygen intake slows down. As a result, tumor cells start experiencing hypoxia, and their glycolysis generates lactic acid end products, which induces Lacto-acidosis. Moreover, abnormal glycoprotein synthesis results in damaged cell surfaces. Cancer cells are liable to grow rather aggressively and do not conform to normal differentiation of tissue formation (Figure 2). These abnormal growth patterns are inclined to interfere with the regular activities of adjacent cells and tissues 26.
A hallmark of cancer is the circumvention of apoptosis, a route of planned cell death in which enzymes disintegrate the cells. The amount of sialic acid in tumor cells also fluctuates, enhancing the negative charge on the surface of the cells and reducing the immune response among individuals 27, 28, 29.
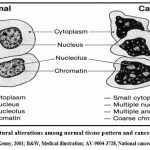 |
Figure 2: Structural alterations among normal tissue pattern and cancer tissue pattern.
|
Gastric carcinoma classification
In order to analyze the severity of gastric cancer Endoscopic ultrasonography (EUS) and (CT) Computed Tomography are typically used to obtain the efficient or precise information needed before operations 30. The classification used to diagnose stomach cancer; According to Borrmann’s classification, which is still widely used, progressive gastric carcinomas exhibit a range of aberrant forms. Gastric carcinomas are divided into four Different Kinds such as polypoid carcinoma (type I), fungating carcinoma (type II), ulcerating carcinoma (type III), and diffusely infiltrating carcinoma (type IV); Particularly type II, then types I and III, make up the bulk of cases of stomach cancer presented 31, 32.
Molecular Basis of Gastric Cancer
Researchers from The Cancer Genome Atlas (TCGA) project, a collaborative effort by the National Human Genome Research Institute to examine DNA, are analyzing cancer. Gastric (stomach) cancer is four separate ailments, rather than just one, as per epigenetic changes in more than thirty different forms of human cancer. The research from TCGA (The Cancer Genome Atlas) and other related projects may eventually lead to a new cancer classification system that organizes cancers according to specific organs or tissues of origin as well as their molecular abnormalities 33, 34.
This comprehensive evaluation of carcinogenesis at the molecular level has been solely possible as a result of modern molecular biology. Oncogenic viruses, in a broad sense, can incorporate their gene into D A of the host cell and hold over the entire host cell’s regulatory regime. Rather than the host genome, the transformed cell will synthesize the viral genome. As a consequence, transformed cells proliferate uncontrollably 35.
Proto-oncogenes are oncogenes found in normal cells. The majority of proto-oncogenes like Ras oncogenes, and the C-myc gene, can be found on several chromosomes. These are normally dormant genes that become active and oncogenes owing to a variety of causes. Oncogenes are overriding genes that mainly encode faulty receptors, kinases, G proteins, and so forth 36, 37.
In the very initial days of the progression of gastroesophageal carcinogenesis, p53-protein overexpression, and gene mutation would develop, Tumor suppressor genes produce regulatory proteins that frequently obstruct cell division. Changes to these genes can lead to the development of tumors. Cancer is mostly caused by mutations in oncogenes and tumor suppressor genes 38.
The transformation of proto-oncogenes to oncogenes is influenced by several mechanisms like insertion of promoters and enhancers, chromosomal translocation, gene amplification, point mutation, and deletion. A viral promoter may be put right upstream of proto-oncogenes when a viral genome gets introduced into the genome of the cell in question. This can lead to the expression of proto-oncogenes and excessive cell proliferation. For example, erb B (Erythroblastic leukemia- oncogene), myb (myoblastosis- oncogene), and abl (Abelson murine leukemia – oncogene) genes are amplified (gene amplification) in some cancers 39, 40.
In gastric cancer patients, a gathering of genetic and molecular abnormalities occurs which includes Oncogene activation, overexpression of growth factors/receptors, inactivation of genes that suppress tumors, DNA repair genes, attachment of cells factors, loss of heterogeneity and point mutations in tumor suppressor genes, and silence of tumor suppressors via CpG island methylation are several instances of oncogene activation 41, 42.
Screening of Tumor and Tumor Markers in Gastric Cancer
Early illness detection is connected with a considerably higher survival rate. This shows that screening may affect disease mortality. This study looks at current screening approaches, ongoing studies, and the future of ovarian cancer screening. Cancer screening often begins with an examination of clinical symptoms, followed by a review of bodily fluids and, finally, radiological imaging. Gastric cancer is screened in the same general fashion as other cancers, including clinical signs, hematology, biopsy, and radio imaging of the target organ/location. Tumor markers are proteins produced by cancer cells that signal the presence of malignancy. A perfect marker would be highly specific to a single tumor type. These might include enzymes, hormones, or surface antigens. They may be quantified in serum, identified in blood, urine, and bodily fluids, and utilized for illness screening, staging, prognosis, and patient follow-up 43, 44.
Tumor markers are categorized according to their origin, chemical composition, and cellular structure. The efficacy in screening GIT (gastrointestinal tract) cancer and several other neoplasia such as breast, ovarian, and pancreatic cancer is thought to be mainly reliant on tumor markers such as CA-72-4 (a glycoprotein with 40% sensitivity), CEA (Carcinoembryonic antigen), and CA-19-9. The markers CA-72-4 and CA-19-9 had normal detection values of 6.9 U/mL and 37 UI/L in peritoneal blood samples among gastric tumor patients, respectively. CEA, a glycoprotein normally produced by embryonic tissue of the stomach, pancreas, and liver, is another frequently used marker, however, it is only used in the earliest phases of a tumor, with a normal value of 3 ng/mL in nonsmokers and 5 ng/mL in smokers among GIT cancer patients. Nevertheless, the sensitivity of all of these indicators in gastric tumor patients is dependent on different phases, samples, and time intervals of testing, thus the data must be juxtaposed with other screening procedures in order to clarify the diagnosis 45, 46.
Many studies have assessed the prognostic usefulness of tumor markers at various stages of Gastric carcinoma, and not all routinely used tumor markers have been encompassed. So far, six types of gastric cancer indicators have been eminently investigated by various researchers, and their expression is strongly regulated throughout tumor growth and cancer metastasis in a manner similar to cancer stem cell marker expression. CD44 (Cluster of differentiation 44), EPCAM (Epithelial cancer adhesion molecules), and ICAM1 (intracellular adhesion molecules) are a few gastric carcinoma markers that are raised during cancer progression, aiding in cancer diagnosis and prognosis, whereas elevated levels of other markers, such as CXCR4 (C-X-C motif chemokine receptor 4), indicates a deprived prognosis and diagnosis. Many researchers believe that tumor markers might be a valuable tool in targeted medication therapy or immune therapy for cancer patients. However, as of now, it is an immature study that has to be advanced further by researchers and medical practitioners 47,48.
In contrast to photofluorography screening, serum pepsinogen measurement is employed for stomach cancer screening. An assay to assess PD-L1(Programmed cell death ligand) expression in patients with gastric cancer is an FDA (Food and Drug Administration)- approved marker for future chemotherapeutics. The most accurate screening includes the upper Gastrointestinal endoscopy with biopsy (a most common /general gastric cancer screening tool). In advanced stages of gastric cancer particular/specific specialized radiological investigation is effectively employed 49, 50.
Table 1: Age-based mortality rates in men and women.
|
Age of patients
|
Combined risk factors |
Mortality Rate in Men |
Mortality Rate in Women |
References |
|
60-80 years |
Viral infections |
~0.02 % |
~0.01% |
Thrumurthy et al., 2013 |
|
≥80 years |
———– |
~67% |
~83% |
Pharoah et al., 2001 |
|
≥80 years |
Breast cancer |
———– |
~90% |
Pharoah et al., 2001 |
Table 2: oncogenic microbial agents associated with gastric cancer and other tumors.
|
Name of the Microbial agent |
Associated cancer type |
References |
|
Helicobacter pylori |
Gastric Cancer |
Gross, 1970; Knudson, 1997 |
|
Epstein Barr Virus (EBV) |
Burkitt’s lymphoma, Stomach cancer |
Costa et al., 2018 |
|
Hepatitis B Virus |
Hepatoma, Liver cancer, colorectal and Stomach cancer |
Costa et al., 2018 |
|
Zika virus |
Brain cancer |
Benavides, 2021 |
Cancer Therapy
Gastric cancer has so far been a silent cause of death due to poor prognosis in the advanced cancerous stage. However, every effort of treatment can work for a few patients to recover from gastric cancer. Gastric cancer is treated at different stages of cancer in various methodologies. In the early stage, only surgery is enough to completely remove cancerous tissue. In the advanced stage, regulated chemotherapy before and after surgery is required along with a postoperative chemoradiation; while during the metastasis stage, the treatment involves more personal care and support in combination with chemotherapy and chemoradiation. Recent breakthroughs in esophageal cancer surgical therapy have enhanced the outlook of early, locally developed esophageal cancer. Primary cancer from an esophageal transplant is uncommon, however, it has been found in long-term survivors. Regular endoscopic evaluations of esophageal gastric grafts in regions with a specific rate of stomach cancer may aid in the earlier detection of primary gastric graft cancer. Reoperation with a colon graft may be an option for treating original gastric graft cancer 51, 52, 53.
Advantages in terms of nutrition are particularly significant in cancer therapy since it is an old and traditional belief in the prevention and treatment of many diseases and illnesses. According to the American Cancer Society and numerous other cancer centers, a number of nutritional supplements used under medical supervision after a cancer diagnosis may be useful for specific cancer locations. Consumption of vitamin C supplements was linked to lower overall and cancer-specific mortality in breast cancer patients. There was limited evidence that taking vitamin D or E supplements was related to a lower risk of breast cancer recurrence. High antioxidant dosages restored the oxidative damage caused by cancer cell therapies. Several demographic and scientific investigations show that a variety of micronutrients, including vitamins and minerals, help to prevent cancer. Diets deficient in certain micronutrients may be linked to an increased risk of cancer. They contain immunological and apoptosis-inducing capabilities, as well as the ability to govern cell proliferation and differentiation. Antioxidants have been proven in laboratory, and in vitro studies to inhibit cancer cell growth via a number of mechanisms, including increased cell differentiation and death 54, 55.
Defects in pathways that regulate signaling are the most prevalent root of cancer and other conditions. The study of cancer, in particular cancer induced by specific viruses, has tremendously aided the comprehension of pathways that transmit signals. Targeting metabolic pathways is essential to control cancer cells. Certain cancer-fighting drugs are commonly used in the therapy of malignancies, which frequently get treated with an assortment of treatments. In recent years, ketogenic diets have gained popularity as a harmless broad-spectrum strategy to target this significant metabolic difference between normal and malignant cells. The efficacy of anticancer medications is related to the amount of cancer cells. Methotrexate, a potential chemotherapeutic drug, is a dihydrofolate reductase inhibitor. The folate derivative works as an adversary inhibitor of the enzyme, binding methotrexate with around 100 times the binding power of dihydrofolate 56, 57, 58.
A gastroenterologist must be knowledgeable about risk factors to carry out safe and practical cancer screening and treatment. To prevent the disease from spreading to other abdominal organs, early identification of gastric cancer is essential. There have reportedly been enormous breakthroughs made with robotics and minimally invasive methods in Asia, among other places 59.
In some neoplasms, viruses can also be therapeutic agents; as a result, some oncolytic viruses with tumor cell-specific tropism have been given the go-ahead for usage in human clinical trials. According to estimates, treating or preventing viral infections could avert at least 1.5 million cancer-related deaths annually 60.
The development of cancer immunotherapeutic techniques is dependent on the identification and confirmation of appropriate target tumor antigens that are tumor-specific and capable of eliciting a rapid and powerful anti-tumor immune response. Chromosomal translocation resulting in abnormal chromosomes is seen in cancer cells. Burkitt’s lymphoma, a rapidly multiplying human B cell cancer, is an example of a reciprocity transposition. A chromosome 8 fragment is torn off and attached to chromosome 14, which holds the myc gene, a metastasis whereby corresponds with cancer of the gastric tract 61.
The disclosure and proper understanding of the molecular events of gastric carcinoma, or any cancer type, lead to the use of cellular pathology in cancer avoidance, early identification, tumor categorizing, and successful therapy. The applications of molecular testing such as the testing of CADHERIN1 and the p53 gene for hereditary diffuse gastric carcinoma (HDGC) activation in stomach malignancies have had an enormous effect on the practice of medicine and have grown into a habit in the treatment of patients 62.
Although cytotoxic chemotherapy remains the mainstay of therapy for metastatic stomach cancer, current developments in molecular understanding of the illness have rekindled hope that specific medicines may be utilized to improve survival while eliminating toxicity. For example, adding trastuzumab to frontline chemotherapy upsurges endurance in patients with human epidermal growth factor-2 (HER2)-positive stomach cancer. Oncologists can nowadays practice using ramucirumab, a VEGF (Vascular endothelial growth factor) receptor inhibitor, as a sole treatment or in amalgamation with chemotherapy in the subsequent line, while the safe checkpoint inhibitor pembrolizumab is licensed in numerous circumstances liable on the Programmed Death Ligand 1 (PD-L1) status. 6-mercapto purine a purine analog, 5-fluoro uracil, L-asparaginase, Vincristine and Vinblastine (alkaloids), Mitomycin (antibiotic) are the other common drugs used in the treatment of cancer in general 63.
It appears to have documented gastric cancer incidence; the first illustration in the region of the Asia-Pacific – an elderly lady got cancer of the gastric tract in her stomach within nine years after laparoscopic mini-gastric surgery for severe weight gain. A big clinical trial found that the medicine nivolumab (OPDIVO) paired with chemotherapy may increase the survival of certain individuals with advanced stomach cancer. The trial also included those with esophageal gastric tumors; the Food and Drug Administration (FDA) has granted quicker authorization to the immunotherapy medicine pembrolizumab (Keytruda®) to be administered in a subset of patients who have metastatic gastric cancer. However, chemotherapy involves common side effects such as fatigue, anemia, and dehydration. few patients were advised to stop treatment because of severe side effects 64, 65, 66.
Conclusion
The basic challenge to combat life-threatening disorders like cancer takes an immense knowledge of its prevention, detection, symptoms, prognosis, and therapeutic advances. In recent years steady safety measures have been endorsed all over the world to avoid cancer. The antioxidants Vitamin E, Beta carotene, Vitamin C, and flavonoids are most significant from a biochemical viewpoint. They prevent the formation of free radicals, which are responsible for cell damage. Antioxidants can detoxify carcinogens. Augmented consumption of fruits and vegetables is advocated to prevent cancer. Gastric cancer is the 7th most common cancer in women and 4th most common cancer in men worldwide. The prevalence and occurrence of stomach cancer are linked to a variety of variables, including geography, diet/regime, and genetic makeup of the host. It is also clear that Helicobacter pylori infection, along with other viral infections, is critical for the emergence of the illness in seriously ill instances. Vast advancements are known to have been developed in various geographical mainly in Asia involving minimal invasive techniques such as laparoscopy and robotics. Various chemotherapy followed by immunotherapy is the most advanced treatment employed for gastric cancer which relies on targeted tumor markers in therapeutics. Finally, this review emphasizes the knowledge of the researchers in cancer therapeutics and also about risk factors needed for a gastroenterologist to implement safe and feasible screening and treatment of cancer helping them to evaluate and create effective screening and treatment methodologies.
Acknowledgement
I would like to express my profound gratitude to the Department of Microbiology and Department of Biochemistry, Nrupathunga University, and Department of Biochemistry, Karnataka State Open University, Mysuru; for their support and contributions to the completion of my review paper titled “A Short Review- Biochemical Aspects and Advancements in gastric cancer”.
Conflict of Interest
No conflict of interest.
Funding Source
No financial assistance was provided for carrying out this study.
Authors’ Contribution
Idea of the title and conceptualization- Principal author 1
Data analysis, original manuscript writing, editing, and drafting- Authors 2
Reviewal of literature resources- Author 3
Formal Approval of the work and supervision- Corresponding author 4*
Data availability statement
All data that support the findings of this study is available as part of the article and no
additional source data are required.
Ethics Approval Statement
Not Applicable
References
- Vasudevan DM, et al. (Eight edition). Text Book of Biochemistry for Medical Students, JaypeeBrothers Medical Publishers (P). LTD; 2013.
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646-674. doi:1016/j.cell.2011.02.013.
- Costa NR, Gil da Costa RMG, Medeiros R. A viral map of gastrointestinal cancers. Life Sci. 2018;199:188-200. doi:1016/j.lfs.2018.02.025.
- Machi J, Takeda J, Sigel B, Kakegawa T. Normal stomach wall and gastric cancer: evaluation with high-resolution operative US. Radiology. 1986;159(1):85-87. doi:1148/radiology.159.1.3513253.
- Correa P. Gastric cancer: overview. Gastroenterol Clin North Am. 2013;42(2):211-217. doi:1016/j.gtc.2013.01.002.
- Keller G, Rudelius M, Vogelsang H, et al. Microsatellite instability and loss of heterozygosity in gastric carcinoma in comparison to family history. Am J Pathol. 1998;152(5):1281-1289.
- Virani SA, Dent S, Brezden-Masley C, et al. Canadian Cardiovascular Society Guidelines for Evaluation and Management of Cardiovascular Complications of Cancer Therapy. Can J Cardiol. 2016;32(7):831-841. doi:10.1016/j.cjca.2016.02.078.
- Vigliani EC, Saita G. Benzene and leukemia. N Engl J Med. 1964;271:872-876. doi:1056/NEJM196410222711703.
- McCormick F. Signalling networks that cause cancer. Trends Cell Biol. 1999;9(12):M53-M56.
- Chung HC, Bang YJ, S Fuchs C, et al. First-line pembrolizumab/placebo plus trastuzumab and chemotherapy in HER2-positive advanced gastric cancer: KEYNOTE-811. Future Oncol. 2021;17(5):491-501. doi:2217/fon-2020-0737.
- Kim YG, Kong SH, Oh SY, et al. Effects of screening on gastric cancer management: comparative analysis of the results in 2006 and in 2011. J Gastric Cancer. 2014;14(2):129-134. doi:5230/jgc.2014.14.2.129.
- Zabaleta J. Multifactorial etiology of gastric cancer. Cancer Epigenetics. Methods Protoc. 2012:411-435.
- Ribeiro J, Oliveira A, Malta M, et al. Clinical and pathological characterization of Epstein-Barr virus-associated gastric carcinomas in Portugal. World J Gastroenterol. 2017;23(40):7292-7302. doi:10.3748/wjg.v23.i40.7292
- Levine AJ. The common mechanisms of transformation by the small DNA tumor viruses: The inactivation of tumor suppressor gene products: p53. Virology. 2009;384(2):285-293. doi:10.1016/j.virol.2008.09.034.
- Kim B, Kim KM. Role of Exosomes and Their Potential as Biomarkers in Epstein-Barr Virus-Associated Gastric Cancer. Cancers (Basel). 2023;15(2):469. Published 2023 Jan 12. doi:10.3390/cancers15020469.
- Bertram JS. The molecular biology of cancer. Mol Aspects Med. 2000;21(6):167-223. doi:10.1016/s0098-2997(00)00007-8
- Russo AE, Strong VE. Gastric cancer etiology and management in Asia and the West. Annu Rev Med. 2019;70:353-367. doi:1146/annurev-med-081117-043436.
- Hu B, El Hajj N, Sittler S, Lammert N, Barnes R, Meloni-Ehrig A. Gastric cancer: classification, histology and application of molecular pathology. J Gastrointest Oncol. 2012;3(3):251-261. doi:3978/j.issn.2078-6891.2012.021.
- Thrumurthy SG, Chaudry MA, Hochhauser D, Mughal M. The diagnosis and management of gastric cancer. BMJ. 2013;347:f6367. doi:1136/bmj.f6367.
- Pharoah PD, Guilford P, Caldas C, International Gastric Cancer Linkage Consortium. Incidence of gastric cancer and breast cancer in CADHERIN1 (E-cadherin) mutation carriers from hereditary diffuse gastric cancer families. Gastroenterology. 2001;121(6):1348-1353. doi:1053/gast.2001.29611.
- Vogelstein B, Kinzler KW. Cancer genes and the pathways they control. Nat Med. 2004;10(8):789-799. doi:1038/nm1087.
- Mirzaei H, Goudarzi H, Eslami G, Faghihloo E. Role of viruses in gastrointestinal cancer. J Cell Physiol. 2018;233(5):4000-4014. doi:10.1002/jcp.26194
- Knudson AG. Hereditary predisposition to cancer. Ann N Y Acad Sci. 1997;833:58-67. doi:10.1111/j.1749-6632.1997.tb48593.x.
- Benavides-Lara A, la Paz Barboza-Arguello M, González-Elizondo M, et al. Zika Virus-Associated Birth Defects, Costa Rica, 2016-2018. Emerg Infect Dis. 2021;27(2):360-371. doi:10.3201/eid2702.202047.
- Loboda AP, Adonin LS, Zvereva SD, et al. BRCA Mutations-The Achilles Heel of Breast, Ovarian, and Other Epithelial Cancers. Int J Mol Sci. 2023;24(5):4982. Published 2023 Mar 5. doi:10.3390/ijms24054982.
- T S gene. Knudson AC 1997. Antioncogenes and human cancer:10914-10921.
- Parsons, S. L., Watson, S. A., Brown, P. D., Collins, H. M., & Steele, R. J. C. (1997). Matrix metalloproteinases. British Journal of Surgery, 84(2), 160-166.
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57-70. doi:10.1016/s0092-8674(00)81683-9.
- Keller G, Rudelius M, Vogelsang H, et al. Microsatellite instability and loss of heterozygosity in gastric carcinoma in comparison to family history. Am J Pathol. 1998;152(5):1281-1289.
- Oliveira C, Suriano G, Ferreira P, et al. Genetic screening for familial gastric cancer. Hered Cancer Clin Pract. 2004;2(2):51-64. doi:1186/1897-4287-2-2-51.
- Carmack SW, Genta RM, Graham DY, Lauwers GY. Management of gastric polyps: a pathology-based guide for gastroenterologists. Nat Rev Gastroenterol Hepatol. 2009;6(6):331-341. doi:1038/nrgastro.2009.70.
- Waldum HL, Fossmark R. Types of Gastric Carcinomas. Int J Mol Sci. 2018;19(12):4109. Published 2018 Dec 18. doi:10.3390/ijms19124109.
- Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17(6):1471-1474. doi:10.1245/s10434-010-0985-4.
- Röcken C. Predictive biomarkers in gastric cancer. J Cancer Res Clin Oncol. 2023;149(1):467-481. doi:10.1007/s00432-022-04408-0.
- Temin HM. Overview of biological effects of addition of DNA molecules to cells. Dev Biol Stand. 1998;93:37-44.
- Shope RE. Evolutionary episodes in the concept of viral oncogenesis. Perspect Biol Med. 1966;9(2):258-274. doi:10.1353/pbm.1966.0022.
- Baserga R. Oncogenes and the strategy of growth factors. Cell. 1994;79(6):927-930. doi:10.1016/0092-8674(94)90023-x.
- Triolo Va. Nineteenth-Century Foundations Of Cancer Research Advances In Tumor Pathology, Nomenclature, And Theories Of Oncogenesis. Cancer Res. 1965;25:75-106.
- Gao H, Wang LD, Zhou Q, Hong JY, Huang TY, Yang CS. p53 tumor suppressor gene mutation in early esophageal precancerous lesions and carcinoma among high-risk populations in Henan, China. Cancer Res. 1994;54(16):4342-4346.
- Knudson AG. Cancer genetics. Am J Med Genet. 2002;111(1):96-102. doi:10.1002/ajmg.10320.
- Ohmura K, Tamura G, Endoh Y, Sakata K, Takahashi T, Motoyama T. Microsatellite alterations in differentiated-type adenocarcinomas and precancerous lesions of the stomach with special reference to cellular phenotype. Hum Pathol. 2000;31(9):1031-1035. doi:10.1053/hupa.2000.16669.
- Takeda Y, Yashima K, Hayashi A, et al. Expression of AID, P53, and Mlh1 proteins in endoscopically resected differentiated-type early gastric cancer. World J Gastrointest Oncol. 2012;4(6):131-137. doi:10.4251/wjgo.v4.i6.131.
- Wu GH, Chen LS, Chang KJ, et al. Evolution of breast cancer screening in countries with intermediate and increasing incidence of breast cancer. J Med Screen. 2006;13 Suppl 1:S23-S27.
- Buys SS, Partridge E, Black A, et al. Effect of screening on ovarian cancer mortality: the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Randomized Controlled Trial. JAMA. 2011;305(22):2295-2303. doi:10.1001/jama.2011.766
- Bhatt AN, Mathur R, Farooque A, Verma A, Dwarakanath BS. Cancer biomarkers – current perspectives. Indian J Med Res. 2010;132:129-149.
- Sun Z, Jia J, Du F, et al. Clinical significance of serum tumor markers for advanced gastric cancer with the first-line chemotherapy. Transl Cancer Res. 2019;8(8):2680-2690. doi:10.21037/tcr.2019.10.27
- Lin T, Peng W, Mai P, Zhang E, Peng L. Human Gastric Cancer Stem Cell (GCSC) Markers Are Prognostic Factors Correlated With Immune Infiltration of Gastric Cancer. Front Mol Biosci. 2021;8:626966. Published 2021 May 25. doi:10.3389/fmolb.2021.626966.
- Li L, Ma B, Liu F, et al. The Number of Positive Tumor Markers (NPTM) Achieves Higher Value in the Prognosis Prediction of Gastric Cancer. Dis Markers. 2022;2022:5145918. Published 2022 Nov 28. doi:10.1155/2022/5145918.
- Sexton RE, Al Hallak MN, Diab M, Azmi AS. Gastric cancer: a comprehensive review of current and future treatment strategies. Cancer Metastasis Rev. 2020;39(4):1179-1203. doi:1007/s10555-020-09925-3.
- Petrovchich I, Ford JM. Genetic predisposition to gastric cancer. Semin Oncol. 2016;43(5):554-559. doi:10.1053/j.seminoncol.2016.08.006.
- Kim N. Chemoprevention of gastric cancer by Helicobacter pylori eradication and its underlying mechanism. J Gastroenterol Hepatol. 2019;34(8):1287-1295. doi:10.1111/jgh.14646.
- Yoon YS, Kim HK, Choi YS, Kim K, Kim J, Shim YM. Primary gastric cancer in an oesophageal gastric graft after oesophagectomy. Eur J Cardiothorac Surg. 2011;40(5):1181-1184. doi:10.1016/j.ejcts.2011.02.061.
- Sasaki A, Nakamura Y, Mishima S, et al. Predictive factors for hyper progressive disease during nivolumab as anti-PD1 treatment in patients with advanced gastric cancer. Gastric Cancer. 2019;22(4):793-802. doi:1007/s10120-018-00922-8.
- Salas S, Cottet V, Dossus L, Fassier P, Ginhac J, Latino-Martel P, Romieu I, Schneider S, Srour B, Touillaud M, et al. Nutritional Factors during and after Cancer: Impacts on Survival and Quality of Life. Nutrients. 2022; 14(14):2958. https://doi.org/10.3390/nu14142958.
- Kamal N, Ilowefah MA, Hilles AR, Anua NA, Awin T, Alshwyeh HA, Aldosary SK, Jambocus NGS, Alosaimi AA, Rahman A, et al. Genesis and Mechanism of Some Cancer Types and an Overview on the Role of Diet and Nutrition in Cancer Prevention. Molecules. 2022; 27(6):1794. https://doi.org/10.3390/molecules27061794
- Leong SP, Naxerova K, Keller L, Pantel K, Witte M. Molecular mechanisms of cancer metastasis via the lymphatic versus the blood vessels. Clin Exp Metastasis. 2022;39(1):159-179. doi:10.1007/s10585-021-10120-z.
- Erickson N, Boscheri A, Linke B, Huebner J. Systematic review: isocaloric ketogenic dietary regimes for cancer patients. Med Oncol. 2017;34(5):72. doi:10.1007/s12032-017-0930-5.
- Klement RJ. The emerging role of ketogenic diets in cancer treatment. Curr Opin Clin Nutr Metab Care. 2019;22(2):129-134. doi:10.1097/MCO.0000000000000540.
- Russo AE, Strong VE. Gastric cancer etiology and management in Asia and the West. Annu Rev Med. 2019;70:353-367. doi:1146/annurev-med-081117-043436.
- Bouza E, Martín Jiménez M, Alemany L, et al. Overview of virus and cancer relationships. Position paper. Rev Esp Quimioter. 2021;34(6):525-555. doi:10.37201/req/058.2021.
- Tagliamonte M, Cavalluzzo B, Mauriello A, et al. Molecular mimicry and cancer vaccine development. Mol Cancer. 2023;22(1):75. Published 2023 Apr 26. doi:10.1186/s12943-023-01776-0.
- Shiotani A, Cen P, Graham DY. Eradication of gastric cancer is now both possible and practical. Semin Cancer Biol. 2013;23(6 Pt B):492-501. doi:10.1016/j.semcancer.2013.07.004.
- Patel TH, Cecchini M. Targeted Therapies in Advanced Gastric Cancer. Curr Treat Options Oncol. 2020;21(9):70. Published 2020 Jul 28. doi:10.1007/s11864-020-00774-4.
- Hagi T, Kurokawa Y, Kawabata R, et al. A multicentre biomarker cohort study on the efficacy of nivolumab treatment for gastric cancer. Br J Cancer. 2020;123(6):965-972. doi:1038/s41416-020-0975-7.
- Janjigian YY, Maron SB, Chatila WK, et al. First-line pembrolizumab and trastuzumab in HER2-positive oesophageal, gastric, or gastro-oesophageal junction cancer: an open-label, single-arm, phase 2 trial. Lancet Oncol. 2020;21(6):821-831. doi:10.1016/S1470-2045(20)30169-8.
- Norman G, Rice S, Spackman E, et al. Trastuzumab for the treatment of HER2-positive metastatic adenocarcinoma of the stomach or gastro-oesophageal junction. Health Technol Assess. 2011;15 Suppl 1:33-42. doi:10.3310/hta15suppl1/04.

This work is licensed under a Creative Commons Attribution 4.0 International License.





