Manuscript accepted on : 19-07-2023
Published online on: 27-10-2023
Plagiarism Check: Yes
Reviewed by: Dr. Thirunahari Ugandhar
Second Review by: Dr. Mushtaq Vaseem Baig
Final Approval by: Dr. Fernando José Cebola Lidon
Beneficial Prospectives: Plant Annona squamosa L.
Shuchi Dave Mehta1* , Sukirti Upadhyay2
, Sukirti Upadhyay2 and Priyanka Rathore3
and Priyanka Rathore3
1Department of Pharmacognosy, Guru Ramdas Khalsa Institute of Science and Technology, Pharmacy, Jabalpur M.P, India.
2Department of Pharmacognosy, School of Pharmaceutical Sciences, Faculty of Pharmacy, IFTM University, Moradabad U.P. India.
3Sagar Lnstitute of Research Technology and Science Pharmacy, Bhopal M.P. India.
Corresponding Author E-mail: Priyankarathore1jan@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/3164
ABSTRACT: Developed and undeveloped countries both are utilizing herbs for maintaining health wellbeing by providing proper treatment and prevention of diseases. The present review gives the Ethnobotanical information linking between plant Annona squamosa L. and human beings were also providing updated facts of phytochemical and pharmacological approaches of the above plant in the present 21st century. Annona squamosa L. is a multipurpose shrub tree that is distributed throughout tropical countries and eminently a desert fruit in India. The plant belongs to the family Annonaceae which in Hindi known as Sitaphal or Sharifa which is utilized for its medicinal properties. The ethnobotanical traditional uses include wound healing, lice repellant, treatment of dysentery and urinary tract infection. Phytochemicals includes alkaloids (benzoxyquinazoline, salsolinol, coclaurine), terpenoids (annomosin A, annosquamosin A), glycoside (quercetin-3-glucoside), essential oil (bicyclogernacrene, T-cadinol), flavonoids (kaempherol, farmarixetin) tannins, and many more responsible for pharmacologically action of plant parts are fruits, seeds, leaves, bark, and flower. Information on medicinal uses and organoleptic properties of various pharmacologically active parts is also provided. The present article discusses the updated information regarding distribution, plant parts used, chemical constituents, traditional uses, morphological and pharmacological importance of this plant hoping for exploring better medicinal value.
KEYWORDS: Annona squamosa L.; Phytochemicals; Pharmacological activity; traditional uses
Download this article as:| Copy the following to cite this article: Mehta S. D, Upadhyay S. Rathore P. Beneficial Prospectives: Plant Annona squamosa L. Biotech Res Asia 2023;20(4). |
| Copy the following to cite this URL: Mehta S. D, Upadhyay S. Rathore P. Beneficial Prospectives: Plant Annona squamosa L. Biotech Res Asia 2023;20(4). Available from: https://bit.ly/477i1lX |
Introduction
Since earliest time, Ayurvedic system of medicine utilizes medicinal plants for maintenance of health and treatment of diseases. The written evidence of utilization of plants as drug was obtained from Mesopotamia.1
Various lead structures which are used for new drug synthesis by the pharmaceutical industry have their origin from natural source i.e. camptothecin, podophyllotoxin, paclitaxel, etc. The collabration of the pharmaceutical industry with academic institutions have created progressive programmers for plant medicine based research2. The new approved structures from plant source continue to play significant role in novel drug discovery3. The plant product shows more structural variation than synthetic product where the majority of bioactive agents are used for new drug discovery. Usually the bioactive guided isolation of plants shows new finding which results in discovery of important drugs4.
Different unknown species in plant kingdom consist of chemical constituents having nutraceutical and therapeutical value. In the catalog of nutritional compounds, fruits and vegetables are always ‘Treasure House’. For the manufacturing of the novel drug in modern pharmaceutical industries, abundant amount of genuine plant are required for herbal formulation. Sophisticated technology of extraction for separation of active constituents are assets for production of drug formulations.
Distribution
It had previously been grown in the Philippines and India by Spanish and Portuguese colonists in the 16th century. When compared to the other Annona species, which are found in warmer tropical and subtropical locations, Annona squamosa L. is the one that is most commonly grown. The word “Annona” comes from the word “anon,” which refers to the cultivation of fruit and means “yearly yield.” The flowering plant Annona squamosa L., a member of the Annonaceae family, is well-known for its mouthwatering fruit. It is a little deciduous tree with erratic branches, about 5 to 6 metres in height. In Hindi, it is known as Sharifa, sitaphal, and in Sanskrit, it is known as shubha, suda, and ganda. In English, it is popularly known as sweetsop, custard apple, and sugar apple.
Leaves are thin, simple, alternate, which are single 5cm to 17cm long and 2cm to 6cm wide. They are rounded at the base and pointed at the tip with oblong-lanceolate in shape with pale green color on both surfaces. Flowers are greenish-yellow about 2.5cm long and occur in spring-early summer6.
Fruits are greenish yellow and size of 5-10cm in diameter in round or heart shaped. The fruit pulp is white tinged and sweetly edible. It may or may not contain carpel. Seeds are 1.3-1.6 cm oblong, shiny which are brown to black in color7.
Plant Parts Used
Fruits, seeds, flower, leaves and bark 8.
Traditional Uses
Leaves of Annona squamosa L. was extensively used as ethnobotanical used for treatment of diabetes, hysteria, intestinal infection whereas bark was conventionally used in diarrhea and dysentery. The plant is also used traditionally as insecticidal, antispasmodic, analgesic and anti-inflammatory etc16, 5.
Chemical Composition
It has been determined that over 100 chemicals from various categories exist as:
Alkaloids: Benzoxyquinazoline, samoquasine A, N-nitrosoxylopine, roemerolidine, duguevalline, meleagrine, chrysogine, dopamine, salsolinol, coclaurine, liriodenine, oxoanalobine. 8-11
Flavanoids: Rutin,isoquercitrin, bullatacin, quercetin, kaempherol, farmarixetin, isorhamnetin8, 12
Entkaurenediterpenoid: Annomosin A Annosquamosin A, annosquamoannosquamosin D, annosquamosin E, annosquamosin F, annoquamosin.
Glycoside: quercetin-3-glucoside13
Essential oil: germacrene, β-elemene, α and β pinene, sabinene, bicyclogernacrene, T-cadinol, T-mucirololspathulenol, bornylacetate, borneol,verbenone14.
Other compounds includes from Glucopyranoside, coumarinoligans annotemoyin-1, annonacin, annonacin A, and annonastatin, as well as Squamocin B to Squamocin N, annotemoyin-2, Squamocin andcholestryl, etc. Fig. 1 showing various constituents of Annona squamosa plants.
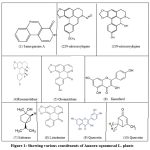 |
Figure 1: Showing various constituents of Annora squamosal L. plants.
|
Organoleptic Characters
Annona squamosa L. have irregularly spreading branches with height of 3-7m Lanceolate to oblong lanceolate, pale green on both sides, and almost globate leaves are found singly, measuring 6–17 × 3-6 cm. They belong to the category of semi-deciduous tree having light brown bark with smoothies fissured in plates.
Table 1: Organoleptic characters various parts of plant Annona squamosa L.
|
Characters |
Leaves |
Fruits |
Seeds |
Stem |
Roots |
|
Taste |
Bitter |
Sweet |
No Taste |
Sight bitter |
Bitter |
|
Odor |
Characteristic
|
Characteristic |
No Odour |
No odor |
No odor |
|
Color |
Green |
Pulp White inside and green outside |
Black |
Brown |
Brown |
Flowers are greenish yellow with fragrance on slender hairy stalk produces singly or in short clusters in 2.5cm length where sepals in 16mm length are pointed, hairy, green in color. The three outer petals are yellow-green, slightly hairy, thick and oblong at the tips whereas inner petelsare ovate and pointed scale. Flowers are greenish-yellow with fragrance, produced singly or in short lateral clusters of 2-4 flowers, about 2.5 cm long, and 2.5 cm wide; sepals are pointed, hairy, green, about 16 mm long; the three outer petals are oblong, thick and rounded at the tips, fleshy, 1.6-2.5 cm long, and 0.6 cm wide; they are yellow-green, slightly hairy, and the interior is light yellow with a purplish or red Scales on the three-minute inner petals are ovoid. Table 1.1 showing morphological features of seeds, leaves, stem, roots and fruits of Annona squamosa L.
Fruits are green-yellow in color, round, ovate, irregular and heart shaped having size 5-10 cm in diameter. When ripen, pulp becomes edible, white and sweet. Seed are oblong, shiny, black, 1.3-1.6cm long numerous in each carpel. Ovary are light green, style is white and crowded and stamen are numerous, white and crowded in 16mm long. Fig. 2, Fig. 3, Fig. 4 and Fig. 5 showing respective fruits, flower, seeds and whole plant.
 |
Figure 2: Annona squamosa L. Fruits
|
 |
Figure 3: Annona squamosa L. flowers.
|
 |
Figure 4: Annona squamosa L. seeds.
|
 |
Figure 5: Annona squamosa L. Plant .
|
Pharmacological Importance of Annona squamosa
A versatile shrub tree that grows in tropical regions and is well-known in India as a fruit for deserts. The plant is a member of the Annonaceae family and is referred to as Sitaphal or Sharifa in Hindi due to its well-known medicinal virtues and commercial use. Typical uses include the treatment of urinary tract infections, dysentery, and wounds. The plant has a number of medical benefits because it contains a variety of phytochemicals, including alkaloids, polysaccharides, fixed oil, tannins, flavonoids, and phenolic compounds. Each component of the plant has strong bioactive ingredients. Recent studies have demonstrated therapeutic activity against cancer, against oxidative stress, against diabetes, against hypertension, against parasites, against malaria, against insects, and against microbes.16
The seeds extract showed the regulation of hyperthyroidism and lipid peroxidation which involves the presence of quercetin through phytochemical analysis including HPLC.
The plant has been reported to possess cytotoxic effect and antidiabetic activity. The saponified petroleum ether extract of bark of Annona squamosa L. showed central and peripheral analgesic and anti-inflammatory activity. Its leaves extract showed protection against aspirin and pyloric ligation induced ulcers. (+)-O-Methylarmepavine, isocorydine and methyl corydaldine are anti-ulcer compounds isolated with anti-secretory properties. 16 beta, 17-dihydroxy-ent-kaurane-19-oic acid is compound have significant activity against H9 lymphocyte cells with HIV replication showing antiviral activity with 0.8μg/ml EC50 value.
Annona squamosa L. reported hepatoprotective activity, hypolipidimic activity, molluscidal properties, antimicrobial activity, Vaso-relaxant activity, antimalarial, antioxidant, anthelmintic, insecticidal activity, wound healing and genotoxic effect.7, 8, 17, 18
Antimicrobial activity
The bacterial membrane destabilization property of methanolic extract of Annona squamosa L. leaves has been evaluated using bacterial strains Staphylococcus aures, Enterobacter cloacae, Klebsiella pneumonia, Pseudomonas aeruginosa, bacillus cereus, Enterococcus faecalis, Shigelladysenteriae, Escherichia coli, Salmonella typhimurium and Salmonella choleraesuis. It was concluded that Annona squamosa L. can be utilized for the treatment of microbial infection.19. Table 1.2 showing antimicrobial activity of Annona squamosa L.
The in-vitro antibacterial evaluation of many leaf extracts from Annona squamosa L., including petroleum ether, methanol, acetone, chloroform, and petroleum ether, was carried out. Both gram positive and gram-negative bacterial strains were used to assess the antibacterial activity. The evaluation techniques for in-vitro antibacterial activity are the agar disc diffusion method and the agar well diffusion method.
The standard antibiotic drugs used was chloramphenicol and ampicillin whereas the standard antifungal drug was nystatin and clotrimazole. The result showed better activity of methanolic extract as compared to other extract of Annona squamosa L. leaves. 20
Table 2: Antimicrobial activity of Annona squamosa.
|
S.No |
Part |
Extract |
Strain |
Method |
Result |
References |
|
|
1. |
Leaves |
Water Alcohol chloroform |
Essherichia coli |
Agar cup method |
Showed inhibition in 5µl and 10µl concentration. |
21 |
|
|
Pseudomonas aeroginosa |
|||||||
|
2 |
Leaves |
Water Alcohol chloroform |
Candid albicans |
Agar cup method |
No inhibition activity |
21 |
|
|
Aspergillusniger |
|||||||
|
3 |
Folier |
Water |
Collosobruschuschinesis |
|
Showed inhibition in 0.07 mg ml−1 concentration |
22 |
|
|
4 |
Seed |
Ethyl acetate Petroleum ether Acetone methanol |
Raj, Castaneumviz CR 1 FSS II CTC-12 |
Larval bioassay |
The petroleum spirit extract of the seed had the highest T. castaneumlarval mortality rate. |
23 |
|
|
5 |
Leaves |
Ethanol |
Sitophilusoryzae pest. |
Insect assayed Contact bioassay |
For concentrations of 1% w/v and 5% w/v, mortality (100%) was attained at 39.61.4 and 14.51.1min, respectively. |
24 |
|
Antibacterial and Insecticidal activity
The antibacterial and insecticidal of seeds activity was investigated for aqueous, ethanol, methanol and acetone extract Annona squamosa L. seeds. The antibacterial activity was performed against P. vulgaris, S. epidermidis, S. aureus, E. coli, P. aerogenes, S.typhi, E.aerogenes and S. typhimuriu. Compared to all four extracts, the methanolic extract showed better antibacterial and insecticidal activity. The possible phytochemical responsible was alkaloids and flavonoids for insecticidal activity. The seeds Annona squamosa L. can also be used as bio-insecticidal.25
Using Aedes albopictus and Culex quinquefasciatus as vectors, the aqueous and oil extracts of Annona squamosa L. and Annona muricata seeds were assessed as a mosquito-controlling agent. Alkaloids and flavonoids were the phytochemicals in responsible. The vectors that cause fever, chikungunya, and dengue include Aedes albopictus and Culex quinquefasciatus. Due to increasing problem of mosquito resistance over chemical insecticides, the two plant of Annonaceae were investigated to control the insects. Both Annona squamosa L. and Annona muricata’s oil and aqueous extract shown potential as insecticides. The Annona muricata extract was shown to be superior to the other two plant extracts. 26
Antifungal activity
Using the agar well diffusion method, the effects of methanol, chloroform, and an aqueous extract of Annona squamosa L. leaves were assessed. Inhibitory minimum concentration for the broth microdilution method using various strains of fungi Aspergillus niger, Microsporum canis, Candida albicans, alternaria alternate and fusarium solani were investigated. The comparison of antimicrobial activity between various parts of Annona squamosa L. such as root, leaf, seeds and cotyledon were made. The antimicrobial activity of methanol extract of above-mentioned plant parts was evaluated against four fungi Trichophyton rubrum, Aspergillus niger, Candida albicans and Aspergillus flavus. Agar well diffusion was used to examine the effects of methanol, chloroform, and an aqueous extract of Annona squamosa L. leaves. the smallest inhibitory dose 27
Anticancer Activity
Evaluation of anticancer on peel, seed and pulp was performed using MTT assay method where sulfated polysaccharide, tannins, flavonoid and triterpenoid was also calculated using RP-HPLC.28 Lebanese Annona squamosa L. seeds were used in the phytochemical screening and cytotoxic activity of the extracts, which demonstrated antioxidant, anti-diabetic, and anti-proliferative properties due to the presence of several secondary metabolites such flavonoids and phenol..29 Table 1.3 showing anticancer activity of Annona squamosa L.
The findings of an evaluation of Annona squamosa L. seeds oil’s anticancer efficacy revealed that the oil inhibits the growth of H22 solid tumours, which may be because of the oil’s unsaturated fatty acid composition and level of total annonaceous acetogenins (ACGs).30
Numerous pathways, including vascular, adipose tissues, inflammation, structural, and physiological, are involved in cellulite. Cellular lipid accumulation, anti-platelet aggregation, and microcirculation was evaluated for three plants where Rosmarinus officinalis, and Annona squamosa L. showed reduction of lipid accumulation whereas Rosmarinus officinalis showed inhibition in platelet aggregation and Zanthoxylum clavaherculis showed reduction of recto-anus coefficient by 79.6% which result in the improvement in microcirculations. This results a formulation with standardized composition which suggested its usage for cellulite.31
Table 3: Anticancer activity of Annona squamosa L.
|
S.No |
Plant part |
Cell lines |
Method |
Result |
References |
|
1. |
Fruit, seed, pulp and peel. |
liver (HepG-2), breast (MCF-7), prostate (PC3), and colon (Caco-2) |
MTT Assay technique |
For four cancer cell lines, seed extracts showed the lowest IC50 values (7.31 0.03 and 15.99 1.25 for PC-3 and MCF-7, respectively). |
28 |
|
2. |
Leaves |
Human Colon |
Assay for 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium |
IC50292.39/mL demonstrated strong cytotoxicity. |
32 |
|
3. |
Seeds |
breast cancer (MCF-7) cells, leukaemia (K-562) cells, nasopharyngeal cancer (KB) cells, lung cancer (A-549) cells |
MTT Assay method |
A panel of cancer cell lines, including nasopharyngeal cancer (KB) cells, lung cancer (A-549) cells, breast cancer (MCF-7) cells, and leukemia (K-562) cells, were evaluated, and the four extracts tested in petroleum ether had the highest levels of cytotoxicity. |
33 |
|
4. |
Leaves |
MDA-MB-231 and MCF-7 breast cancer cell lines
|
Nuclear staining, MTT, and LDH tests |
At a concentration of 100μg/mL, showed strong antiproliferative and cytotoxic effects |
34 |
|
5. |
Seeds |
HT-29 colon cancer cell lines, ovary cancer cell lines, breast cancer cell lines, cervix cancer cell lines (SiHa), liver cancer cell lines.
|
sulforhodamine B assay method |
The Ovcar-5 cell line showed the greatest activity (69.72), and considerable activity was also shown against the HT and SiHa cell lines. |
35 |
|
6. |
Seeds |
MCF-7, Hep G2 cells H(22) tumor cells |
H(22) hepatoma cells transplanted into a tumour model in vivo and (MTT) cytotoxicity in vitro |
(MTT) cytotoxicity in vitro and MCF-7 transplanted tumour model using H(22) hepatoma cells (IC50). Hep G2 (0.25% g/ml) (IC50). cells in vitro at 0.36 g/ml. (MTT) cytotoxicity in vitro and MCF-7 transplanted tumour model using H(22) hepatoma cells (IC50). Hep G2 (0.25% g/ml) (IC50). cells in vitro at 0.36 g/ml..
|
36 |
|
7. |
Leaves |
T47D cells |
MTT assay |
IC50 value was 174.25 µg/ml |
38 |
|
8. |
Leaves |
LNCap-FGC Prostate tumour in a human, A549 A human lung cancer cell line and human kidney cell lines HEK-293 |
MTT assay |
IC50 value of 200 mg/ml for all the three tested cell lines |
36 |
|
9. |
Bark and Leaves |
MCF-7 cells, breast cancer cell lines |
MTT assay |
A. squamosa suppresses the growth of MCF-7 via causing apoptosis, according to an IC50 value of 10 ug/ml. |
40 |
Immunostimulant Activity
Haematological tests were used to assess the immunostimulant activity of Annona squamosa L. aqueous extract in which phytochemical analysis was performed, and the findings showed that alkaloids, steroids, tannins, phenols, reducing sugar, saponin, and flavonoids were present. The findings supported the plant’s immunomodulatory properties.41
Gas chromatography-mass chromatography (GC-MS) and gas chromatography-flame ionisation detector (GC-FID) were used to evaluate the chemical makeup of the essential oil of Annona squamosa L. leaves from northern India. The majority of sesquiterpenoids and oxygenated sesquiterpenoid were obtained. (E)-caryophyllene, epi-α-cadinol, β-elemene, (Z) caryophyllene, α-humulene, viridiflorene, caryophyllene oxide, spathulenol, (2Z,6Z)-farnesal and many more sesquiterpenoids were also obtained. The monoterpenoids present in the essential oil were p-cymene, limonene and bornyl acetate. The major constituents of sesquiterpenoids were (E)-caryophylene, epi-α-cadinol, (Z)-caryophyllene, α-humulene, γ-cardinene, viridiflorene and γ-muurolene.42
Significant impact especially in relation to an average of antibody titer where gumboro (like HIV) affects the profile of antibody titers from ethanol extract of leaves [43] whereas fruits ethanol extract showed cell mediated and humoral immunity in mice as compared to control animal which confirms good potential of immunomodulatory activity.44
Antioxidant activity
The ability of extracts to reduce and scavenge free radicals were both studied. Comparable to chloroform and aqueous extract, the methanolic extract demonstrated stronger antioxidant activity. It was shown that the antifungal activity varied depending on the dosage. 46
A papaya seed extract and Annona squamosa L. extract was studied for their antioxidant potential. Additionally, the quantity of total flavonoids and phenols was calculated. The papaya seed extract from Carica Papaya had the highest antioxidant activity.45
Antiulcer property
Pyloric ligation was used to test an aqueous extract of Annona squamosa L. seeds. Gastric juice volume, pH, free acidity, total acidity, and ulcer index were the variables examined. Increases in the dosage of Annona squamosa L. aqueous extract resulted in a reduction in the ulcer index. Ranitidine served as the reference standard drug. Ranitidine’s ulcer index was 0.71 for ranitidine and 1.10 for the 200 mg/kg aqueous extract of Annona squamosa L. seeds.47
Hepatoprotective activity
The hepatotoxicity caused by diethylnitrosamine at a level of 200 mg/kg was examined in an ethanol extract of Annona squamosa L. leaves. Total protein, serum and tissue glutamyloxaloacetae transaminase, serum and tissue glutamyl pyruvate transaminase (GPT), serum and tissue alkaline phosphatase (ALP), serum acid phosphatase (ACP), alpha fetoprotein (AFP), and bilirubin were among the biochemical markers examined. The histopathological studies of the liver of mice revealed normal pattern of blood in the spaces which confirmed the hepatoprotective activity. The lowering of the GOT, GPT, ALP, AFP, total, and direct bilirubin levels with the chosen dose of Annona squamosa L. leaves further confirmed the hepatoprotective potential.48, 56, 57
Carbon tetrachloride-induced liver damaged with Rats were used to test the hydroalcoholic extract of Annona squamosa L. seeds for hepatoprotective efficacy. As Annona squamosa L. seeds contain flavonoids and phenols, a hydroalcoholic extract of the seeds has hepatoprotective properties.49
Antidiabetic activity
Recent research indicates that the root extract of Annona squamosa L. has anti-diabetic properties in streptozotocin (STZ) induced diabetes mellitus and insulin insufficiency lead to elevated glucose level. In mice with diabetes that had been induced by the drug streptozocin (STZ), the aqueous extract of Annona squamosa L. leaves significantly reduced blood sugar levels and increased antioxidant activity.52
Antimalarial activity
In recent research on Annona squamosa L., all compounds shown modest effectiveness against a chloroquine-sensitive strain and a chloroquine-resistant strain of Plasmodium falcifarum. Isolation of antimalarial compound including N-Nitrosoxylopine (1), Roemerolidine (2) and Duguevalline which was isolated from bark showed moderate activity against chlorquine resistant strain.53
Table 4: Hepatoprotective activity of Annona squamosa
|
S.no |
Parts |
extract |
Method |
Result |
References |
|
1 |
Seeds |
Ethanolic |
Biochemical indicators, including albumin, total protein, ALT, AST, ALP, LDH, SBL, and total cholesterol; |
Indicated the protective effect against alcohol liver induced injury |
50 |
|
2 |
Leaves |
Ethanol water |
Total bilirubin, protein,ALP, AST, ALT and γ-GT |
to be useful in controlling hepatic injury in drug induced hepatotoxicity |
51 |
|
3 |
Seeds |
Hydroalcoholic |
SGOT, ALP, SGPT and total bilirubin |
the possibility of liver protection by lowering the levels of SGOT, SGPT, ALP, and total bilirubin |
49 |
|
4 |
Leaves |
water |
ALT, AST, GGT, ALP, creatinine kinase, bilirubin, serum creatinine, albumin, globulin and total protein |
hepatoprotective effect at dose dependent manner. |
50 |
Anti-inflammatory property
In order to study the effects of an ethanolic extract of Annona squamosa L. seeds, indomethacin was utilised as the reference medication and a technique to produce hind paw edoema with carrageenan was employed. The results of the phytochemical screening indicated the presence of amino acids, reducing sugar, phenolic compounds, alkaloids, and tannins. A 100mg/kg dose of Annona squamosa L. seeds resulted in substantial anti-inflammatory effects.51
Anthelmintic activity
Utilizing a variety of models, the anthelmintic properties of Annona squamosa L. and its leaf extract have been investigated. In order to determine the duration of paralysis and the time until death, the crude drug’s extracts in hexane, ethyl acetate, and ethanol were examined at various concentrations. When compound 1 was extracted from ethyl acetate extract, it reduced the hatching of Haemanthus contortus eggs and the seeds of Annona squamosa L. demonstrated anthelmintic efficacy against this parasite at 25 mg ml-1. Based on spectroscopic investigation, it was discovered that had a C37 trihydroxy adjacent bistetrahydrofuran acetogenin structure.54 The anthelminthic activity from Annona squamosa L. were evaluated by using Indian earthworm Pheretima posthuma demonstrated that the isolated compounds (AS-4 and AS-5) had good paralysis times that are equivalent to albendazole standards.55
Against genotoxicity activity
Research on Annona squamosa L. potential for against genotoxicity has revealed that blood enzyme levels under oxidative stress were considerably changed by the treatment with plant extract.17
Novel Formulation Approaches Annona squamosa L.
Plant extracts and isolates have improved over past years in the creation of novel medication delivery methods. Annona squamosa L.showed significant antimicrobial activity, antibacterial, insecticidal activity, anticancer Activity, heptoprotective, anti-genotoxic, anthelmintic activity, antimalarial activity, Antidiabetic activity, anti-inflammatory property, and many more pharmacological activities. To justify various therapeutic activities and effective therapeutic treatment involving better absorption of the drug with minimum risk of complications which was to be the main basis for the production of herbal formulation.
Tetranychus urticae was tested for fatal toxicity after a formulation of Annona squamosa L. extract microencapsulation. The microencapsulation was shown to be effective in killing the two-spotted spider mite and might be utilised to control it.58
In streptozotocin-induced diabetic rats, the effects of a polyherbal formulation containing Annona squamosa L. and Nigella sativa on blood sugar, plasma insulin, tissue lipid profile, and lipid peroxidation were examined.59 Emulgel loaded with Annona squamosa L. extract with and without penetration enhancer where the formulation was evaluated at various storage conditions. The result showed good pharmaceutical stability that help to utilize in the cosmetic and pharmaceutical and cosmetic industry.60
Conclusion
Medicinal plants are considered as rich sources for the phytochemicals which can be utilized for drug formulation and development. The natural medicines are reliable and safe approach for treatment and prevention of various diseases as compared to synthetic drug. Additionally, herbal drugs are economic due to low cost compared to synthetic drug.
Annona squamosa L. has become more well-known as a result of recent research and studies that have been undertaken on the bioactivities and health benefits of various plant components, including the seeds, bark, leaves, and fruits. It has a long history of usage as a purgative, vermicide, insecticide, and therapy for abscesses, infertility, and cancer. The reported pharmacological properties include antidiabetic activity, hepatoprotective activity, antimalarial activity, antibacterial, antiulcer, wound healing, anti-inflammatory, anti-microbial and several other medicinal properties. Phytoconstituents investigations considered as alkaloids i.e. samoquasine A, N-nitrosoxylopine etc., flavonoids i.e. rutin, quercetin, bullaracin etc., entkaurene diterpenoid i.e. Annomosin A, Annosquamosin etc., glycoside i.e. quercetin-3glucoside, essential oil i.e. germacrene, β-elemene etc. as secondary metabolites.
According to research and development, novel drug delivery and polyherbal formulations have a number of advantages over conventional formulations, including improved solubility, bioavailability, and protection from toxicity, pharmacological activity, stability, improved tissue macrophage distribution, sustained delivery, and defense against physical and chemical deterioration.
Annona squamosa L. fruit with medicinal properties but also quality of ice confectionery that can be utilized as one of the ingredients in the food industry and nutraceutical industry which can be helpful for human civilization.61,62. It has been utilised in traditional folk medicine across the globe and is probably used in the food industry. Vitamin B1 (thiamine), dietary fibre, potassium, and sodium are all present in significant levels in the pulp, which is utilised as an ice cream flavouring due to the fact that between 50 and 80 percent of the fruit is edible.
The future investigation of phytochemistry and pharmacological studies of the plant will have fruitful scope with justified evidence based herbal formulation as phytotherapeutic medicine for the purpose of prevention and treatment of severe disease. In further future, Annona squamosa L. plant needs to explore investigation in various emerging areas with possible clinical studies that can be useful for welfare of the mankind.
Acknowledgement
We would like to acknowledge all colleagues who directly and indirectly conducting review.
Conflicts of Interest
Authors declares no conflict of interest.
Funding Sources
There is no funding for this article
References
- Koehn F E and Carter G T. “The evolving role of natural products in drug discovery,”in Nature Review Drug Discovery,2005, 4(3) 4206-220, doi:10.1038/nrd1657
CrossRef - Patwardhan B., Vaidhya A., Chorghade M, “Ayurveda and Natural Products Drug Discovery”, Current Science, 2004, 86, 789-799.
CrossRef - Chin Y.W., Balunas M. J., Chai and King- horn H. B.,“Drug Discovery from Natural Sources”, The American Association of Pharmaceutical Scientists , 2006, 8, 239-242.
CrossRef - Lahlou M., Screening of Natural Product for Drug Discovery, Expert Opinion on Drug Discovery, 2006, 2, 689-,700.
CrossRef - Gajalakshmi S., Divya R., Deepika V., Mythili S., Sathiavelu A., “Pharmacological activities of annonasquamosa: a review”, International Journal of Pharmaceutical Sciences Review and Research, 2011, 10, 2.
- Kral R. “Annona Squmosa Linnaeus, http://floranorthamerica.org/Annona_squamosa, 2020.
- Orwa C., Mutua A., Kindt R., Jamnadass R., Simons A. Agroforestry Tree Database, World agroforestry centre, Kenya, https://www.worldagroforestry.org/publication/agroforestree-database-tree-reference-and-selection-guide-version, 2010;40.
- Saha R.,“Pharmacognosy and pharmacology of Annona squamosa : A review,”International journal of pharmacy and life sciences, 2011, 2, 1183-1189.
- Morita H., Sato Y., Chan K. L., Choo C. Y., Itokawa H., Takeya K., & Kobayashi J., Samoquasine A, “A benzoquinazoline alkaloid from the seeds of Annona squamosa L.”, Journal of natural products, 2000, 63,1707–1708, https://doi.org/10.1021/np000342i
CrossRef - Johns, T., A. Windust, T. Jurgens and S.M. Mansor, “Antimalarial alkaloids isolated from Annona squamosa L.”, Phytopharmacology, 2011, 1, 49–53.
- Yunianto, P., Rusman, Y., Saepudin, E., Suwarso, W. P., & Sumaryono, W., “ Alkaloid (Meleagrine and Chrysogine) from endophytic fungi (Penicillium sp.) of Annona squamosa ”, Pakistan journal of biological sciences, 2014, 17, 667–674, https://doi.org/10.3923/pjbs.2014.667.674
CrossRef - Soni H., Malik S.S. and Singhai A.K., “Evaluation of leaf of aqueous extract of MeOH extract of Annona squamosa L.on cell viability”, American. Journal of Pharmaceutical. Technology and. Research, 2012, 2, 936-944.
- , S, and Anand Kar., “Antidiabetic and antioxidative effects of Annona squamosa L. leaves are possibly mediated through quercetin-3-O-glucoside, BioFactors” (Oxford, England) ,2007, 31, 3-4, 201-10. doi:10.1002/biof.5520310307.
CrossRef - Yves Pelissier, Chantal Marion, Annick Dezeuze & Jean-Marie Bessiere,“Volatile Components of Annona squamosa L.”, Journal of Essential Oil Research, 1993, 5, 557-560, DOI: 10.1080/10412905.1993.9698278
CrossRef - Chen, Y., Chen, J.W., Zhai, J.H., Wang, Y., Wang, S.L. and Li, X., “Antitumor activity and toxicity relationship of annonaceous acetogenins”, Food and chemical toxicology, 2013, 58, 394-400.
CrossRef - Pandey M, Debnath M., Gupta S, Chikara S.K,“Phytomedicine: an ancient approach turning into future potential source of therapeutics”, Journal of Pharmacognosy and Phytotherapy, 2011, 3(3), 27-37.
- Bhattacharya A and Chakraverty R, “The pharmacological properties of Annona squamosa L.: A Review”, International Journal of pharmacy and engineering, 2016, 4(2), 692-699.
- Win Min Oo, Myat Mon Khine, “Pharmacological Activities of Annona squamosa L.: Updated Review”, International Journal of Pharmacy and Chemistry, 2017; 3(6), 86-93, doi: 10.11648/j.ijpc.20170306.14.
CrossRef - Pinto N. C. C., Silva J. B. , Menegati L. M., Clara M. R., Marques L. B., Silva T. P. D., Milo R. C. N., Elaine M., Desouza F., Marcos J. S., Elita S., Rodrigo L. F., “Cytotoxicity and bacterial membrane destabilization induced by Squamosa L. extracts”, Annals of the Brazilian Academy of Sciences,2017, 89(3), 2053-2073.
CrossRef - Pawaskar S. M. and K. C. Sasangan,“Preliminary Phytochemical and In-vitro-Antimicrobial analysis of Squamosa Linn. leaf extract”, Journal of Pharmaceutical Science and Research, 2017, 9, 5, 618-623.
- Neethu Simon K., Santhoshkumar R., Neethu S. Kumar.,“Phytochemical analysis and antimicrobial activities of Annona squamosa L. leaf extracts”, Journal of Pharmacognosy and Phytochemistry, 2016, 5(4), 128-131.
CrossRef - Hemlata M. Kotkar, Sangeetha V. G .S. Sadan, Shipra R. Jha, Shripad M. Upasani , Vijay L Maheshwari, “Antimicrobial and pesticidal activity of partially purified flavonoids of Annona squamosa L. “ , Pest management science, 2002, 58(1), 33-37.
- Khalequzzaman M. and Shajia Sultana S, Insecticidal Activity of Annona squamosa L.Seed Extracts against the Red Flour Beetle, Triboliumcastaneum (Herbst), Journal of Bio-Science, 2007, 14, 107-112.
CrossRef - Kumar A, Rekha T., Shyamala Devi S., Kannan M., Jaswanth A., Gopal V., Insecticidal Activity of Ethanolic Extract of Leaves of Annona squamosa L., Journal Chemical and Pharmaceutical Research, 2010, 2(50), 177-180,.
- Kulkarni C.P, Antibacterial and Insecticidal Activity of Crude Seeds Extracts of SquamosaL., International Journal of Pharmaceutical Science Invention, 2017, 6(9), 25-29.
- Ravaomanarivo L. H. R., Razafindralev H. A., Raharimalala F. N, Rasoahantaveloniaina B., Ravelonandro P. H., Mavingui P., “Efficacy of seeds extracts of Squamosa andAnnonamuricata (Annonaceae) for the control of Aedesalbopictus and Culexquinquefasciatus (Culicidae)” , Asian pacific journal of Tropical biomedicine, 2014 4(10), 798-806.
CrossRef - Vidyasagar G. M. and Shivakumar Singh P., “A Comparative antimicrobial activity of methanolic root, leaf, seeds, cotyledon extracts of Annona squamosa L.; International Journal of Pharmacy and Pharmaceutical Sciences, 2012, 4(5), 289-292.
- Shehata, M. G., Abu-Serie, M. M., Abd El-Aziz, N. M., & El-Sohaimy, S. A. , “Nutritional, phytochemical, and in vitro anticancer potential of sugar apple (Annona squamosa ) fruits” ; Scientific reports, 2021,11(1), 6224.
CrossRef - Sabbah A, Mohamad N, Akram H, Hassan R, Ghassan N; “Phytochemical Screening and Cytotoxic Activity of Two Extracts from Seeds of Lebanese Squamosa L”; International Journal of Pharma Research and Health Sciences, 2017, 5(1), 1586-1591.
- Ma C., Chen Y., Chen J., Li X., Chen Y.,“A Review on Annona squamosa L., Phytochemicals and Biological Activities”, American Journal of Chinese Medicine, 2017, 45(5), 933-964, doi: 10.1142/S0192415X17500501. PMID: 28659034.
- Yimam M., Lee Y. C., Jiao P., Hong M., Brownell L., Jia Q. A.,“Standardized Composition Comprised of Extracts from Rosmarinusofficinalis, Annona squamosa L. and Zanthoxylumclava-herculis for Cellulite”, Pharmacognosy Research, 2017, 9(4), 319-324.
CrossRef - Fadholly A., Proboningrat A., DewiIskandar R.P., Rantam F.A., Sudjarwo S.A., “In vitroanticancer activity Annonasquamosa extract nanoparticle on WiDr cells”, Journal of Advance Pharmaceutical Technology Research, 2019, 10(4), pp.149-154.
CrossRef - Biba Vikas, Sukumaran Anil, P .Remani, “Cytotoxicity Profiling of Annona squamosa L.in Cancer Cell Lines”, Asian Pacific Journal of Cancer Prevention, , 2019, 20(9), 2831-2840, doi: 10.31557/APJCP.2019.20.9.2831.
CrossRef - Younes, Maria, “The selective anti-proliferative and pro-apoptotic effect of A. cherimola on MDA-MB-231 breast cancer cell line”, BMC complementary medicine and therapies, 2020, 20(1), 343, doi:10.1186/s12906-020-03120-1.
CrossRef - Mehta S. D. & Paliwal S., “Phytochemical analysis, liquid chromatography, and mass spectroscopy and in vitro anticancer activity of Annona squamosa L.seeds linn”, Asian Journal of Pharmaceutical and Clinical Research, 2018,11(3), 101–103, https://doi.org/10.22159/ajpcr.2018.v11i3.22808.
CrossRef - Chen Y., Xu S.S., Chen J.W., “Anti-tumor activity of Annona squamosa L. seeds extract containing annonaceous acetogenin compounds”, Journal of Ethnopharmacology, 2012, 142(2), 462-466.
CrossRef - Al-Nemari, R.; Ben Bacha, A.; Al-Senaidy, A.; Arafah, M.; Al-Saran, H., Abutaha, N.; Semlali, A. “Selective Cytotoxic Effects of Annona squamosa L. Leaves against Breast Cancer Cells via Apoptotic Signaling Proteins”, 2020, 05, 02-12 (doi: 10.20944/preprints 202005.0212.v1).
CrossRef - Amin, M., Saleh, I., & Hidayat, R, “The Anticancer Activity of Srikaya Leaves Fraction (Annona squamosa L.) An In Vitro Study, BioscientiaMedicina” Journal of Biomedicine and Translational Research, 2019, 3(4), 02, 39-44
CrossRef - Sanjeeva Kumar A, Gayathri B., GeethaBala Y, “Evaluation of In-vitro Anti-cancer Activity of Annona squamosa L. Leaves”, Inventi Impact : Ethnopharmacology, 2015, 4, 128-131.
- Veerakumar S., “ Anti-cancer efficacy of ethanolic extracts from various parts of Annona squamosa L. on MCF-7 cell line” , Journal of Pharmacognosy and Phytotherapy, 2016, 8(7), 147-154.
CrossRef - Paul R., Bhandari R. , Khanna A., Yadav, Gautam D., “Phytochemical screening of Squamosa and haematological studies in clariasbatrachus” , World journal of pharmacy and pharmaceutical sciences, 2016, 5(8), 1121-1131.
- Verma R.S., Joshi N., Padalia R .C., Singh V. R., Goswami P., “Characterization of the Leaf Essential Oil Composition of Annona squamosa L. from Foothills of North India” , Medicinal and Aromatic Plants (Los Angel), , 2016, 5(5), 270.
CrossRef - Hair B. M., Salina S., Hafiza H. and Julaida S.,“In ovo vaccination againts infectious bursal disease in broiler chickens, Potential of Sweetsop Annona squamosa L. Toward Physiological Aspects of Immune System in The Gumboro Infected Broilers (HIV Like Infection)” . J. Vet. Malaysia , 2010,2000(10), 1.
- Dhasarathan P., Gomathi R., Theriappan P. and Paulsi S, “Immunomodulatory Activity of Alcoholic Extract of Different Fruits in Mice” , Journal of Applied Sciences Research, 2010, 6(8), 1056-1059.
- Kothari and Seshadri, S., “Antioxidant activity of seed extracts of Annona squamosa L. and Carica papaya; Nutrition & Food Science”; 2010, 40(4), 403-408.
CrossRef - Kalidindi N., Thimmaiah N. V., Nandeep N .V., Swetha S., Kalidindi B.,“Antifungal and antioxidant activities of organic and aqueous extracts of Annona squamosa L. Leaves”, Journal of food and drug analysis, 2015, 23, 795 -802.
CrossRef - KushwahP, KayandeN,“Antiulcer Activity of Annona squamosa L. Seed Extract in Experimental Rats”, PharmaTutor, 2014, 2(6), 174-176.
- Raj D. S., Vennila J. J., Aiyavu C., Panneerselvam K., “The hepatoprotective effect of alcoholic extract of Annona squamosa L. leaves on experimentally induced liver injury in swiss albino mice”; International journal of integrative biology , 2009, 5(3),183.
- Mehta S.D., Paliwal S,“Hepatoprotective Activity of Hydroalcohilic Extract of Annona squamosa L. Seeds”, International Journal of Pharmacognosy and Phytochemical Research , 2017, 9(7), 997-1000.v1
CrossRef - Neha Sonkar, Anil K. Yadav, Pushpesh K. Mishra, P. K. Jain, Chandana V. Rao, World Journal of pharmacy and pharmaceutical science,“Evaluation of hepatoprotective activity of Annona squamosa L. leaves and bark extract against carbon tetrachloride liver damage in wistar rats”; 2016, 5(8), 1353-1360.
- Umamaheshwari A, Arunkumara, VedhahariB. N., Suryaprabha D and Punitha S; Phytochemical evaluation and antinflammatory activity of seed extract of Annona squamosa ; International Journal Chemical Sciences; 2008, 6(3), 1594-1599.
- Kaleem M, Asif M, Ahmed Q.U, Bano B., “Antidiabetic and antioxidant activity of Annona squamosa L. extract in streptozotocin-induced diabetic rats”, Singapore Med J., 2006, 47(8), 670-675.
- Tylor J, Arnold W , Thomas J, Saleh M M, “Antimalarial alkaloids isolated from Annona squamosa L.”,Phytopharmacology, 2011, 1(3), 49-53.
- Souza M.M., Bevilaqua C.M., Morais S.M., Costa C.T., Silva A.R., Braz-Filho R., “Anthelmintic acetogenin from Annona squamosa L. Seeds”, An Acad Bras Cienc., 2008 80(2), 271-277. doi:10.1590/s0001-37652008000200005.
CrossRef - Sandeep and Mittal A, “In vitro anthelminthic and antioxidant activity of isolated compounds from Annona squamosa L. bark”International Journal of Pharmaceutical Sciences and Research, 20189, 6, 2535-2539.
- Zahid M., Arif M., Rahman M.A., Mujahid M. “Hepatoprotective and antioxidant activities of Annona squamosa L. seed extract against alcohol-induced liver injury in Sprague Dawley rats”, Drug Chem. Toxicol., 2020, 43(6), 588-594.
CrossRef - Mohamed Saleem T.S., Christina A.J.M., Chidambaranathan N., Ravi V., Gauthaman, “Hepatoprotective activity of Annona squamosa L. on experimental animal model” International Journal of Applied Research in Natural Product, 2008, 1(3),1-7.
- Anilde da G.S.M., Roseane C.P.T., Irinaldo D. B., Antonio E G. S., Jose S, Lucas Adriel T. Santos, Edmilson S. Silva, Johnnatan D. de Freitas, Ticiano G. do Nascimento,“Microencapsulation of Annona squamosa L. (Annonaceae) seed extract and lethal toxicity to Tetranychus urticae” (Koch, 1836) (Acari: Tetranychidae), Industrial Crops and Products, 2019, 127, 251-259.
CrossRef - Meer S., Aslam S., Abbasi S A., Tahir,“Preparation and Characterization of Natural Antioxidant Emulgels Loaded with Annona squamosa L. Extract with and without Penetration Enhancer”, Asian Journal of Research in Dermatological Science, 2020, 3(3), 1-10.
- Singh S., Manvi F. V., Basavraj N. , Nema R.K.“ Antihyperlipidemic Screening of Polyherbal Formulation of Annona squamosa L. and Nigella sativa”, International Journal of Toxicological and Pharmacological Research, 2010, 2, (1),1-5.
- Kumari N, Prakash S, Kumar M, Radha, Zhang B, Sheri V, Rais N, Chandran D, Dey A, Sarkar T, Dhumal S, Kumar S, Mahato DK, Vishvanathan M, Mohankumar P, Pateiro M, Lorenzo JM. Seed Waste from Custard Apple (Annona squamosa ): A Comprehensive Insight on Bioactive Compounds, Health Promoting Activity and Safety Profile. Processes. 2022; 10(10):2119. https://doi.org/10.3390/pr10102119
CrossRef - Chikhalikar, Ninad & Sahoo, A. & Singhal, Rekha & Kulkarni, Pushpa; Studies on frozen pourable custard apple (Annona squamosa L.) pulp using cryoprotectants. Journal of the Science of Food and Agriculture; 2000; 80. 1339 – 1342.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





