Manuscript accepted on : 24-11-2023
Published online on: 06-12-2023
Plagiarism Check: Yes
Reviewed by: Dr. Sanchita Choubey
Second Review by: Dr. Ahmed Salah Naser
Final Approval by: Dr. Ali Mohamed Elshafei
Department of Biotechnology, Bodoland University, Kokrajhar, India.
Corresponding Author E-mail:jonaliowary@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/3196
ABSTRACT: Flax seed oil is a vegetable oils with a rich source of fatty acids especially omega-3-fatty acid α-linolenic acid. Extraction of flaxseed oil employes many techniques including solvent extraction. The present work estimated the effect of a total of six solvents, both polar and non-polar on the physio-chemical characteristics of flax seed oil. Amongst the polar solvents, isopropanol showed better iodine value and DPPH free radical scavenging values. Overall, hexane showed the highest oil content of 45% (w/w). This study indicated that hexane a non-polar solvent showed the most efficient indicators in terms of iodine value (IV) (25.3 ± 0.41 iodine/ 100 g oil), peroxide value (PV) (21.2 ± 0.7 meq KOH/kg), saponification value (SV) (203.0 mg KOH/g oil) as well as DPPH free radical scavenging activity at 76.7 ± 2.32 % compared to the other polar solvents.
KEYWORDS: Flaxseed; Oil; Polar, Non-Polar; Physiochemical Parameters; Solvent Extraction
Download this article as:| Copy the following to cite this article: Ekka S, Owary J. Extraction Effect of Polar and Non-Polar Solvents on the Physio Chemical Characteristics of Flaxseed Oil. Biotech Res Asia 2023;20(4). |
| Copy the following to cite this URL: Ekka S, Owary J. Extraction Effect of Polar and Non-Polar Solvents on the Physio Chemical Characteristics of Flaxseed Oil. Biotech Res Asia 2023;20(4). Available from: https://bit.ly/3GvglYd |
Introduction
Flaxseed or Linseed (Linum usitatissimum) is one of the oldest and most important crops cultivated in the world, especially in China and Canada1. Flax belongs to the Linaceae family. Flaxseed is used mainly for industrial and feed purposes such as the production of paints, inks, cosmetics, etc1. It has gained popularity for its several health benefits. It is a great source of omega-3 fatty acids, alpha-linolenic acid, short-chain polyunsaturated fatty acids, phytoestrogens lignans, proteins, and antioxidants2. Flaxseed is widely grown because of its health-promoting benefits and is used in a variety of medicinal ways, including topically as a skin salve, reducing cancer, orally for constipation, weight loss, heart disease, mainly the prostate and mammary gland. The major countries that grow flaxseeds are Canada, the US, China, India, and Ethiopia. There are two types of varieties of flaxseeds- brown and golden. Flax seed is presently grown for its oil in addition, flax is a rich source of fatty acid and has increasing uses in foods3. The seed contains oil that is used for edible purposes2. It nearly contains about 25 % protein, 30 % dietary fibre and 45% oil4. Flaxseed oil contain essential fatty acid higher than any other vegetable oils4. This oil is rich in polyunsaturated fatty acid, and monosaturated fatty acid, and low in saturated fatty acid. The oil of flaxseed is oxidizable when extracted and purified. Among the oilseeds, flaxseed is considered unique because the content of α- linolenic acid (ALA) is very high5.
Mechanical press, solvent extraction with organic solvents and conventional mechanical screw presses are used for edible oil extraction. However, these techniques have their own disadvantages such as presence of fatty acids in oil extracted by conventional mechanical press; mechanical screw press on the other hand is reported to be inefficient as it leaves an amount of oil in the de-oiled cake6. However, the solvent extraction method is the most common technique used for the extraction of oil from the seeds7. Most of the available literature or source on flaxseed oil extraction reported on the use of hexane, and isopropanol4, hexane and ethanol8, petroleum ether2, Hexane, dichloromethane, petroleum ether, ethanol, acetone, methanol6. Even though hexane is considered a hazardous pollutant, hexane is used in most of the research because of its high oil extraction capacity. Other solvents used for extraction of oil from different seeds can be performed using the Soxhlet method are chloroform, cyclohexane, ethyl ether, ethyl acetone, and diethyl ether7. The present study aimed to compare the oil extraction capacity of different solvents and to determine the effect of different solvents on the different physiochemical parameters such as moisture content, peroxide value, free fatty acid, acid value, saponification value, and iodine value and also to study the antioxidant activity of oil extracted from polar and nonpolar solvents.
Materials and Method
Sample collection
Flaxseeds (5 Kg) was collected from the local market of Kokrajhar in the state of Assam, India. The collected sample was taken to the laboratory of Department of Biotechnology, Bodoland University, India for carrying out the experiments.
Sample preparation
Flaxseed was washed with water then it was sun-dried. After the seed dried it was ground in a grinding machine to get the powdery form and was stored in an air-tight container until further experiments. All the experiments were carried out in triplicates.
Moisture content
The moisture content of flaxseed was estimated by AOAC (2012)9. Initial weights of petri plates were noted down. 5g of sample was taken in the petri plates (done in triplicates) and weight was noted down respectively. The Petri plates containing the flaxseeds powder were placed in the hot air oven for 4 hours at 105°C. After 4 hours, the Petri plates were taken out from the oven and kept aside for a while to cool down. Then the petri plates containing the sample were weighed again and the weight was noted down. The final weight was subtracted from the pre-weighted petri dish and the final moisture content was estimated using the formula:
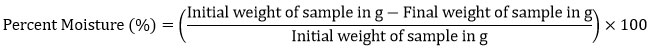
Extraction of Oil
Soxhlet apparatus (Merck, India) was used for extraction of oil. Both polar and non-polar solvents were used for extraction. The solvents used for extraction were commercially obtained. In total six different solvents under polar and non-polar category were used for extraction of oil. The polar solvents used for extraction were Methanol (Merck, India), n-Butanol (Fisher Scientific, India), Isopropanol (SRL, India), Acetone (Merck, India) and Ethanol (Merck, India) and Non-polar solvent used was Hexane (Rankem, India). The oil extraction was done by using the AOAC standard method.
The ground flaxseed (5gm) was placed in a thimble and placed in the Soxhlet Apparatus with a round bottle flask and a water condenser. The oil seed extraction was performed for 4hrs using polar solvents (methanol, n-butanol, isopropanol, acetone, and ethanol) as well as non- polar solvent (hexane) at a temperature of 70°C. After the extraction, the extractor that has solvent in it was placed inside a rotary evaporator (Equitron, India) to evaporate the solvent. The oil obtained was stored in the refrigerator for further analysis.
Estimation of peroxide value (PV)
The peroxide value (PV) is the measurement of oxidative deterioration of oils10. The peroxide value of oil was estimated by AOAC (1995) method11. It is determined by titrating iodine liberated from potassium iodide with sodium thiosulphate solution. The oil sample weighted 1.25g was taken in the conical flask. 7.5ml of glacial acetic acid: chloroform (1:2) mixture was added to the oil sample and mixed well followed by adding 0.125ml of potassium iodide (KI). The flask was placed in a shady chamber for 1min, then 7.5ml of distilled water was added to stop the reaction. A few drops of the starch indicator were added and titrated against 0.1N Sodium thiosulphate. The peroxide value is determined as follows:
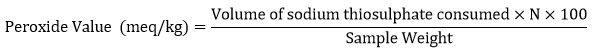
where, N = normality of sodium thiosulphate solution
Estimation of Acid Value (AV)
The acid value is the number of milligrams of Potassium hydroxide required to neutralize the free fatty acids present in one gram of fat. It is a relative measure of rancidity as free fatty acids are normally formed during decomposition of triglycerides12. The free fatty acid value of oil was estimated by AOAC (1995) method11. Neutralization of alcohol was carried out by titrating against 0.1N potassium hydroxide solution. Oil sample of 0.5g was taken in the conical flask and 50ml neutralized alcohol was added into the flask and was heated on the hot plate. It was followed by the addition of phenolphthalein indicator. The mixer was titrated against 0.25N of potassium hydroxide solution. The acid value was determined as follows:
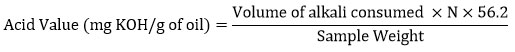
Where, N = normality of potassium hydroxide solution
56.2 = molecular weight of potassium hydroxide
Estimation of iodine value (IV)
The Iodine value can be determined by the uptake of halogen. It gives the measure of unsaturation of oil components. The greater the iodine value, the more unsaturation and the higher the susceptibility to oxidation13. The iodine value of oil was estimated by AOAC (1995) method11. Stoppered glass flasks (3 Nos.) were taken as blank and 3 Nos. contained 0.5g of oil sample. 5ml of chloroform was added to all 6 glass flasks which was followed by 12 ml of iodine solution. Then it was kept in a dark room for 30 minutes. 10 ml of potassium iodide (KI) was added to the glass flask followed by addition of distilled water. It was then titrated with 0.1N sodium thiosulfate until the yellow color disappeared. A few drops of the starch indicator were added to the glass flask. Again, it was titrated until the blue color disappeared. The iodine value was determined as follows:
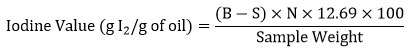
Where, B = volume of the standard solution needed for blank
S = volume of the standard solution needed for the sample
N = normality of sodium thiosulphate solution
Estimation of saponification value (SV)
The saponification value is the number of milligrams of KOH needed to neutralize the fatty acids obtained by complete hydrolysis of 1gram of an oil sample13. A small saponification value indicates long chain fatty acids on the glycerol backbone in a sample; on the contrary, a high saponification value indicates triacylglycerols with shorter fatty acyl chains15. The saponification value of oil was estimated by AOAC (1995) method11. For standard, 0.2 g of oil sample was taken into the conical flask, followed by the addition of 10ml of 0.5N ethanolic Potassium hydroxide (KOH). Then it was placed into the water bath for 30mins at 80-85°C. After 30 mins the conical flask was cooled down to between 30-40°C liquid state and then a few drops of phenolphthalein indicator were added. It was titrated with 0.5N HCl standard solution and the saponification value was determined as follows:
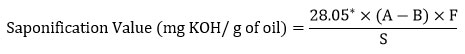
where, S = sample weight
B = titration volume of sample
⁕ = half of molecular weight of KOH
A = titration volume of blank
F = factor of 0.5N HCl standard solution
Estimation of DPPH free radical scavenging activity
The 2,2-Diphenyl-1-picrylhydrazyl (DPPH) is used for the measurement of antioxidant properties that includes the use of the free radicals used for assessing the potential of substances to serve as hydrogen providers or free-radical scavengers16. The DPPH assay of the flaxseed oil was carried out as described by Okoh et al., 201417. A solution of 0.02g DPPH (SRL, India) in 50ml of methanol was prepared. 1 ml of DPPH was added to 3 ml of the sample. For standard, 1ml of DPPH was added to 3ml of methanol. The mixer was vigorously vortexed and was kept in a dark room for 30 minutes. Then the absorbance was measured at 517 nm by using a UV visible spectrophotometer. DPPH scavenging effect was determined as follows:
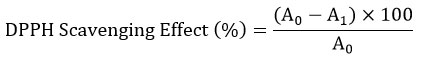
Where, A0 = absorbance of the standard solution
A1 = absorbance of the sample in the standard solution
Statistical Analysis
All the test were carried out in triplicates and the results obtained from the analysis were expressed as mean value ± standard deviation of three replicates.
Results and Discussion
Moisture content
The moisture content in flax seeds before the extraction of oil was found to be 4.53%. Rizvi et al. 2021 found nearly 6.7% of moisture present in flaxseeds before oil was extracted from them.
Oil Extraction
In this study, polar solvents (methanol, acetone, n-butanol, isopropanol, ethanol) and non-polar solvent (hexane) was used for oil extraction. The oil content for the polar solvents was obtained as 30 % (w/w) for methanol, 38 % (w/w) for acetone, 25 % (w/w) for n-butanol, 35 % (w/w) for isopropanol and 38 % (w/w) for ethanol. For the non-polar solvent hexane, the oil content obtained was 45 % (w/w).
Peroxide value (PV)
The peroxide value for the oil extracted under different solvent extraction was found to be lowest in ethanol extract (19.1 ± 1.0 meq KOH/kg) followed by hexane (21.2 ± 0.7 meq KOH/kg), n-butanol (28.2 ± 1.0 meq KOH/kg), acetone (32.8 ± 1.4 meq KOH/kg), methanol (62.4 ± 1.2 meq KOH/kg) and highest in isopropanol extract (74.0 ± 1.0 meq KOH/kg). Rizvi et al. 2021 reported the peroxide value of the extracted flax oil extracted in petroleum ether as 2.75 meq KOH/g which is very much high compared to the current study. However, Ghosh et al. 2019 reported a comparatively low peroxide value of the oil extracted using isopropanol was 0.95 meq/kg oil and the oil extracted using hexane was 0.99 meq/kg oil.
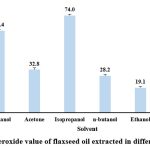 |
Figure 1: Peroxide value of flaxseed oil extracted in different solvent
|
Acid value (AV)
The acid value obtained ranged from 4.3 ± 0.04 mg KOH/ g oil for n- butanol to 9.3 ± 0.09 mg KOH/ g oil for hexane. The FAO designated maximum level of acid value for edible oil is 4.0 mg KOH/g oil which indicates that the extracted oils under different solvents have high free fatty acids formed by the decomposition of triglycerides. Other similar studies reported he acid value of the extracted oil in petroleum ether as 1.3±0.02 mg KOH/g2. Ghosh et al. 2019 also reported acid value of the oil extracted using isopropanol as 0.80 mg KOH/g oil and the oil extracted using hexane as 0.84 mg KOH/g oil which is comparatively way less than the current study.
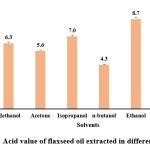 |
Figure 2: Acid value of flaxseed oil extracted in different solvent.
|
Iodine value (IV)
Iodine value was obtained lowest in hexane oil extract (25.3 ± 0.41 iodine/ 100 g oil) followed by many fold increase in the iodine value in extracts of isopropanol (75.7 ± 0.62 iodine/100 g oil), ethanol (101.8 ± 0.97 iodine/100 g oil), n-butanol (118.4 ± 0.77 iodine/100 g oil), methanol (125.6 ± 1.13 iodine/100 g oil) and acetone (170.4 ± 1.48 iodine/100 g oil). Hexane showed the lowest iodine value and hence can be used for extraction purposes. Results reported by Rizvi et al., 2021 showed iodine value of the extracted oil in petroleum ether as 165-169±0.03 g l2/100g. Similarly, high values were obtained by Ghosh et al. 2019, for the oil extracted using isopropanol (172 iodine/100g oil) and the oil extracted using hexane was (180 iodine/100g oil).
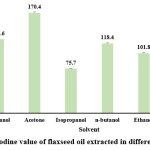 |
Figure 3: Iodine value of flaxseed oil extracted in different solvents.
|
Saponification value (SV)
The high saponification value of the oil extracts from acetone (475.8 ± 2.4 mg KOH/g oil), methanol (465.4 ± 2.65 mg KOH/g oil), ethanol (420.3 ± 1.3 mg KOH/g oil), isopropanol (376.4 ± 2.01 mg KOH/g oil) and n-butanol (303.8 ± 0.78 mg KOH/g oil) indicate the presence of shorter chain fatty acid chain and a lower molecular weight. However, hexane (203.0 mg KOH/g oil) showed comparatively lower saponification value but yet it was higher than the standard saponification value of flaxseed oil i.e., 186-196 mg KOH/g oil. The saponification value of the extracted oil was reported to be 192 mg KOH/g by Rizvi et al. 2021 and Ghosh et al., got the values at 180 mg KOH/g oil and 192 mg KOH/g oil for oil extracted using isopropanol hexane respectively.
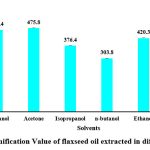 |
Figure 4: Saponification Value of flaxseed oil extracted in different solvents
|
DPPH free radical scavenging activity
Ghosh et al. 2019 reported the DPPH free radical scavenging activity of the oil extracted using isopropanol was 80% and the oil extracted using hexane was 82%. The current study got the free radical scavenging activity to be highest in hexane at 76.7 ± 2.32 % followed by 61.1 ± 1.08 % for isopropanol, 52.5 ± 0.97 s% for acetone, 42.1 ± 1.26 % for n-butanol, 26.6 ± 0.67 % for ethanol and lowest at 14.7 ± 0.55 % for methanol.
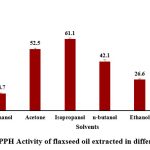 |
Figure 5: DPPH Activity of flaxseed oil extracted in different solvents
|
Conclusion
This study indicated that a substantial amount of effect is generated on the characteristics of the oil due to solvent. All the solvents showed varied range of values for the different physio-chemical parameters. This study also indicated that quality and antioxidant activity of non-polar solvent extracted flaxseed oil is better compared to the polar solvents. Hexane, a non-polar solvent showed the best results amongst all the solvents in terms of peroxide value, iodine value, saponification and DPPH free radical scavenging activity.
Acknowledgement
The authors would like to express their sincere thanks to the Department of Biotechnology, Bodoland University for providing the necessary laboratory facilities for completion of this work.
Conflict of Interest
The authors declare that there is no conflict of interest.
Funding Sources
There is no funding Sources.
References
- Zhang, Z.S., Wang, L.J., Li, D., Li, S.J and Ozkan, N. Characteristics of flaxseed oil from two different flax plants. International Journal of food properties. 2011; 14:1286-1296.
CrossRef - Rizvi, Q.E.H., Sharma, V., Shams, R., Dar, A.H., Jan, B and Manzoor, A. Extraction of oil from flaxseed using three-phase partitioning techniques. Journal of postharvest technology. 2021; 09(1):35-45.
- Akbar, S., Baig, M.N and Amruta, D. Flaxseed: A Review. International Journal of innovative research in Technology. 2021; 8 (3).
- Ghosh, S., Bhattacharyya, D.K and Ghosh, M. Comparative study of chemical characteristics, phytochemical contents, and antioxidant activity of polar and nonpolar solvent extracted flaxseed oil. Acta Pharmaceutical Sciencia. 2019; 57(3).
CrossRef - Soni, R.P., Katoch, M., Kumar, A and Verma, P. Flaxseed-composition and its health benefits. Research in environment and life sciences. 2016; 9(3), 310-316
- Gutte, K.B., Sahoo, A.K., and Ranveer, R.C. Effect of ultrasonic treatment on extraction and fatty acid profile of flaxseed oil. Oil seeds & fats crops and lipids. 2015; 22(6) D606.
CrossRef - Dasari, S.R and Goud,V. Effect of pre-treatment on solvents extraction and physico-chemical properties of caster seed oil. Journal of Renewable and sustainable energy. 2014; 6, 063108.
CrossRef - Naz, S., Shabbir, M.A., Khan, M.R. and Shahid, M. Comparison of flaxseed oil characteristics of three Pakistani varieties obtained by supercritical CO2 and two conventional extraction methods. International food research journal. 2019; 26(5): 1599-1607.
- Official Method of Analysis: Association of Analytical Chemists. 19th Edition. Washington DC. 2012.
- H. Gordon. Factors affecting lipid oxidation. Understanding and measuring the shelf life of food, 2004. Woodhead Publishing. Pp 128-141.
CrossRef - Official Method of Analysis: Association of Analytical Chemists. 17th Edition. Washington DC. 1995.
- Manual of methods of analysis of food. Oils and Fats. New Delhi. 2021.pp 16-21.
- T.H. Groundnut oil. In: Encyclopaedia of Food Sciences and Nutrition, Second Edition. 2003, pp 2967-2974.
CrossRef - O., Charles. M.Agricultural Waste Diversity and Sustainability Issues Sub-Saharan Africa as a Case Study. In: New approach and prospects of agro-waste resources conversion for energy systems performance and development, 2021, pp 97-118.
CrossRef - Ivanova M, Hanganu A, Dumitriu R, Tociu M, Ivanov G, Stavarache C, Popescu L, Ghendov-Mosanu A, Sturza R, Deleanu C, Chira NA. Saponification Value of Fats and Oils as Determined from 1H-NMR Data: The Case of Dairy Fats. 2022 May 18;11(10):1466.
CrossRef - Baliyan S, Mukherjee R, Priyadarshini A, Vibhuti A, Gupta A, Pandey RP, Chang CM. Determination of Antioxidants by DPPH Radical Scavenging Activity and Quantitative Phytochemical Analysis of Ficus religiosa. 2022 Feb 16;27(4):1326.
CrossRef - Okoh, S.O., Asekun, O.T., Familoni, O.B and Afolayan, A.J. 2014. Antioxidant and free radical scavenging capacity of seed and shell essential oils extracted from Abrus precatorius (L). Antioxidants. 2014,3,278-287.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





