Manuscript accepted on : 06-07-2023
Published online on: 27-10-2023
Plagiarism Check: Yes
Reviewed by: Dr. Ranjan Singh
Second Review by: Dr Manita Paneri
Final Approval by: Dr. Ahmad Ali
Estimation of Thiocyanate Content from Selected Cruciferous Vegetables
Aaliya Shaikh , Rhea Thomas
, Rhea Thomas and Sara Khan*
and Sara Khan*
Department of Biochemistry, Shri Vile Parle Kelvani Mandal’s Mithibai College of Arts, Chauhan Institute of Science and Amrutben Jivanlal College of Commerce and Economics (Autonomous), Vile-Parle (West), Mumbai, Maharashtra, India.
Corresponding Author E-mail: sara.khan@mithibai.ac.in
DOI : http://dx.doi.org/10.13005/bbra/3185
ABSTRACT: As per the studies conducted by the Indian Thyroid Society, it is suggested that one out of ten people in India suffer from hypothyroidism and these figures are likely to increase in the near future. According to the survey, women in the post-menopausal age group are more affected in comparison to men. Globally, the prevalence of hypothyroidism is 4-5%. Taking the same into consideration, a comparative study was undertaken to estimate the thiocyanate content in cruciferous vegetables such as Raphanus sativus (radish), Brassica rapa (turnip), Brassica okracea var. botrytis (cauliflower) Brassica oleracea (broccoli), Brassica clearccea var. botrytis (cabbage). Increased levels of thiocyanate often cause imbalance of the thyroid hormones by inhibiting iodine uptake thereby leading to hypothyroidism. Thiocyanate is therefore also employed in the treatment of hyperthyroidism. This study used 10 gram of sample extract in triplicates that were obtained from different markets, treated with ferric chloride leading to the formation of a colored complex and the amounts of thiocyanate was evaluated colorimetrically. It was observed that the thiocyanate content in cabbage was the highest followed by radish, turnip, broccoli and cauliflower. High thiocyanate content coupled with low iodine content go a long way in leading to goiter which is a clinical manifestation of any abnormality associated with thyroid gland. This leads us to very important conclusion that while ascertaining causes of any abnormalities of the thyroid gland that a person’s diet needs to be taken into consideration.
KEYWORDS: Goiter; Goitrogen; Hypothyroidism; Post-menopausal; Thiocyanate
Download this article as:| Copy the following to cite this article: Shaikh A, Thomas R, Khan S. Estimation of Thiocyanate Content from Selected Cruciferous Vegetables. Biotech Res Asia 2023;20(4). |
| Copy the following to cite this URL: Shaikh A, Thomas R, Khan S. Estimation of Thiocyanate Content from Selected Cruciferous Vegetables. Biotech Res Asia 2023;20(4). Available from: https://bit.ly/45J5Cn3 |
Introduction
Thyroid diseases are one of the most widespread diseases in the world. The same holds true for the Indian scenario. Studies suggest an increasing incidence of thyroid disorders. As per a meta-analysis conducted by Anne and Rahiman (2022) it was concluded that 1 in 1031 neonates in India, suffered from congenital hypothyroidism which is significantly higher than developed countries like Japan, Germany and United Kingdom where the prevalence is 1 in 2500 to 3500, 1 in 3330 and 1 in 1887 respectively.1 As per the guidelines set by the American Thyroid Association (2023), the RDA for iodine is 150µg/day for healthy adults.2
Thyroid hormones are of vital importance in the normal metabolism and virtually affect every cell of the body. The thyroid gland is found in the neck and consist of two lobes connected by the isthmus.2 It produces two related hormones 3,5,3’,5’-tetraiodothyronine (T4) and 3,5,3’-triiodothyronine (T3). Tyrosine an amino acid that is synthesized in the body in sufficient amounts, is the major substrate that combines with iodine to form these hormones. Unlike tyrosine that is produced in the body 3, iodine is primarily supplied through diet especially in meat products that contain up to 400 µg/kg. 4 Iodine that is absorbed in the blood stream is taken up by several glands such as the salivary glands and thyroid glands in the form of I– using the Na+/I– symporter which is further oxidized to iodine. 5
Thyroid hormones are known to have a pronounced effect on the metabolism of various biomolecules. It was reported that increased levels of thyroid hormones increase the rate of glucose metabolism by increasing the transcription of insulin; a hormone necessary for absorption of glucose by the body cells. Increased levels of insulin lead to increase in its secondary effects on the overall body.6 In case of increased thyroid hormone levels, the rate of glycogenolysis and gluconeogenesis also increase. It can thus be concluded that increased thyroid hormone levels lead to a hyperglycemic state. It was pointed out that since the metabolism of glucose and thyroid functions are interlinked it is implausible to rule out the connection of iodine deficiency and diabetes mellitus.7
It was reported that T3 and T4 stimulate lipolysis owing to an increased mobilization of triglycerides stored in adipose tissue. Increased levels of Thyroid Stimulating Hormone (TSH) are positively correlated to both, Low Density Lipoproteins (LDL) and total cholesterol levels in serum. 8
A study conducted by Ibad et al., (2023) suggested that increased levels of free thyroxine in the blood stream can be positively correlated to sarcopenia which is indicative of their catabolic effect at higher concentration. 9
A study carried out by Yoo et al., (2021) inferred that local regulation of thyroid hormones in the Central Nervous System may physiologically regulate appetite. Increased thyroid hormone levels may increase the metabolic rate thereby increase the appetite of an individual whereas decrease in the thyroid levels may slow down the basal metabolic rate thereby decreasing the appetite of an individual. 10
The increase in the levels of thyroid hormones both T3 and T4 is known as hyperthyroidism conversely a decrease in their levels is known as hypothyroidism.
The diseases that are chronically related to the thyroid gland are hyperthyroidism, Hashimoto’s thyroiditis, 11 Grave’s disease, 12 cretinism 13 to name a few. Goitre is the clinical manifestation of any abnormality associated with the thyroid gland.
A myriad of chemical compounds in the environment and some drugs are known to hinder the functions of the thyroid gland thus posing the danger of diseases of thyroid gland.14 Further, substances found in the environment such as water, food sources that lead to goitre are called environmental goitrogens who’s exposure or consumption should be limited as their intake over an acceptable limit can be deleterious to health.15 Some of the examples of goitrogens are thiocyanates, perchlorates, thiourea sulfdamethoxines.
Perchlorates are substances used in the development of various explosives pose severe threat to the thyroid gland as they act as a competitive inhibitor of iodide thereby hindering its uptake.16 Recently, perchlorates have come under great inspection as studies report that there is a greater environmental distribution especially in potable water than what was previously known.17 Based on the study conducted by Legakis et al., (2022) exposure to perchlorates through smoking, radiation may increase the risk of thyroid cancer that accounts for about 90% of all endocrine cancers18
Thiourylenes inhibit the iodination of monoiodotyrosine and thereby block the coupling reaction. A study concluded that 6-n-propylthiouracil which is a derivative of thiourea is a safe and potent drug for treatment of hyperthyroidism. Clinically used drugs such as propylthiouracil and methimazole cause hinderance in the iodination of tyrosine. They act as competitive inhibitors of tyrosine and themselves get iodinated. Further propylthiouracil also inhibits 5’ DI thereby reducing the conversion of T4 to T3 in many extra-thyroid tissues.19 Drugs such as methimazole and carbimazole cause inhibition of the enzyme thyroperoxidase.20
Thiocyanates are anions that have antithyroid activity. They inhibit the uptake of iodine by acting as competitive inhibitors of the sodium/iodine symporter.21 It thus worsens iodine deficiency by inhibition of thyroidal uptake of iodine. Thiocyanate is found in large concentration in the cruciferous vegetables.22 It is found in its progoitrogen form as glucosinolates which on cleavage by myrosinase enzyme leads to the production of thiocyanate.23 A recent study reported that thiocyanate exposure along with other endocrine disruptors even in low concentration could alter the thyroid hormone homeostasis in adults in China.24
The primary objective of this research is to compare the thiocyanate content in different cruciferous vegetables based on purchases from various markets of the city. The vegetables included were radish, turnip, cauliflower and cabbage. Various established, sensitive and specific methods were tested for the estimation of thiocyanate content but out of all, the modified Johnston and Jones method proved convenient. This method employed the formation of a red coloured complex of iron-thiocyanate whose extinction was measured at 530nm.
Various other techniques are available one of which, involves formation of a pink coloured pyridine-benzidine complex in the presence of thiocyanate and the absorbency is measured at 525nm. This method was employed to estimate the concentration of thiocyanate in urine. 25
A colorimetric estimation was carried out by Duch et al., (2019) which measured a red coloured complex concentration that was a result of Fe(SCN)63- complex formation when the extract was treated with trichloroacetic acid and ferric ions. 26
A similar study conducted for finding the concentration of thiocyanate colorimetrically made use ferric nitrate which yield a complex whose absorbance was measured at 470nm.27
Material and Method
Materials
Instruments: The instruments that had been used are as follows:
Table 1: Instruments used.
|
Instrument |
Name of the Company |
|
Colorimeter |
Equiptronics |
Reagents
Potassium Thiocyanate [KCNS]: Working standard of KCNS (30µg/mL) was prepared and serially diluted with distilled water to obtain concentrations of 5 µg/mL, 10 µg/mL, 15 µg/mL, 20 µg/mL, 25 µg/mL and 30 µg/mL. Standard Iron chloride [FeCl3] of concentration 0.4M was prepared in distilled water. 5% mercuric chloride was prepared in 1N HCl. All chemicals used were of AR/GR grade.
Methods
Sample preparation
Vegetable samples were obtained from 6 different local vegetable markets of Mumbai. 3 samples of each vegetable were acquired from every market. Therefore, a sample size of 18 for every vegetable was procured for analysis. Each sample was taken in triplicates (10g each), crushed, extracted with 10 mL distilled water and refluxed for 45 minutes in a conical flask. The refluxed samples were allowed to cool until room temperature was obtained and were then filtered using muslin cloth and filter papers. The residues were given repeated washings with distilled water and the volumes of the filtrates were made up to 50mL.
In order to negate the green colour imparted to the extract of certain vegetables, 6mL of the elute was taken and distributed in 2 different test tubes. 2-3 drops of 5% mercuric chloride were added to one of the tubes to it to block thiocyanate. Ferric chloride was then added to the tubes and the extinction was measured.
Estimation of thiocyanate
Thiocyanate estimation was done using modified Johnston and Jones method. This method involves the formation of a red colour complex which is a result of the reaction between SCN– ions from the vegetable sample and Fe from FeCl3 in the +3 oxidation state
The extinction values of blank, standard and test samples were measured at 530nm.
The concentrations of the unknown samples were computed from the standard curve.
The series of standard tubes were prepared in the following manner:
Table 2: Dilution table for blank, standard and unknown tubes
|
Tube no. |
Working Standard (0.03mg/mL) |
Distilled Water (mL) |
FeCl3 (mL) |
|
Blank |
– |
3.0 |
1.5 |
|
1 |
0.5 |
2.5 |
1.5 |
|
2 |
1.0 |
2.0 |
1.5 |
|
3 |
1.5 |
1.5 |
1.5 |
|
4 |
2.0 |
1.0 |
1.5 |
|
5 |
2.5 |
0.5 |
1.5 |
|
6 |
3.0 |
– |
1.5 |
|
Unknown |
3.0 |
– |
1.5 |
Results
The amount of thiocyanate from various vegetables was determined using a standard curve in which the value obtained in µg/10g has been scaled up to report the value in mg/100gm. A wide range of thiocyanate content was observed across different cruciferous vegetables. The lowest mean level of thiocyanate yield was observed in cauliflower (2.44±0.03 mg/100gm) as compared to the other vegetables chosen for the experiment. The difference in the thiocyanate concentration of the outer and inner leaves of cabbage may be attributed to the fact that the outer leaves are more mature as compared to the inner leaves. The average thiocyanate content in the selected vegetables is as depicted in table 2. In order to negate the bias of sampling, we compared the total thiocyanate content of vegetables that were purchased from six different local markets.
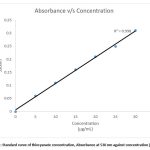 |
Figure 1: Standard curve of thiocyanate concentration, Absorbance at 530 nm against concentration (μg/mL).
|
Table 3: Thiocyanate content in vegetables per 100gm wet weight obtained from six different markets
|
Vegetables |
Thiocyanate content obtained from different markets (mg/100gm) |
|||||
|
Market 1 |
Market 2 |
Market 3 |
Market 4 |
Market 5 |
Market 6 |
|
|
Turnip |
1.72 |
1.82 |
1.72 |
1.72 |
1.82 |
1.82 |
|
Cabbage (Outer leaves) |
2.51 | 2.32 |
2.42 |
2.12 |
2.68 |
2.64 |
|
Cabbage (Inner leaves) |
2.22 |
2.28 |
2.16 |
1.85 |
2.48 |
2.42 |
|
Radish |
2.42 |
2.35 |
2.42 |
1.92 |
2.51 |
2.37 |
|
Cauliflower |
0.86 |
1.13 |
0.71 |
1.23 |
1.73 |
1.29 |
|
Broccoli |
1.42 |
2.07 |
1.92 |
1.95 |
1.66 |
2.42 |
The values are mean ± S.D of 3 samples obtained from a particular market in Mumbai.
Table 4: Average thiocyanate content in vegetables per 100gm wet weight
|
S No. |
Sample |
Thiocyanate (-SCN) (mg/100gm) |
|
1. |
Turnip Brassica rapa |
1.77±0.009 |
|
2. |
Radish Raphanus sativus |
2.33±0.03 |
|
3.
|
Cabbage Brassica cleraccea var. capitate (a) Inner leaves (b)Outer leaves |
2.23±0.03 2.44±0.03 |
|
4. |
Cauliflower Brassica okracea var. botrytis |
1.13±0.05 |
|
5. |
Broccoli Brassica oleracea |
1.90±0.05 |
The values are mean ± S.D of 6 samples obtained from different markets in Mumbai.
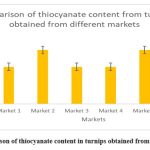 |
Figure 2: Comparison of thiocyanate content in turnips obtained from different markets
|
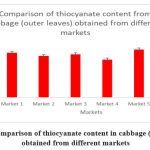 |
Figure 3: Comparison of thiocyanate content in cabbage (outer leaves) obtained from different markets
|
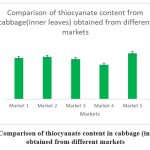 |
Figure 4: Comparison of thiocyanate content in cabbage (inner leaves) obtained from different markets.
|
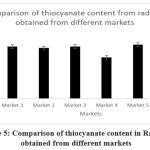 |
Figure 5: Comparison of thiocyanate content in raddish obtained from different markets
|
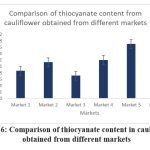 |
Figure 6: Comparison of thiocyanate content in cauliflower obtained from different markets
|
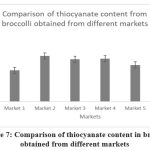 |
Figure 7: Comparison of thiocyanate content in broccolli obtained from different markets
|
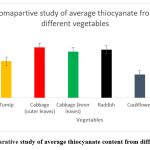 |
Figure 8: Comparative study of average thiocyanate content from different vegetables.
|
Table 5: Two-way ANOVA test for the thiocyanate concentration of different vegetables obtained from 6 markets in Mumbai.
|
Source |
DF |
Sum of Square (SS) |
Mean Square (MS) |
F Statistic (df1,df2) |
P-value |
|
Factor A – Vegetables |
5 |
7.0431 |
1.4086 |
29.008 (5,25) |
1.204e-9 |
|
Factor B – Markets |
5 |
0.6632 |
0.1326 |
2.7315 (5,25) |
0.04212 |
A two-way ANOVA was conducted to check for the difference in the thiocyanate concentration for different vegetables obtained from different markets in Mumbai. There is a statistically significant difference seen in the thiocyanate concentration of different vegetables (F =29.008, p-value=1.204e-9). Thus, the null hypothesis is rejected. The concentration of thiocyanate seen in different vegetables is statistically significant.
There is a statistically significant difference seen in the thiocyanate concentration of samples of any particular vegetable obtained from 6 different markets (F= 2.7315, p-value= 0.042). Thus, the null hypothesis was rejected. The thiocyanate concentration of a particular vegetable obtained from 6 different markets in Mumbai is statistically significant.
Discussion
The current study aimed to evaluate the thiocyanate concentration of different cruciferous vegetables. The method employed was a modified Johnston and Jones method. A similar colorimetric estimation conducted in Poland by Przybylska et al., (2022) reported that cabbage has a thiocyanate concentration of 1.194±0.001 mg/100gm whereas broccoli has a thiocyanate concentration of 1.034±0.001 mg/100gm. These results match the findings of our study as they suggest that cabbage has a higher concentration of thiocyanate as compared to broccoli.28 The results of our study were also in line with a similar study conducted by Sun and Chen in the year 2019 on members of the cruciferous family which suggests that broccoli has a higher thiocyanate concentration than cauliflower.29 The study conducted on Allium vegetables by Czech et al., (2022) such as garlic, yellow onion, red onion, and leek reported a lower thiocyanate concentration as compared to the vegetables of the cruciferous family included in our study which suggests that thiocyanate content is found more in members of the cruciferous family.27 Differences in thiocyanate yield amongst individual cruciferous vegetables may be chalked up to environmental factors and hereditary factors, which determine the content of glucosinolates and/or the ratio of thiocyanate-producing glucosinolates to the other glucosinolates in cruciferous vegetables as reported by Liu et al., (2020).30 Differences in thiocyanate concentration found in different vegetables in our study can be attributed to the difference in glucosinolates as a study conducted by Abbaoui et al., (2018) reported that different concentrations in glucosinolates yield different amounts of isothiocyanates, thiocyanates and other metabolites.31
Conclusion
In this study, the vegetables examined include members of the cruciferous family that are very integral to Indian cuisine. Thus, the data obtained from this study can be used to assess the exposure of the population to thiocyanate especially through their diet. It was reported that repeated intake of small quantities of these vegetables over a prolonged period of time have a negatory effect on the thyroid gland, especially coupled with a low iodine diet.25 Repeated consumption of thiocyanate through diet can lead to an increase in the urinary thiocyanate levels which can lead to a decrease in the triiodothyronine levels.32 Also, thiocyanate can act as a competitive inhibitor of sodium/iodine symporter 20, in high concentrations can lead to decrease in thyroid hormones synthesis and could also inhibit the uptake of iodine in infants, resulting in a decrease iodine available to them.33 A reduction in the regular consumption of thiocyanate can thus go a long way in treatment of hypothyroidism. Due to the extreme fluctuations in the concentration of iodine found in different sources of food and water, it was suggested that in order to combat iodine deficiency, making salt iodination mandatory is the best way moving forward.34 Although several national and international endeavours have been undertaken to increase the intake of iodine either volitionally or through mandated iodination of salt and have achieved success in many countries, the problems of iodine deficiency still remain in countries like Russia, Australia, Africa and few countries of Asia. Therefore, from this study we effectively conclude that when ascertaining causes of any thyroid gland abnormalities, an individual’s diet needs to be taken into consideration.
Acknowledgement
Financial support to the department from Shri Vile Parle Kelvani Mandal (SVKM) is gratefully acknowledged.
Conflict of interest
The authors declare that there are no conflicts of interest in the course of conducting the research. All the authors had final decision regarding the manuscript and decision to submit the findings for publication.
Funding Source
Financial support to the Department of Biochemistry from Shri Vile Parle Kelvani Mandal (SVKM) is gratefully acknowledged.
References
- Anne, R. P and Rahiman, E. A. Congenital hypothyroidism in India: A systematic review and meta-analysis of prevalence, screen positivity rates, and etiology. The Lancet Regional Health-Southeast Asia., 2022; 5:100040.
CrossRef - https://www.thyroid.org/
- Fitzpatrick, P. F. The aromatic amino acid hydroxylases: Structures, catalysis, and regulation of phenylalanine hydroxylase, tyrosine hydroxylase, and tryptophan hydroxylase. Archives of Biochemistry and Biophysics., 2023; 735.
CrossRef - Duborská, E, Urík, M, and Šeda, M. Iodine biofortification of vegetables could improve iodine supplementation status. Agronomy., 2020; 10: 1574.
CrossRef - Riesco-Eizaguirre G, Santisteban P and De la Vieja A. The complex regulation of NIS expression and activity in thyroid and extrathyroidal tissues. Endocrine-Related Cancer., 2021; 28: 141–165.
CrossRef - Thakur M and Prasad C.B. Associations between Glucose Homeostasis and Thyroid Diseases. European Journal of Molecular & Clinical Medicine., 2023; 10(04): 1481-1494.
- Ilias I, Milionis C, Zabuliene, L and Rizzo M. Does Iodine Influence the Metabolism of Glucose?. Medicina., 2023; 59:189.
CrossRef - Ravikant S, Tak A. K. B. N. K, Yogi J. P, Jat R. K, and Choudhary S. Association Of Thyroid And Lipids Profile In Euthyroid, Hypothyroidism And Hyperthyroidism Subjects. European Journal of Molecular & Clinical Medicine., 2023; 10(01): 3965-3970.
- Ibad H. A, Mammen J. S, Simonsick E. M, Kwoh C. K, Guermazi A, and Demehri S. Higher thyroid hormone has a negative association with lower limb lean body mass in euthyroid older adults: Analysis from the Baltimore Longitudinal study of aging. Frontiers in aging., 2023; 4: 115064.
CrossRef - Yoo E. S, Yu J, and Sohn J. W. Neuroendocrine control of appetite and metabolism. Experimental & molecular medicine., 2021; 53(4): 505-516.
CrossRef - Ragusa F, Fallahi P, Elia G, Gonnella D, Paparo S. R, Giusti C, and Antonelli A. Hashimotos’ thyroiditis: Epidemiology, pathogenesis, clinic and therapy. Best Practice & Research Clinical Endocrinology & Metabolism., 2019; 33(6): 101367.
CrossRef - Smith, T. J. and Hegedüs L. Graves’ disease. New England Journal of Medicine., 2016; 375: 1552-1565.
CrossRef - Bennaoui F, Slitine N. E. I, and Maoulainine, F. M. R. Cretinism: Case report. J Clin Images Med Case Rep., 2022; 3(1): 1534.
- Muzzaffar S, Nazir T, Bhat M. M, Wani I. A, and Masoodi F. A. Goitrogens. In Handbook of Plant and Animal Toxins in Food., 2022; 125-154.
CrossRef - Oladejo A. A, Okesola M. A, Oyerinde A. S, Jaiyesimi K. F, and Kolawole J. A. Evaluation of Goitrogenic Content of Common Vegetables in South West Nigeria. Asian Food Science Journal., 2018; 4(1): 1-7.
CrossRef - Eder S, Hermann C, Lamkowski A, Kinoshita M, Yamamoto T, Abend M, and Rump A. A comparison of thyroidal protection by stable iodine or perchlorate in the case of acute or prolonged radioiodine exposure. Archives of Toxicology., 2020; 94: 3231-3247.
CrossRef - Cao F, Jaunat J, Sturchio N, Cances B, Morvan X, Devos A, an Ollivier P. Worldwide occurrence and origin of perchlorate ion in waters: A review. Science of the Total Environment., 2019; 661: 737-749.
CrossRef - Legakis I, Barbouni A, and Chrousos G. The importance of toxic environmental substances in the development of thyroid cancer. Toxicology and Environmental Health Sciences., 2022; 14(2): 101-109.
CrossRef - Ruslan A. and Okosieme O. E. Non-thionamide antithyroid drug options in Graves’ hyperthyroidism. Expert Review of Endocrinology & Metabolism., 2023; 18: 67-79.
CrossRef - Goura R, Manubolu S.S.B, Katari N.K, Kodanda A.R, & Rebelly P. Scalable Process of Methimazole. Organic Process Research & Development., 2022; 27: 54-64.
CrossRef - Lo, D., Wang HsinI, W. H., Wu WanJen, W. W., & Yang RayYu, Y. R. Anti-nutrient components and their concentrations in edible parts in vegetable families. CABI Reviews., 2018; 1-30.
CrossRef - Ağagündüz D, Şahin T. Ö, Yılmaz B, Ekenci K. D, Duyar Özer, Ş, and Capasso R. (2022). Cruciferous vegetables and their bioactive metabolites: from prevention to novel therapies of colorectal cancer. Evidence-Based Complementary and Alternative Medicine., 2022; 2022.
CrossRef - Qin H, King G. J, Borpatragohain P. and Zou J. Developing multifunctional crops by engineering Brassicaceae glucosinolate pathways. Plant Communications., 2023; 100565.
CrossRef - Yue B, Ning S, Miao H, Fang C, Li J, Zhang L. and Wu Y. (2023). Human exposure to a mixture of endocrine disruptors and serum levels of thyroid hormones: A cross-sectional study. Journal of Environmental Sciences., 2023; 125: 641-649.
CrossRef - Johnston T. D, and Jones D. I. H. Variations in the thiocyanate content of kale varieties. Journal of the Science of Food and Agriculture., 1966; 17, 70-71.
CrossRef - Mondal C and Chandra A. K. (2019). Goitrogenic/antithyroidal potential of moringa leaves (Moringa oleifera) and spinach (Spinacia oleracea) of Indian origin on thyroid status in male albino rats. Brazilian Journal of Pharmaceutical Sciences., 2019; 55.
CrossRef - Kapusta-Duch J, Szeląg-Sikora A, Sikora J, Niemiec M, Gródek-Szostak Z, Kuboń M and Borczak B. (2019). Health-promoting properties of fresh and processed purple cauliflower. Sustainability., 2019; 11(15).
CrossRef - Czech A, Szmigielski M, and Sembratowicz I. (2022). Nutritional value and antioxidant capacity of organic and conventional vegetables of the genus Allium. Scientific Reports., 2022; 12(1).
CrossRef - Przybylska A, Mariańska, A, & Koba M. Analiza zawartości tiocyjanianów w wybranych warzywach krzyżowych (Cruciferae). Bromatologia i Chemia Toksykologiczna., 2021; 54(4): 265-278.
- Sun, J., & Chen, P. Quantification of total glucosinolates and isothiocyanates for common Brassicaceous vegetables consumed in the US market using cyclocondensation and thiocyanate ion measurement methods. Journal of Analysis and Testing., 2019; 3(4): 313-321.
CrossRef - Liu Y, Rossi M, Liang X, Zhang H, Zou L, and Ong C. N. (2020). An integrated metabolomics study of glucosinolate metabolism in different Brassicaceae genera. Metabolites., 2020; 10(8).
CrossRef - Abbaoui, C. R. Lucas, K. M. Riedl, S. K. Clinton, and A. Mortazavi. Cruciferous vegetables, isothiocyanates, and bladder cancer prevention. Molecular Nutrition and Food Research., 2018; 62(18).
CrossRef - King L, Wang Q, Xia L, Wang P, Jiang G, Li W and Liu L. Environmental exposure to perchlorate, nitrate and thiocyanate, and thyroid function in Chinese adults: A community-based cross-sectional study. Environment International., 2023; 171: 107713.
CrossRef - Hatch-McChesney A, and Lieberman H. R. Iodine and iodine deficiency: a comprehensive review of a re-emerging issue. Nutrients., 2022; 14(17), 3474.
CrossRef - Krela-Kaźmierczak I, Czarnywojtek A, Skoracka K, Rychter A. M, Ratajczak A. E, Szymczak-Tomczak, A and Dobrowolska A. Is there an ideal diet to protect against iodine deficiency?. Nutrients., 2021; 13(2), 513.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.