Manuscript accepted on : 23-11-2023
Published online on: 22-12-2023
Plagiarism Check: Yes
Reviewed by: Dr. Bhavana Gundavarapu
Second Review by: Dr. Abhishek Raj
Final Approval by: Dr. Hifzur R. Siddique
Formulation of Drug-polyelectrolyte Complex for Enhancement of Solubility of Hydrochlorthiazide
Swati Talele1 , Priyanka Kajale 2, Shital Bhil1, Rushikesh Patil1, Amit Gaikwad1 and Laxmikant Borse1
, Priyanka Kajale 2, Shital Bhil1, Rushikesh Patil1, Amit Gaikwad1 and Laxmikant Borse1
1Department of Pharmaceutics, Sandip Institute of Pharmaceutical Sciences, Sandip Foundation, Nashik, Maharashtra, India.
2Sandip Institute of pharmaceutical sciences, Nashik, Maharashtra, India
Corresponding Author E-mail: swatitalele77@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/3176
ABSTRACT: The objective of this work is to formulate Nanoplex formulation which enhances the drug solubility and also to enhance the dissolution rate of the formulation which were prepared. Complex nanoplex is formed when positively or negatively charged molecules of the drug react with oppositely charged polyelectrolyte. In this study, we use hydrochlorothiazide as a study drug and dextran sulphate as anionic polyelectrolyte. During the procedure crystalline nanoplex is formed due to electrostatic interactions between API and polyelecrolyte. The nanoplex shaped by this method upgrades dissolvability and disintegration rate of inadequately water solvent medication. It was assessed for complexation effectiveness, % yield, molecule size, zeta potential, SEM, TEM, XRD, soaked solvency study and so forth. This gives particles having molecule size in the scope of 100-200nm.Amongst different ratio, 1:1 showed highest Complexation efficiency as 96.3%, % yield and drug loading as 82 % and 77.69 % respectively. Solubility and stability of hydrochlorothiazide, poorly water-soluble molecule can be increased with formulation of hydrochlorothiazide nanoplex.
KEYWORDS: Dextran; Hydrochlorothiazid; Nanoplex; Polyelectrolyte; Solubility Enhancement
Download this article as:| Copy the following to cite this article: Talele S, Kajale P, Bhil S, Patil R, Gaikwad A, Borse L. Formulation of Drug-polyelectrolyte Complex for Enhancement of Solubility of Hydrochlorthiazide. Biotech Res Asia 2023;20(4). |
| Copy the following to cite this URL: Talele S, Kajale P, Bhil S, Patil R, Gaikwad A, Borse L. Formulation of Drug-polyelectrolyte Complex for Enhancement of Solubility of Hydrochlorthiazide. Biotech Res Asia 2023;20(4). Available from: https://bit.ly/3tw0VQD |
Introduction
The Drug delivery via oral route is a very common and most utilized method of drug delivery due to easy and convenient intake of formulation. Orally controlled medications are totally assimilated just when they demonstrate reasonable solvency in gastric medium and such medications indicates great bioavailability. Nanoscience and nanotechnology deals with the extremely small things which can be used in biology, physics, chemistry, material science, and engineering. In particular, nanotechnology accompanied at the nanoscale i.e., about 1 to 100 nanometers and with the process which occurs at the molecular level. Red blood corpuscles (RBC), DNA, viruses, and water molecules are some of the structures with nano-dimensions. Nanotechnology is an emerging technology which comprises of multidisciplinary approach to basic and applied scientific principles. Nanotechnology has an influential effect on numerous fields of medicines such as immunology, endocrinology, oncology, ophthalmology, cardiology, and pneumology.
Lot of current research in API formulation has focused on improvement of water solubility in various poorly water soluble drugs1,2,3. The solubility of a medication decides the dissolution behavior of an Active pharmaceutical ingredient (API) in the definition and also remedial adequacy of the medication. The solubility constrained assimilation (characteristic dissolvability controlled), the plan methodology is usually Used to upgrade the solvency of the API and this methodology additionally incorporates the utilization of surfactants in the definition (strong scatterings), non-crystalline materials, and distinctive salt types of API, however current studies demonstrate, improved water solubility and dissolution rate with nanoplex formulation.4
Amphoteric drug molecules are broken up to ionized components in a media where drug pKa is nearer to medium pH. Ionized fraction is largely water insoluble, a nanoplex formulation allows ionized fraction of the drug to combine with oppositely charged polyelectrolyte to render the molecules unionized thus increases the water solubility. Electrostatic interactions between the API molecule and polyelectrolyte molecule prevents precipitation of the drug thus forming individual polyelectrolyte-nanoparticle complex. In the present study improvement of solubility of hydrochlorothiazide, BCS class IV drug was attempted with the novel nanoplex formulation methodology as delineated in fig. 15 .
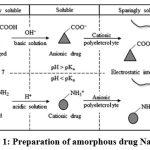 |
Figure 1: Preparation of amorphous drug Nanoplex
|
The drug molecules are combination of rapid precipitation and long-lasting electrostatic interactions between the drug and the polyelectrolyte. An amorphous drug-polyelectrolyte nanoparticle complex is formed as a result.
Intermolecular revulsions between like-charged PE chains inhibit three-dimensional interactions in the absence of salt. The same can be said for the drug, with charged drug molecules repelling each other in the absence of salt, preventing a compact assembly of drug molecules. The drug’s higher solubility in a lower ionic strength solution contributes to the lack of inter-drug hydrophobic interactions (Singh, Caram-Lelham, 1998). However, the salt’s charge-shielding effect must not be too strong, or it will
(i)inhibit the electrostatic interactions between the drug molecules and the PE involved in complex formation, or
(ii)neutralize the charge interactions between the drug and the PE in the already formed complex, resulting in decomplexation.
Rationale of nanoplex Formulation given below:
Nanoplex preparation method is simple.
In this, only mixing of drug and polyelectrolyte is required.
There is no need to use heavy solvents.
It is a fast process.
The energy required for the formulation of nanoplex is minimum than the other NPs.
Also, for nanoplex formulation, there is no need to use a sophisticated instrument.
Aim of this research is to increases the solubilizing property of hydrochlorothiazide by novel approach as nanoplex.
Material and Methods
Materials
Glenmark Pharmaceutical, Nashik, provided a free sample of the drug (hydrochlorothiazide) (fig.4). Himedia Laboratories Ltd, Mumbai, supplied the Pluronic F 68, dextran sulphate. Modern Science Pvt. Ltd., Nashik, also supplied glacial acetic acid and sodium chloride.
Preparation of Nanoplex: 4, 5, 6, 7, 8
Drug suspended in sodium chloride and glacial acetic acid was mixed with equivalent weight by volume of dextran solution (10mg/ml) to avoid precipitation. The resultant solution was treated with pluronic F68 a surfactant molecule. Thus, obtaining a stabilize suspension of nanoplex. It was ensured that no white precipitate formed in the mixture. The suspension was allowed to form nanoplex complexes over 3 hours. The suspension there after was washed with pluronic F68 to remove excess of drug molecule. The resulting suspension was freeze dried to obtained dry powder.
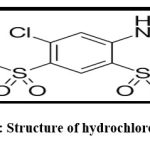 |
Figure 2: Structure of hydrochlorothiazide
|
Physical characterization of Hydrochlorothiazide nanoplex suspension:
Complexation Efficiency was analyzed. It is the proportion of drug molecule forming the nanoplex complex with respect to initial amount of drug molecule and was calculated by measuring optical density of supernatant liquid after first centrifugation of nanoplex suspension. Production yield was as well analyzed. It is ratio of weight of nanoplex formulation formed after freeze drying to initially addtotal amount of drug and polyelectrolyte.
% production yield = wt. of Nanoplex/ drug + polyelectrolyte * 100
Drug loading can be calculated as the effective amount of drug molecule present in nanoplex powder and is derived by absorbance of solution of 5mg nanoplex powder in 20 mg ethanol after centrifugation and filtration.
Physical Characterization of Nanoplex Powder
The FT-IR spectrum of drug and polymer were recorded by FTIR spectrophotometer (Jasco FT-IR -460 plus). Weighed samples of drug and polymers were placed in FTIR spectrophotometer and scanned in between wave number 500-4000 cm-1. Photon correlation spectroscopy was used to analyse the particle size of nanoparticle powder on a particle size analyzer (DelsaNanoC, Particle analyzer) with water as the dispersion medium and quarts cuvettes as the sample holders. The particle size was determined by scanning the sample 100 times. Photon zettaliter was used to calculate the zeta potential of formulation ingredients and formulation (Nanophox). Before analysis, the samples were diluted ten times with solvent. As a sample holder, a fiber cuvette was used. DSC was carried out using a DSC instrument. (Mettler — Toledo, India, DSC 30S) 2 mg sample was placed in shallow aluminium sample holder and heated at the rate of 10°c/mm over temp range 40°C – 300°C. Powder X-ray diffraction patterns were determined for samples using a Powder X-ray diffractometer (Bruker, D- 08 Advance, Germany) at a scan rate of 10 min -1. The value of 2 ranges from 10 to 80. This provides information about the nature of the sample. The sample’s surface morphology was investigated (Hitachi S-4800 Type II).
Saturation solubility study
Excess drug was added to nanoplex suspension with phosphate buffer of pH 6.8 in 5ml glass vial. The vial were subjected to undergo centrifugation after the equilibrium was achieved. The resulting supernatant liquid was removed by filtration. Remaining solution was analyzed for saturation solubility study using spectrophotometry study.
Dissolution rate study
The study drug solution and nanoplex suspension was analyzed for dissolution rate study using dialysis bag method. At pH 6.8 rpm and 37% temperature with 15 of sampling interval. The dissolution was estimated with UV Spectroscopy.
Stability testing
For one month, 50 mg of nanoplex powder was placed in an environmental test chamber at 75% RH and 25°C temperature and tested for drug content.
Result and Discussion
Complexation Efficiency, % Yield and Drug Loading
Hydrochlorothiazide nanoplexes with drug: polyelectrolyte ratios of 1:1 were found to be higher than those with drug: polyelectrolyte ratios of 1:0.5 and 1:1.5, as shown in table 1. The ratio 1:1 demonstrated the highest Complexation efficiency, yield, and drug loading, with values of 96.3%, 82%, and 77.69%, respectively. As a result, when the drug and polyelectrolyte are at the same concentration, we can confidently state that nanoplex produces good results in terms of complexation efficiency, percentage yield, and drug loading.
Table 1: Drug: polyelectrolyte optimization of nanoplex
|
Sr. No. |
D:P ratio |
CE (%) |
% Yield |
Drug loading (%) |
|
1. |
1:0.5 |
86.7 |
78.29 |
51.36 |
|
2. |
1:1 |
96.3 |
82 |
77.69 |
|
3. |
1:1.5 |
91.2 |
44.54 |
41.08 |
FT-IR of Nanoplex
Figures 3 and 4 show the FT-IR spectrums of HCTZ pure drug and Dextran sulphate, respectively. The FT-IR of nanoplex shows peaks of both HCTZ and dextran sulphate, indicating that it contains both HCTZ and dextran sulphate. Figure 5 represents FT-IR spectra of formulated Nanoplex. FT-IR spectra of nanoplex shows presence of main peaks of both HCTZ and dextran sulfate at 778.31 cm-1 of C-Cl stretching, 1600.99cm-1 of C=C aromatic stretching, 3362.07cm-1 of NH2 stretching 1059.93cm-1of S=O stretching, 1558.55cm-1of N-H bending. FT-IR spectra of nanoplex shows absence of peak at 3362.07 cm-1 indicates complex formation of Hydrochlorothiazide and dextran sulphate by H bonding of NH2 group with dextran.
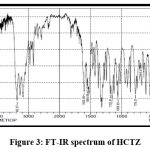 |
Figure 3: FT-IR spectrum of HCTZ
|
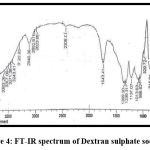 |
Figure 4: FT-IR spectrum of Dextran sulphate sodium.
|
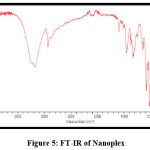 |
Figure 5: FT-IR of Nanoplex
|
Particle Size Analysis:7, 8
Figure 6 shows that the wavelength was 110.77 nm. The degree of particle size distribution is indicated by the polydispersibility index. A higher polydispersibility index value suggests a wide particle magnitude arrangement, whereas a tight size distribution is required to avoid particle growth owing to Ostwald ripening and to ensure nanoplex stability. Long-term stability was observed in batches with reduced polydispersity levels. The polydispersibility index was found to be 0.80, indicating that the particles were monodisperse.
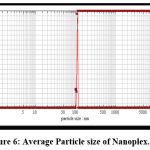 |
Figure 6: Average Particle size of Nanoplex.
|
Zeta Potential analysis:17
The surface properties of nanoplex were investigated using zeta potential analysis. The zeta potential is an important parameter for predicting nanoplex stability. The zeta potential of Hydrochlorothiazide nanoplex was found to be -32.45, as shown in fig. 7, indicating that the nanoplex is moderately stable.
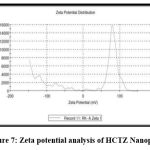 |
Figure 7: Zeta potential analysis of HCTZ Nanoplex.
|
DSC analysis
The DSC thermogram of HCTZ which shows an endothermic sharp peak at 273ºC indicates crystalline nature of HCTZ (fig. 8). Represents a broad exothermic peak at 218ºC indicates melting point of dextran sulfate (fig. 9). The DSC thermogram of nanoplex which shows absence of both exothermic and endothermic peak indicates successfully formation of complex of HCTZ and dextran sulfate as shown in (fig. 10).
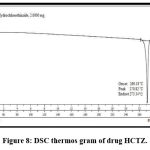 |
Figure 8: DSC thermos gram of drug HCTZ.
|
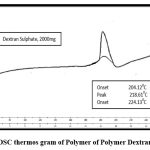 |
Figure 9: DSC thermos gram of Polymer of Polymer Dextran Sulphate.
|
 |
Figure 10: DSC of Nanoplex.
|
X-Ray Diffraction analysis
The XRD analysis provides information on the nature of the chemical. As indicated in the XRD of HCTZ as shown in fig.11, which shows distinct peaks with maximum intensity up to 3000, the medication is crystalline. Whereas in fig 12 intensity of peak somehow reduced interpreting amorphous nature of nanoplex of hydrochlorothiazide.
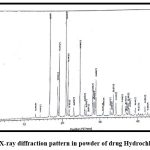 |
Figure 11: X-ray diffraction pattern in powder of drug Hydrochlorothiazide
|
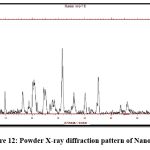 |
Figure 12: Powder X-ray diffraction pattern of Nanoplex.
|
FE-SEM Analysis
The FE-SEM analysis provides information about the structure of the particles. The plex structure was discovered by the FE-SEM images displayed in HCTZ figures 13 and 14. The images clearly show the nanoplex of HCTZ. The SEM image of nanoplex indicates the size of nanoplex is 5μm.
 |
Figure 13: SEM image if hydrochlorothiazide nanoplex
|
 |
Figure 14: SEM image of Hydrochlorothiazide Nanoplex.
|
Transmission Electron Microscopy (TEM)
TEM analysis gives idea about particle size. The TEM images of HCTZ Nanoplex are shown in (Fig. 15) and (Fig.16) which indicates size as 25 nm.
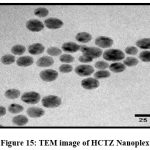 |
Figure 15: TEM image of HCTZ Nanoplex
|
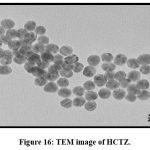 |
Figure 16: TEM image of HCTZ.
|
Saturation solubility study
In distilled water, PH 6.8 buffers, and 0.1N HCl solutions, the saturation solubility of the pure drug and nanoplex was studied. Table 2 demonstrates that medication solubility increased by more than 30 times.
Table 2: Saturation solubility of drug and nanoplex in different solvent.
|
Sr. No. |
Solvent |
Conc. (mg/ml) in Drug |
Conc. (mg/ml) in Nanoplex |
Enhancement Ratio |
|
1. |
Phosphate Buffer pH6.8 |
0.45 |
15.96 |
1:35.47 |
|
2. |
0.1N HCl |
0.60 |
16.89 |
1:28.15 |
|
3. |
Distilled Water |
0.30 |
3.892 |
1:12.97 |
Dissolution study
The pure medication and nanoplex were dissolved in a pH 6.8 buffer. As shown in table 3, drug and nanoplex demonstrate complete dissolution at the end of 210 and 150 minutes, respectively, implying that the dissolution rate was increased due to an increase in drug solubility in nanoplex formulation.
Table 3: % Drug release study of drug and nanoplex
|
Sr. No. |
Time (min) |
% CDR of Drug |
% CDR of Nanoplex |
|
1. |
0 |
0 |
0 |
|
2. |
15 |
8.804 |
11.142 |
|
3. |
30 |
8.948 |
32.216 |
|
4. |
45 |
26.111 |
43.896 |
|
5. |
60 |
26.767 |
60.689 |
|
6. |
90 |
27.521 |
74.725 |
|
7. |
120 |
28.252 |
84.412 |
|
8. |
150 |
28.613 |
91.118 |
|
9. |
180 |
31.679 |
92.34 |
|
10. |
210 |
39.177 |
98.91 |
Stability Study
The stability of optimal nanoplex formulation was supported at Relative Humidity 75% ± 5% & temperature 40ºC ± 2ºC. Initial Drug content was 61.2 %, after15 days storage drug content found was 60.7% and after 30 days storage it was 60.1 % respectively. Since there was no major change in drug content so we can definitely say that nanoplex formulation was stable for at least one month.
Drug release kinetics:18
The drug release kinetic gives information about drug release mechanism. There are various mathematical models which indicate mechanism of drug release i.e. Zero order kinetic model, first order kinetic model, Higuchi model for drug release, Korsemeyer peppas kinetic model, Matrix type model, Hixon Crowell kinetic model. The data of comparison of different models is shown in Table 4. From the observed R2 values, it was determined that API or drug releases profile of optimized batch followed Higuchi model and release patterns respectively.
The R2 value were in the following order viz, Higuchi > Zero order >Korsemeyarpeppas> First order >HixonCrowel Model. Higuchi defines drug release as a time-dependent diffusion process based on Fick’s law. The accumulated % medication release vs square root of time data were plotted. The model is used to investigate the release of pharmaceuticals that are both water soluble and poorly soluble from a range of matrices, including solids and semisolids.
Table 4: Drug release kinetics of nanoplex
|
Models |
R |
K |
|
Zero Order |
0.9392 |
0.7202 |
|
1st Order |
0.9959 |
-0.0156 |
|
Matrix type model |
0.9714 |
7.3210 |
|
Koresmeyers Peppas model |
0.9670 |
1.2485 |
|
Hixon Crowell model |
0.9951 |
-0.0039 |
Conclusion
When compared to the raw medication, the nanoplex formulation of hydrochlothiazide exhibits a 30 fold increase in solubility with a faster dissolving rate, resulting in increased bioavailability. The complexation method consists of a simple mixing of drug and polyelectrolyte solution, is wholly aqueous in nature, is rapid, and has a high complexation efficiency, drug loading, and manufacturing yield. Self-assembly resulted in the successful synthesis of stable hydrochlothiazide nanoplexes.
Acknowledgements
The authors of these article are grateful and thankfulss to Principal and Management of Sandip Institute of Pharmaceutical Sciences, Nashik for providing necessary facilities. I am as well thankful to Saif STIC laboratories, Cochin, India of SEM facility.
Conflict of Interest
The authors declare that they have no any conflicts of interest.
Funding Sources
there is no funding source.
References
- Dressman J, Reppas C. Drug solubility: how to measure it, how to improve it. Advanced drug delivery reviews. 2007;7(59):531-2.
CrossRef - Pouton CW. Formulation of poorly water-soluble drugs for oral administration: physicochemical and physiological issues and the lipid formulation classification system. European journal of pharmaceutical sciences. 2006 Nov 1;29(3-4):278-87.
CrossRef - Hörter D, Dressman JB. Influence of physicochemical properties on dissolution of drugs in the gastrointestinal tract. Advanced Drug Delivery Reviews. 1997 Apr 14;25(1):3-14
CrossRef - Chavan M. Pande V., A novel nanoplex mediated solubility and dissolution rate enhancement of CefpodoximeProxetil. Literati journal of Pharmaceutical Drug Delivery tech., 01 (02), 2015, 56-60.
- Rupali K., Shendge R., Pande V., A review of nanotechnology with an emphasis of nanoplexes. Brazilian journal of pharmaceutical sciences, April / june2015, vol. 51.
CrossRef - Cheow WS, Hadinoto K. Green amorphous nanoplex as a new supersaturating drug delivery system. Langmuir. 2012 Apr 17;28(15):6265-75.
CrossRef - Cheow WS, Hadinoto K. Green preparation of antibiotic nanoparticle complex as potential anti-biofilm therapeutics via self-assembly amphiphile–polyelectrolyte complexation with dextran sulfate. Colloids and Surfaces B: Biointerfaces. 2012 Apr 1;92:55-63
CrossRef - Cheow WS, Hadinoto K. Self-assembled amorphous drug–polyelectrolyte nanoparticle complex with enhanced dissolution rate and saturation solubility. Journal of colloid and interface science. 2012 Feb 1;367(1):518-26.
CrossRef - Wairkar SM, Gaud RS. Solid dispersions: Solubility enhancement technique for poorly soluble drugs. International Journal of Research in Pharmaceutical and Biomedical Sciences. 2013 Jul;4(3):847.
- Khan A, Iqbal Z, Shah Y, Ahmad L, Ullah Z, Ullah A. Enhancement of dissolution rate of class II drugs (Hydrochlorothiazide); a comparative study of the two novel approaches; solid dispersion and liqui-solid techniques. Saudi Pharmaceutical Journal. 2015 Nov 1;23(6):650-7.
CrossRef - Shendge RS, Pande VV, Kadnor NA, Jamdhade AA, Kadam RN. A FACILE APPROACH TO ENHANCE SOLUBILITY AND DISSOLUTION RATE OF NORFLOXACIN BY NANOPLEX. Pharmacophore. 2014 May 1;5(3).
- Dhumal RS, Biradar SV, Yamamura S, Paradkar AR, York P. Preparation of amorphous cefuroxime axetil nanoparticles by sonoprecipitation for enhancement of bioavailability. European Journal of Pharmaceutics and Biopharmaceutics. 2008 Sep 1;70(1):109-15.
CrossRef - Wang Y, Zhu L, Dong Z, Xie S, Chen X, Lu M, Wang X, Li X, Zhou W. Preparation and stability study of norfloxacin-loaded solid lipid nanoparticle suspensions. Colloids and Surfaces B: Biointerfaces. 2012 Oct 1;98:105-11.
CrossRef - Anitha A, Deepagan VG, Rani VD, Menon D, Nair SV, Jayakumar R. Preparation, characterization, in vitro drug release and biological studies of curcumin loaded dextran sulphate–chitosan nanoparticles. Carbohydrate Polymers. 2011 Mar 17;84(3):1158-64.
CrossRef - Bonoiu A, Mahajan SD, Ye L, Kumar R, Ding H, Yong KT, Roy I, Aalinkeel R, Nair B, Reynolds JL, Sykes DE. MMP-9 gene silencing by a quantum dot–siRNA nanoplex delivery to maintain the integrity of the blood brain barrier. Brain research. 2009 Jul 28;1282:142-55.
CrossRef - Ranjan A, Pothayee N, Seleem M, Jain N, Sriranganathan N, Riffle JS, Kasimanickam R. Drug delivery using novel nanoplexes against a Salmonella mouse infection model. Journal of Nanoparticle Research. 2010 Mar;12:905-14.
CrossRef - Woitiski C, Ribeiro A, Neufeld R, Veiga F. Bioencapsulation into nanoplex carrier for oral insulin delivery. InINTERNATIONAL WORKSHOP ON BIOENCAPSULATION 2007 (Vol. 15, pp. 720-744).
- Honary S, Zahir F. Effect of zeta potential on the properties of nano-drug delivery systems-a review (Part 1). Tropical journal of pharmaceutical research. 2013 May 9;12(2):255-64.
CrossRef - Ramteke KH, Dighe PA, Kharat AR, Patil SV. Mathematical models of drug dissolution: a review. Sch. Acad. J. Pharm. 2014 Jan;3(5):388-96.
- Talele SG, Gedam SS, Bakliwal AA. An Overview of Polyelectrolyte-Based Nanoplex. Green Chemistry and Sustainable Technology. 2020 Aug 23:137-55.
CrossRef
(Visited 63 times, 1 visits today)

This work is licensed under a Creative Commons Attribution 4.0 International License.





