Manuscript accepted on : 14-11-2023
Published online on: 14-12-2023
Plagiarism Check: Yes
Reviewed by: Dr Randa Salah Gomaa Mahmoud
Second Review by: Dr. Mohammed Najim Abed
Final Approval by: Dr Ghulam Md Ashraf
Shankar Ganesh M , Satheesh S*
, Satheesh S* and Aravindhan S
and Aravindhan S
Department of pharmacy practice, JKKN college of pharmacy, Kumarapalayam, Namakkal, Tamil Nadu, India.
Corresponding Author E-mail:dr.satheeshsaravanan.pharmd@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/3170
ABSTRACT: Polycythemia, characterized by an excess of red blood cells (RBCs), is a common occurrence in individuals with chronic obstructive pulmonary disease (COPD) due to chronic hypoxia resulting from impaired lung function. While polycythemia may have some protective effects for COPD patients on long-term oxygen therapy, it can also increase the risk of life-threatening complications such as pulmonary embolism (PE). This review explores the impact of polycythemia on COPD patients, delving into its epidemiology, risk factors, and potential consequences. The prevalence of polycythemia in COPD varies from 6% to 10.2% in outpatient settings and is associated with factors like smoking, male gender, impaired lung function, and severe hypoxemia. The elevated red blood cell count can thicken the blood, increasing the risk of blood clots and cardiovascular complications. Proper management of polycythemic COPD involves addressing increased blood viscosity, managing thromboembolic risks, and optimizing cardiovascular health. Therapeutic interventions include phlebotomy, low-dose aspirin, and oxygen therapy to reduce complications and improve outcomes. Additionally, new cytoreductive therapies may offer promising treatment options for primary polycythemia. However, further research is required to enhance our understanding and refine care for individuals with polycythemic COPD, with the ultimate goal of mitigating complications, improving quality of life, and enhancing long-term prognosis. Clinicians must remain vigilant in managing polycythemia in COPD to optimize patient outcomes and reduce the burden of associated complications.
KEYWORDS: COPD; Hypoxia; Hydroxyurea; Low dose aspirin; Polycythemia; Phlebotomy
Download this article as:| Copy the following to cite this article: Ganesh M S, Satheesh S, Aravindhan S. Insights into the Pathophysiology and Therapeutic Targets of Consequences Induced by Polycythemia in COPD. Biotech Res Asia 2023;20(4). |
| Copy the following to cite this URL: Ganesh M S, Satheesh S, Aravindhan S. Insights into the Pathophysiology and Therapeutic Targets of Consequences Induced by Polycythemia in COPD. Biotech Res Asia 2023;20(4). Available from: https://bit.ly/4alWxnW |
Introduction
Polycythemia is a condition where the blood has too many (RBC) red blood cells.1 It can occur in people with COPD (chronic obstructive pulmonary disease) as a result of chronic low oxygen levels. Polycythemia may have some protective effects for COPD patients on long-term oxygen therapy.3,4 However, it can also increase the risk of pulmonary embolism (PE), which is a life-threatening blood clot in the lungs. Polycythemia, a condition where the body produces many red blood cells, has been identified as an independent risk factor for death in COPD patients who also have pulmonary embolism. A study found that among COPD outpatients, the occurrence of polycythemia varies from 6% to 10.2% when measured by a hemoglobin level of at least 17 g/dL for males and at least 15 g/dL for females.2 Additionally, in a group of severe COPD patients undergoing long-term oxygen therapy, 8.4% had a hematocrit level of at least 55%.3 In COPD patients, there are several factors that can increase the likelihood of developing secondary polycythemia. These include being male, smoking, having a reduced ability to transfer carbon monoxide from the lungs to the blood (impaired DLCO), experiencing low levels of oxygen in the blood (severe hypoxemia), and living at high altitudes. Secondary polycythemia can worsen COPD symptoms by hindering the lungs’ ability to oxygenate the blood, leading to shortness of breath, fatigue, and other complications. Furthermore, Polycythemia can increase the likelihood of developing blood clots, which can pose an added risk for people with COPD.19 Hence, this review aims to examine the impact of polycythemia on individuals with COPD, delving into the pathophysiology and potential therapeutic targets. The objectives include providing a thorough overview of the current understanding of polycythemia in COPD, encompassing its prevalence, risk factors, and potential consequences. Additionally, the review seeks to identify areas where further research is necessary to enhance our understanding and management of polycythemic COPD patients.
Polycythemia: Etiology
secondary polycythemia, is a common occurrence in individuals with COPD due to chronic hypoxia, characterized by low oxygen levels resulting from impaired lung function. In COPD, the damaged lungs hinder proper airflow and gas exchange, leading to a reduced supply of oxygen to the body’s tissues.76 In response, the body initiates a compensatory mechanism by increasing the production of red blood cells to enhance oxygen-carrying capacity. This response is triggered by the hormone erythropoietin, which is produced by the kidneys in response to low oxygen levels.77,78 Consequently, higher levels of red blood cells lead to polycythemia. However, this condition can thicken the blood, increasing the risk of blood clots and cardiovascular complications. Managing polycythemia in COPD involves addressing the underlying lung disease and employing therapeutic interventions to improve oxygenation levels.79
Risk factors in polycythemic COPD
Table 1: Risk factors in polycythemic COPD.
| S.No | Risk Factor | Description |
| 1. | Male sex | Men have a higher risk of developing polycythemia in COPD compared to women. |
| 2. | Smoking | Cigarette smoking is a significant risk factor for developing polycythemia in COPD. 67 |
| 3. | Impaired DLCO | a reduced ability to transfer carbon monoxide from the lungs to the blood (impaired DLCO) is associated with an increased risk of developing polycythemia. 67 |
| 4. | Hypoxemia severity | The severity of chronic hypoxemia is positively correlated with polycythemia risk. 2 |
| 5. | Altitude | High-altitude living can raise the risk of secondary polycythemia. |
| 6. | Chronic lung diseases | Polycythemia can be exacerbated by other chronic lung illnesses, such as interstitial lung disease. |
| 7. | Cyanotic heart diseases | Certain heart conditions with cyanosis can lead to polycythemia. |
| 8. | Post-renal transplant | After a kidney transplant, some patients may develop hypertension and erythrocytosis. |
| 9. | ACEIs/ARB’s treatment resistance | In post-renal transplant patients, resistance to ACE inhibitors or ARBs can be a risk factor. |
Differential Diagnoses of Polycythemia
In Adults
Polycythemia is a medical disorder characterized by an abnormally high number of red blood cells in the body.19 This condition can be divided into two main categories: primary and secondary polycythemia. Primary polycythemia also referred to as polycythemia vera, is caused by a genetic mutation in the JAK2 gene that results in uncontrolled production of red blood cells.26 In contrast, secondary polycythemia is a response to chronic low oxygen levels in the body, which triggers the production of additional red blood cells to help carry more oxygen.[28] Gaisbock’s syndrome, also known as stress polycythemia, is a type of relative polycythemia that is often seen in individuals with essential hypertension. This condition is characterized by an increase in the number of red blood cells, despite having a normal circulating red blood cell mass and a decreased plasma volume.29
Types of polycythemia in COPD
Table 2: Types of polycythemia in COPD.
| S.No | Category | Primary Polycythemia | Secondary Polycythemia | Relative polycythemia |
| 1. | Causes | A genetic disorder resulting from a mutation in the bone marrow cells. 27 | Excess of erythropoietin (EPO) | Burns, Stress and dehydration |
| 2. | Erythropoietin (EPO) level | Low.28 | High | Normal |
| 3. | Red blood cell count | High.28 | High | High |
In Neonates
Hypoxia: When a fetus experiences inadequate oxygen delivery, known as hypoxia, it may respond by producing more red blood cells as a way to compensate. This can occur acutely due to complications during birth or chronically when the fetus is exposed to maternal consequences such as hypertension, diabetes, and smoking.35,36
Umbilical cord stripping: When the umbilical cord is clamped later and stripped towards the baby, the remaining blood in the cord and placenta can enter the fetal circulation, leading to an increase in blood volume.37,38
Twin-to-twin transfusion syndrome: a condition that can occur during a twin pregnancy, the twin receiving blood (the recipient) may develop polycythemia.39,40
Pathophysiology of Polycythemic COPD
In COPD patients, about 6-10 percent of people with COPD develop polycythemia. Polycythemic COPD is a subtype of COPD characterized by an increase in hematocrit count in response to chronic hypoxemia.2 The onset of secondary polycythemia can be influenced by elevated carboxyhemoglobin (COHb) levels in individuals who smoke and persistent hypoxemia, or low oxygen levels in the blood, in those with COPD. It is associated with reduced lung function and respiratory symptoms such as cough, mucus production, and shortness of breath.5,6 Polycythemia is generally referred to as “secondary polycythemia” and is caused by chronically low oxygen levels. About 6-10% of patients with COPD develop polycythemia. Secondary polycythemia in COPD is associated with consequences such as smoking, being male, being of non-Hispanic white ethnicity, having a reduced ability of the lungs to transfer carbon monoxide (impaired DLCO), and experiencing severe hypoxemia, or low oxygen levels in the blood.2,14,15 Polycythemic COPD patients are highly vulnerable to complications like pulmonary hypertension and blood clot formation.[7,8] While polycythemia increases blood volume, patients remain at risk of dehydration due to chronic hypoxemia and increased respiratory water loss during exertion.9 Additionally, the elevated blood viscosity can lead to fluid retention and edema.10 This can disrupt fluid balance and contribute to swelling in the legs and ankles. Management of fluid balance in polycythemic COPD patients requires careful monitoring of hydration levels, considering individual factors such as respiratory water loss and edema, and ensuring adequate fluid intake to prevent dehydration and mitigate excessive fluid retention.11 Consequences of dehydration in polycythemic COPD patients can include worsened circulation and impaired oxygen delivery due to thicker blood and difficulties in mucus clearance, leading to respiratory infections and exacerbations.12,13 Proper hydration is essential to mitigate these risks and maintain overall health and well-being.
Pathophysiology of Polycythemic COPD
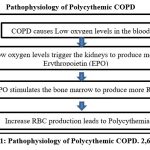 |
Figure 1: Pathophysiology of Polycythemic COPD. 2,6,17,18 |
Consequences of Polycythemic COPD
Hematological consequences
Polycythemia, a condition characterized by an excess of RBC or Hematocrit, can develop in individuals with COPD due to chronic low oxygen levels. While polycythemia may offer some protective benefits for COPD patients on long-term oxygen therapy, it can also heighten the risk of pulmonary embolism (PE), a life-threatening blood clot in the lungs, and is an independent risk factor for mortality in COPD patients with PE.35 Elevated erythrocytes can cause the blood to thicken abnormally, increasing the risk of (VTE) venous thromboembolism and potentially leading to the development of pulmonary hypertension.45
Constituents of hematological consequences
Table 3: Constituents of hematological consequences.
| S.No | Condition | Consequence |
| 1. | Pulmonary Embolism | A blood clot that forms in a deep vein in the leg and travels to the lung, blocking an artery and stopping blood flow, is known as a pulmonary embolism. This condition can be life-threatening and is often characterized by symptoms such as shortness of breath, chest pain, fainting, coughing up blood, rapid or irregular heartbeat, lightheadedness or dizziness, excessive sweating, and fever.20,21 |
| 2. | Venous Thromboembolism | A blood clot that forms in a vein deep in the body. Most often occurs in the legs. Can travel to the lungs and cause a pulmonary embolism. Symptoms can include swollen and tender legs that are painful to the touch, shortness of breath, and pain when breathing.22,23 |
| 3. | Pulmonary Hypertension | Elevated pressure within the blood vessels that carry blood to your lungs can lead to heart failure. Symptoms of this condition may include difficulty breathing (especially after physical activity), swelling, coughing up blood, and fainting.24,25 |
Systemic consequences
In patients with COPD, chronic inflammation and oxidative stress can contribute to the development of secondary polycythemia. Inflammation and oxidative stress can lead to tissue damage and hypoxia, or low oxygen levels, where the production of erythropoietin, a hormone that stimulates the production of red blood cells, can be stimulated, leading to an increase in red blood cell count known as polycythemia.80 Cigarette smoke is a major risk factor for COPD development and has been associated with increased oxidant burden on multiple cell types in the lungs. Elevated levels of reactive oxygen species (ROS) may significantly affect the expression of biological molecules, signaling pathways, and the function of antioxidant defenses. Mitochondrial dysfunction plays a critical role in ROS production due to oxidative phosphorylation. Excess oxidative stress is able to alter mitochondrial function, morphology, and RNA and protein content.81
Some common complications of polycythemia in COPD include
Progression to leukemia: Approximately 5% of cases may develop into acute myeloid leukemia (AML), a condition that is typically challenging to treat. Certain drugs, including chlorambucil, pipobroman, and radioactive phosphorous, have been linked to AML development.30,31
Bleeding: Iron deficiency anemia can occur in recurrent nosebleeds or gastrointestinal bleeding, which can complicate the interpretation of clinical findings, including changes in the appearance of bone marrow.32
Thrombosis: Increased blood viscosity can increase arterial and venous thrombosis risk. Arterial thrombosis can cause digital infarcts and cerebral ischemic infarcts, especially in watershed areas. Venous thrombosis, including diseases such as Budd-Chiari syndrome, can also develop.33,34
Cardiovascular Complications: The increased blood viscosity and workload on the heart can lead to various cardiovascular issues, including hypertension, heart failure, and increased risk of stroke.68
Reduced Oxygen Delivery: Despite increased RBC count, the transport of oxygen to tissues might still be impaired due to decreased lung function in COPD, resulting in tissue hypoxia.69
Enlarged Spleen (Splenomegaly): The body’s attempt to accommodate increased red blood cells might lead to an enlarged spleen.70
Pathophysiology of Polycythemia induced consequences
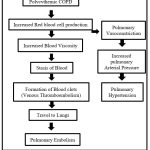 |
Figure 2: Polycythemic COPD induced Hematological consequence.41,42,43,44,45 |
Therapeutic Targets for Polycythemic COPD
Phlebotomy
Phlebotomy is a medical treatment that involves taking blood from a patient’s body. The primary therapy for the blood disorder polycythemia vera was established by a trial conducted by the Polycythemia Vera Study Group. The study revealed that phlebotomy alone was associated with a greater median survival rate when compared to other medications such as Radioactive phosphorous or chlorambucil.46 The purpose of phlebotomy is to reduce hyperviscosity by removing blood and generating an iron deficit, which can help restrict red-cell growth. In practice, Blood of 500 mL is obtained each week until a hematocrit of less than 45% is achieved. The CYTO-PV investigation indicated that patients who were kept below this threshold had a significantly lower incidence of cardiovascular death and severe thrombotic events.47 Secondary polycythemias are treated with phlebotomy in conditions such as chronic lung disease, cyanotic heart disease, and Patients with hypertension after undergoing a kidney transplant and erythrocytosis who do not respond to proper ACEI or ARB doses.48
Hydroxyurea
Hydroxyurea is frequently used as a last-resort treatment. Studies, including one by the Polycythemia Vera Study Group, have shown that it can reduce the risk of blood clots when compared to phlebotomy alone.49 Despite concerns, there is no evidence that hydroxyurea use increases the incidence of leukemia.50 Phlebotomy is not feasible or practical or when other symptoms such as severe thrombocytosis or intractable itching are present, it is used. The usual dose is 500 – 1500 mg per/day, calibrated to keep platelet levels below 500,000/mcL while maintaining absolute neutrophil counts over 2000/microliter.
Ruxolitinib
Ruxolitinib, a JAK2 inhibitor, is used when individuals do not respond to or tolerate hydroxyurea. The COMFORT investigations found evidence for its use in myeloproliferative disorders, with significant reductions in spleen size, decreased symptoms, and enhanced survival compared to placebo or the best available treatment.51 However, the use of Ruxolitinib may increase the risk of anemia and thrombocytopenia. For polycythemia vera, the typical dosage is 10 mg taken two times a day; however, it may need to be lowered if hemoglobin levels fall below 12 g/dl. If hemoglobin levels fall below 8 g/dl, dosage should be temporarily halted.
Low-flow oxygen therapy
In COPD patients, the disease can lead to hypoxia or low oxygen levels. This can stimulate the production of erythrocytes in an attempt to increase the oxygen-carrying capacity of the blood, leading to polycythemia. Low-flow oxygen therapy can help to increase the oxygen levels in the blood, reducing the stimulus for erythrocyte production and potentially improving symptoms associated with polycythemia.71,72
Low-Dose Aspirin
The first PVSG study found that patients who only received phlebotomy treatment had a higher risk of developing blood clots during the first three years of treatment. This suggests that using antiplatelet or anticoagulant drugs may be beneficial. While earlier experiments employing greater dosages of aspirin resulted in unacceptable gastrointestinal hemorrhage, subsequent investigations discovered that lower doses of aspirin may be safely employed.52 When microvascular symptoms are not well treated after obtaining the targeted erythrocytes or when additional cardiovascular risk factors are present, aspirin is now recommended. The recommended dose is low, ranging from 40 to 100 mg/day.
Treatment of Polycythemia during Pregnancy
In most situations, the conventional therapy for polycythemia during pregnancy is low-dose aspirin and phlebotomy. Some high-risk women may also require the inclusion of pegylated interferon-alpha.53
Treatment of Neonatal Polycythemia
The majority of newborn polycythemia patients do not require therapy. However, in situations of hyperviscosity, an exchange transfusion may be required.73
Cytoreductive therapy
Cytoreductive therapy is used to treat primary polycythemia, or polycythemia vera, a clonal disorder affecting hematopoietic stem/progenitor cells. The goal of this therapy is to decrease the production of red blood cells in the bone marrow and maintain a consistent blood cell count. In contrast to phlebotomy, which lowers the blood cell count temporarily before it rises again, cytoreductive therapy aims to prevent fluctuations in the blood cell count. Givinostat has the potential to become the new standard first-line cytoreductive treatment for polycythemia vera patients, replacing hydroxyurea and changing the current therapeutic guidelines for managing PV.74,75
Management of polycythemic COPD consequences
Table 4: Management of polycythemic COPD consequences.
| S.No | Consequences of Polycythemia in COPD | Treatment |
| 1. | Increased Blood Viscosity. 54,55 | Adequate Hydration |
| Low-dose Aspirin | ||
| 2. | Venous Thromboembolism (VTE). 56-58 | Phlebotomy (Blood Removal) |
|
Anticoagulant Medications. 64 |
||
|
Compression Stockings |
||
| 3. | Pulmonary Hypertension.56-61 | Inferior Vena Cava Filter. 59 |
| Oxygen Therapy (at least 16 hr/day) | ||
| Pulmonary Vasodilators | ||
| Diuretics | ||
| 4. | Increased Risk of Cardiovascular Events.54 | Endothelin Receptor Antagonists.63 |
| Lifestyle Modifications (e.g., Exercise,
Diet, Smoking Cessation) |
||
| 5. | Increased Risk of Thrombotic Complications.57,58 | Medications for Cardiovascular Health. |
| Anticoagulant Medications.65 | ||
| 6. | Increased Risk of Stroke. 60,61 | Phlebotomy (Blood Removal) |
| Anticoagulant Medications | ||
| 7. | Impaired Oxygen Transport | Supplemental Oxygen Therapy |
| Bronchodilators and Inhaled Medications.66 |
Dietary Modulation
Dietary modulation of oxidative stress in chronic obstructive pulmonary disease (COPD) patients is a topic of interest in the medical community.82 Barnes et al., discusses the role of oxidative stress in COPD and how dietary modulation can help reduce it. The article highlights that there is a marked increase in oxidative stress in the lungs of patients with COPD, as measured by increased exhaled 8-isoprostane, ethane, and hydrogen peroxide in the breath. The lung may be exposed to exogenous oxidative stress from cigarette smoking and indoor or outdoor air pollution and to endogenous oxidative stress from reactive oxygen species released from activated inflammatory cells, particularly neutrophils and macrophages, in the lungs. Oxidative stress in COPD may be amplified by a reduction in endogenous antioxidants and poor intake of dietary antioxidants.83
The review article suggests that dietary modulation can help reduce oxidative stress in COPD patients. Protective roles have emerged for healthy dietary patterns to include consumption of antioxidants, anti-inflammatory foods like fruits and vegetables, other sources of dietary fiber (e.g., legumes), certain micronutrients (vitamins D and E), dietary supplements (i.e., N-acetylcysteine), and avoidance of unhealthful fats and simple carbohydrates.
Conclusion
Polycythemia in COPD is a significant medical condition characterized by an excess of erythrocyte due to chronic low oxygen levels. While it may offer some protective effects for COPD patients on long-term oxygen therapy, it can also lead to severe complications and increased risks, including pulmonary embolism and cardiovascular events. This review highlights the epidemiology, risk factors, and consequences of polycythemia in COPD patients, shedding light on the pathophysiology and potential therapeutic targets. Proper management of polycythemic COPD involves addressing increased blood viscosity, managing thromboembolic risks, and optimizing cardiovascular health. Treatments such as phlebotomy, low-dose aspirin, and oxygen therapy are vital in reducing complications and improving outcomes. However, further research is needed to enhance understanding and refine the care for individuals with polycythemic COPD, striving to mitigate the complications associated with this condition and ultimately improve patients’ quality of life and long-term prognosis.
Acknowledgement
We would like to express our sincere gratitude to all the researchers and scientists whose work has contributed to our understanding of Polycythemia-induced Consequences in COPD. Their insights into the pathophysiology and therapeutic targets have been invaluable in the preparation of this review. We would also like to thank our colleagues and peers for their constructive feedback and support throughout the writing process.
Conflicting Interest
There is no conflict of interest
Funding Sources
There are no funding sources
References
- Wikipedia Contributors. Polycythemia. Wikipedia. Published November 30, 2019. Accessed on July 11, 2023.
- Zhang J, DeMeo DL, Silverman EK, et al. Secondary polycythemia in chronic obstructive pulmonary disease: prevalence and risk factors. BMC Pulm Med. 2021;21(1):235. Published 2021 Jul 14. doi:10.1186/s12890-021-01585-5.
- Barbui T, Thiele J, Gisslinger H, Finazzi G, Vannucchi AM, Tefferi A. The 2016 revision of WHO classification of myeloproliferative neoplasms: Clinical and molecular advances. Blood Rev. 2016;30(6):453-459. doi: 10.1016/j.blre.2016.06.001.
- Martin L. Hematocrit levels: Definition, low levels, high levels, and more. www.medicalnewstoday.com. Published May 25, 2021. Accessed on July 10, 2023.
- Foster LJ, Corrigan K, Goldman AL. Effectiveness of Oxygen Therapy in Hypoxic Polycythemic Smokers. Chest. 1978;73(5):572-576.
- Peter M.A. Calverley, Leggett RJ, McElderry LA, Flenley DC. Cigarette Smoking and Secondary Polycythemia in Hypoxic Cor Pulmonale1,2. The American review of respiratory disease. 2015;125(5):507-510.
- Medrek SK, Sharafkhaneh A, Spiegelman AM, Kak A, Pandit LM. Admission for COPD Exacerbation Is Associated with the Clinical Diagnosis of Pulmonary Hypertension: Results from a Retrospective Longitudinal Study of a Veteran Population. COPD: Journal of Chronic Obstructive Pulmonary Disease. 2017;14(5):484-489.
- Sekine Y, Behnia M, Fujisawa T. Impact of COPD on pulmonary complications and on long-term survival of patients undergoing surgery for NSCLC. Lung Cancer. 2002;37(1):95-101.
- McGrath RL, Weil JV. Adverse effects of normovolemic polycythemia and hypoxia on hemodynamics in the dog. 1978;43(5):793-798.
- Nihill MR, McNamara DG, Vick RL. The effects of increased blood viscosity on pulmonary vascular resistance. 1976;92(1):65-72.
- Shaheen NA, Alqahtani AA, Assiri H, Alkhodair R, Hussein MA. Public knowledge of dehydration and fluid intake practices: Variation by participants’ characteristics. BMC Public Health. 2018;18(1):1-8.
- Cote C, Zilberberg MD, Mody SH, Dordelly LJ, Celli B. Haemoglobin level and its clinical impact in a cohort of patients with COPD. Eur Respir J. 2007;29(5):923-929. doi:10.1183/09031936.00137106.
- Ashraf Fawzy MD. Polycythemia is Associated with Lower Incidence of Severe COPD Exacerbations in the SPIROMICS Study. Chronic Obstructive Pulmonary Diseases:Journal of the COPD Foundation. 8(3):326-335. Accessed July 10, 2023.
- Polycythemia is Associated with Lower Incidence of Severe COPD Exacerbations in SPIROMICS Study. Chronic Obstructive Pulmonary Diseases: Journal of the COPD Foundation. Published online 2021.
- Fischer LG, Aken HV, Bu [Combining Diaeresis] rkle H. Management of Pulmonary Hypertension: Physiological and Pharmacological Considerations for Anesthesiologists. Anesthesia & Analgesia. 2003;96(6):1603-1616.
- Alkhedaide AQ. Tobacco smoking causes secondary polycythemia and a mild leukocytosis among heavy smokers in Taif City in Saudi Arabia. Saudi Journal of Biological Sciences. 2020;27(1):407-411.
- Gordana Pavliša, Veljko Vrbanić, Vesna Kušec, Branimir Jakšić. Erythropoietin response after correction of severe hypoxaemia due to acute respiratory failure in chronic obstructive pulmonary disease patients. 2004;106(1):43-51.
- Balter M, Daniak N, Chapman KR, Sorba S, Rebuck AS. Erythropoietin Response to Acute Hypoxemia in Patients with Chronic Pulmonary Disease. 1992;102(2):482-485.
- Pillai AA, Babiker HM. Polycythemia. PubMed. Published 2020.
- Mayo Clinic. Pulmonary embolism: Take measures to lower your risk – Symptoms and causes. Mayo Clinic. Published 2018.
- Shankarappa RK, Math RS, Srinivas Papaiah, Yeriswamy Mogalahally Channabasappa, Satish Karur, Nanjappa MC. Free floating right atrial thrombus with massive pulmonary embolism:near catastrophic course following thrombolytic therapy. 2013;65(4):460-463.
- Phillippe HM. Overview of venous thromboembolism. Am J Manag Care. 2017;23(20 Suppl): S376-S382.
- Tritschler T, Kraaijpoel N, Le Gal G, Wells PS. Venous Thromboembolism: Advances in Diagnosis and Treatment [published correction appears in JAMA. 2018 Dec 18;320(23):2486]. JAMA. 2018;320(15):1583-1594. doi:10.1001/jama.2018.14346.
- Mandras SA, Mehta HS, Vaidya A. Pulmonary Hypertension: A Brief Guide for Clinicians. Mayo Clin Proc. 2020;95(9):1978-1988. doi: 10.1016/j.mayocp.2020.04.039.
- Hoeper MM, Ghofrani HA, Grünig E, Klose H, Olschewski H, Rosenkranz S. Pulmonary Hypertension. Dtsch Arztebl Int. 2017;114(5):73-84. doi:10.3238/arztebl.2017.0073.
- Polycythemia vera classification – wikidoc. www.wikidoc.org. Accessed July 22, 2023.
- Prchal JT. Primary polycythemias. Current Opinion in Hematology. 1995;2(2):146-152.
- Mithoowani S, Laureano M, Crowther MA, Hillis CM. Investigation and management of erythrocytosis. CMAJ. 2020;192(32): E913-E918. doi:10.1503/cmaj.191587.
- Stefanini M, Urbas JV, Urbas JE. Gaisböck’s syndrome: its hematologic, biochemical and hormonal parameters. Angiology. 1978;29(7):520-533. doi:10.1177/000331977802900703.
- Appelbaum FR. Age and acute myeloid leukemia. Blood. 2006;107(9):3481-3485.
- Boddu P, Kantarjian HM, Garcia-Manero G, et al. Treated secondary acute myeloid leukemia: a distinct high-risk subset of AML with adverse prognosis. Blood Advances. 2017;1(17):1312-1323.
- Annibale B, Capurso G, Chistolini A, et al. Gastrointestinal causes of refractory iron deficiency anemia in patients without gastrointestinal symptoms. The American Journal of Medicine. 2001;111(6):439-445.
- Roque Pifarré. THROMBOSIS AND CARDIOVASCULAR DISEASE. 1998;82(3):511-522.
- Rautou PE. Budd-Chiari syndrome. Clinical Liver Disease. 2014;3(6):133-136.
- Risso A, Fabbro D, Damante G, Antonutto G. Expression of fetal hemoglobin in adult humans exposed to high altitude hypoxia. Blood Cells, Molecules, and Diseases. 2012;48(3):147-153.
- POLVI H, PIRHONEN J, ERKKOLA R. The hemodynamic effects of maternal hypo and hyperoxygenation in healthy term pregnancies. Obstetrics & Gynecology. 1995;86(5):795-799.
- Duley L, Dorling J, Gyte G. When should the umbilical cord be clamped? BMJ. Published online September 9, 2015:h4206.
- Mccausland Am, Holmes F, Schumann Wr. Management of cord and placental blood and its effect upon the newborn-part I. Obstetrical & Gynecological Survey. 1950;5(2):178-179.
- Slaghekke F, van den Wijngaard JPHM, Akkermans J, et al. Intrauterine transfusion combined with partial exchange transfusion for twin anemia polycythemia sequence: Modeling a novel technique. Placenta. 2015;36(5):599-602.
- Suzuki T, Kagami K, Mitani Y, Yamazaki R, Ono M, Fujiwara H. Twin anemia‐polycythemia sequence with blood chimerism in monochorionic dizygotic opposite‐sex twins. Journal of Obstetrics and Gynaecology Research. 2019;45(6):1201-1204.
- Petit RD, Warburton RR, Ou LC, Hill N. Pulmonary vascular adaptations to augmented polycythemia during chronic hypoxia. 1995;79(1):229-235.
- Nihill MR, McNamara DG, Vick RL. The effects of increased blood viscosity on pulmonary vascular resistance. 1976;92(1):65-72.
- Bhardwaj B, Jacob D, Sharma A, Ghanimeh MA, Baweja P. Pulmonary vein thrombosis in a patient with polycythemia vera. World Journal of Cardiology. 2016;8(11):684-688.
- Huang P, Li Y. Polycythemia vera presenting with pulmonary embolism and splenic infarction: a case report. Journal of International Medical Research. 2022;50(1):030006052110728.
- Brandt M, Giokoglu E, Garlapati V, et al. Pulmonary Arterial Hypertension and Endothelial Dysfunction Is Linked to NADPH Oxidase-Derived Superoxide Formation in Venous Thrombosis and Pulmonary Embolism in Mice. Oxidative Medicine and Cellular Longevity. 2018; 2018:1-10.
- Berk PD, Goldberg JD, Donovan PB, Fruchtman SM, Berlin NI, Wasserman LR. Therapeutic recommendations in polycythemia vera based on Polycythemia Vera Study Group protocols. Semin Hematol. 1986;23(2):132-143.
- Marchioli R, Finazzi G, Specchia G, Masciulli A, Mennitto MR, Barbui T. The CYTO-PV: A Large-Scale Trial Testing the Intensity of CYTOreductive Therapy to Prevent Cardiovascular Events in Patients with Polycythemia Vera. Thrombosis. 2011; 2011:794240. doi:10.1155/2011/794240.
- Assi TB, Baz E. Current applications of therapeutic phlebotomy. Blood Transfus. 2014;12 Suppl 1(Suppl 1): s75-s83. doi:10.2450/2013.0299-12.
- Fruchtman SM, Mack K, Kaplan ME, Peterson P, Berk PD, Wasserman LR. From efficacy to safety: a Polycythemia Vera Study group report on hydroxyurea in patients with polycythemia vera. Semin Hematol. 1997;34(1):17-23.
- Tefferi A, Rumi E, Finazzi G, et al. Survival and prognosis among 1545 patients with contemporary polycythemia vera: an international study. Leukemia. 2013;27(9):1874-1881. doi:10.1038/leu.2013.163.
- Harrison C, Kiladjian JJ, Al-Ali HK, et al. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med. 2012;366(9):787-798. doi:10.1056/NEJMoa1110556.
- Landolfi R, Marchioli R, Kutti J, et al. Efficacy and safety of low-dose aspirin in polycythemia vera. N Engl J Med. 2004;350(2):114-124. doi:10.1056/NEJMoa035572.
- Tefferi A, Vannucchi AM, Barbui T. Polycythemia vera treatment algorithm 2018. Blood Cancer J. 2018;8(1):3. Published 2018 Jan 10. doi:10.1038/s41408-017-0042-7.
- Doi T, Sakurai M, Hamada K, et al. Plasma volume and blood viscosity during 4 h sitting in a dry environment: effect of prehydration. 2004;75(6):500-504.
- Corrigan T, O’Malley L, Bailey D, et al. Changes in the Physical and Mechanical Properties of Human Blood with Sustained Prophylactic Use of Acetylsalicylic Acid (Aspirin)—A Rheological Study. Open Journal of Fluid Dynamics. 2021;11(04):167-176.
- Alizadeh RF, Sujatha-Bhaskar S, Li S, Stamos MJ, Nguyen NT. Venous thromboembolism in common laparoscopic abdominal surgical operations. The American Journal of Surgery. 2017;214(6):1127-1132.
- Schastlivtsev I, Lobastov K, Tsaplin S, et al. Rivaroxaban in the treatment of upper extremity deep vein thrombosis: A single-center experience and review of the literature. Thrombosis Research. 2019; 181:24-28.
- Lewis CE, Antoine J, Mueller C, Talbot WA, Swaroop R, Edwards WSterling. Elastic compression in the prevention of venous stasis. The American Journal of Surgery. 1976;132(6):739-743.
- Strauss EJ, Egol KA, M. Alaia, D. Flemming Hansen, Bashar M, Steiger D. The use of retrievable inferior vena cava filters in orthopaedic patients. 2008;90-B (5):662-667.
- Chaouat A, Kraemer JP., Canuet M, et al. Hypertension pulmonaire des affections respiratoires chroniques. La Presse Médicale. 2005;34(19):1465-1474.
- Haj RM, Cinco JE, Mazer CD. Treatment of pulmonary hypertension with selective pulmonary vasodilators. Current Opinion in Anaesthesiology. 2006;19(1):88-95.
- Fukuda M, Kimura G. Pathophysiology of Antihypertensive Therapy with Diuretics. Hypertension Research. 2006;29(9):645-653.
- Giannessi D, Del Ry S, Vitale RL. The role of endothelins and their receptors in heart failure. Pharmacological Research. 2001;43(2):111-126.
- Yau Y, Lee PD, Wilson A, Jenkins A. Prosthetic valve endocarditis: what is the evidence for anticoagulant therapy? 2011;41(11):795-797.
- Virdee MS, Stewart D. Optimizing the use of oral anticoagulant therapy for atrial fibrilation in primary care: a pharmacist-led intervention. International Journal of Clinical Pharmacy. 2017;39(1):173-180.
- Cukier A, Ferreira CAS, Stelmach R, Ribeiro M, Cortopassi F, Calverley PMA. The effect of bronchodilators and oxygen alone and in combination on self-paced exercise performance in stable COPD. Respiratory Medicine. 2007;101(4):746-753.
- Peter M.A. Calverley, Leggett RJ, McElderry LA, Flenley DC. Cigarette Smoking and Secondary Polycythemia in Hypoxic Cor Pulmonale1,2. The American review of respiratory disease. 2015;125(5):507-510.
- Koenig W, Sund M, Ernst E, Matrai A, Keil U, Rosenthal J. Is Increased Plasma Viscosity A Risk Factor for High Blood Pressure? Angiology. 1989;40(3):153-163. doi: https://doi.org/10.1177/000331978904000301.
- Spector JI, Zaroulis CG, Pivacek LE, Emerson CP, Valeri CR. Physiologic effects of normal-or low-oxygen-affinity red cells in hypoxic baboons. 1977;232(1):H79-H84.
- Agarwal D, Mittal A. Hematology – A diagnostic tool in cases of splenomegaly. International Journal of Biomedical and Advance Research. 2016;7(9):413.
- Iyer VN. Low-dose oxygen therapy in COPD patients. Current Opinion in Pulmonary Medicine. 2018;24(2):187-190.
- Esteban A, Cerda E, Cal MADL, Lorente JA. Hemodynamic Effects of Oxygen Therapy in Patients with Acute Exacerbations of Chronic Obstructive Pulmonary Disease. Chest. 1993;104(2):471-475.
- Hakanson DO, Oski FA. 58 NEONATAL HYPERVISCOSITY SYNDROME: LONG-TERM BENEFIT OF PARTIAL PLASMA EXCHANGE TRANSFUSION. Pediatric Research. 1981; 15:449-449.
- Chifotides HT, Bose P, Verstovsek S. Givinostat: an emerging treatment for polycythemia vera. Expert Opinion on Investigational Drugs. 2020;29(6):525-536.
- A Barbasch, Higby DJ, Brass CA, et al. High-dose cytoreductive therapy with autologous bone marrow transplantation in advanced malignancies. 1983;67(2):143-148.
- Boyer L, Chaar V, Pelle G, et al. Effects of polycythemia on systemic endothelial function in chronic hypoxic lung disease. 2011;110(5):1196-1203.
- LEWIS CS, SAMUELS AJ, DAINES MC, HECHT HH. Chronic Lung Disease, Polycythemia and Congestive Heart Failure. Circulation. 1952;6(6):874-887.
- Alkhedaide AQ. Tobacco smoking causes secondary polycythemia and a mild leukocytosis among heavy smokers in Taif City in Saudi Arabia. Saudi Journal of Biological Sciences. 2020;27(1):407-411.
- Chetty KG, Brown SJ, Light RW. Improved exercise tolerance of the polycythemic lung patient following phlebotomy. 1983;74(3):415-420.
- Sethi GS, Dharwal V, Naura AS. Immunological Basis of Oxidative Stress-Induced Lung Inflammation in Asthma and COPD. Oxidative Stress in Lung Diseases. Published online 2019:195-223.
- Wiegman CH, Li F, Ryffel B, Togbe D, Chung KF. Oxidative Stress in Ozone-Induced Chronic Lung Inflammation and Emphysema: A Facet of Chronic Obstructive Pulmonary Disease. Frontiers in Immunology. 2020;11.
- De Batlle J, Barreiro E, Romieu I, et al. Dietary modulation of oxidative stress in chronic obstructive pulmonary disease patients. Free Radical Research. 2010;44(11):1296-1303.
- Barnes PJ. Oxidative Stress in Chronic Obstructive Pulmonary Disease. Antioxidants. 2022;11(5):965. doi: https://doi.org/10.3390/antiox11050965.

This work is licensed under a Creative Commons Attribution 4.0 International License.





