Manuscript accepted on : 23-11-2023
Published online on: 14-12-2023
Plagiarism Check: Yes
Reviewed by: Dr. Hind Shakir Ahmed
Second Review by: Dr. Mohamed Reda Abdo Abdel Latif
Final Approval by: Dr. Ghulam Md Ashraf
Abinash R1, Karthick, A1 and Jegan G2*
1Department of Biotechnology, Dr. M.G.R. Education and Research Institute University, Maduravoyal, Chennai, Tamil Nadu, India
2Department of Biotechnology, Apollo Arts and Science College, Poonamallee, Chennai, Tamil Nadu, India.
Corresponding Author E-mail: jeganacademic@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/3190
ABSTRACT: Antibiotics are one of our most important weapons in fighting with bacterial infections and diabetics. Drugs were derived from natural sources that play an important role in the treatment and prevention of human diseases. Traditional fermented foods containing lactic acid bacteria such as milk, yogurt, curd, etc., have historically been consumed by human. Lactobacilli are well known friendly bacteria for their probiotic activities against pathogens. In this investigation cow and goat milk samples were selected and collected for the study. The gram stain was used for morphological identification of Lactobacillus sp. in the two samples. The two isolated Lactobacillus sp. from goat and cow milk samples were subjected to the extraction of secondary metabolites using the solvent ethyl acetate. The two extracts of Lactobacillus spp. those were isolated from cow and goat milk samples, showed antibacterial activity against Escherichia coli, Staphylococcus aureus, Bacillus subtilis and Pseudomonas aeruginosa. The zone of inhibition was assessed by mm. The good results of zones were observed at 1000 and 2000 µg in cow milk and 2000 µg in goat milk extraction. These metabolites were also showed good results in antidiabetic activity using the alpha amylase inhibition activity. The IC50 value has showed moderate activity in the two crude extractions when compared to acarbose standard drug. Therefore, in this investigation the Lactobacillus sp. producing secondary metabolites was good source for the activity of antibacterial and antidiabetic activity.
KEYWORDS: Antimicrobial; α-amylase inhibition; Milk; Lactobacillus spp; Secondary Metabolites
Download this article as:| Copy the following to cite this article: Abinash R, Karthick A, Jegan G. Antibacterial and Anti Diabetic Activities of Ethyl Acetate Fractions of Lactobacillus Isolated from Cow and Goat Milk. Biotech Res Asia 2023;20(4). |
| Copy the following to cite this URL: Abinash R, Karthick A, Jegan G. Antibacterial and Anti Diabetic Activities of Ethyl Acetate Fractions of Lactobacillus Isolated from Cow and Goat Milk. Biotech Res Asia 2023;20(4). Available from: https://bit.ly/41sTuWV |
Introduction
The Multi-drug resistant bacteria are the cause of the disease which results in numerous clinical problems throughout the world. The use of bacterial antibiotic resistance has been considered difficulties nowadays, because of the extensive use of classical antibiotics in treatment of animal and human diseases1, 2. As a result of the various resistant strains has been appeared and extended causing difficulties and use of the antibiotics as growth promoters have been restricted. Therefore, the continuous improvement of new classes of the anti-microbial agents has become increasing significance to medicine3, 4. Diabetes, also known as diabetes mellitus (DM), is a set of illnesses brought on by the presence of excessive amounts of sugar in our blood cells, which causes high blood sugar levels. The 1.5 million people deaths because of diabetes and which result that 48% of death occurred due to DM in 2019 and which happened the age before 70 years5. The acarbose and miglitol are the inhibitors for diabetes which is using in clinical at present. Although the use of these inhibitors are causes some gastrointestinal problems6. Therefore, the new pharmacological active agents from medicinal plants, animals, microorganisms or their extracts can lead to efficient for many diseases7.
The Probiotics are live bacteria or yeasts cells which make a health benefit to the host when it given sufficient proportions8, 9. The majority of probiotic species and strains are from the Lactobacillus and Bifidobacterium genus10, 11. Generally the Lactic acid bacteria (LAB) are safe bacteria and which is used to develop products with useful and probiotic properties due to their resistance to low pH and bile salts at the intestine12. The Lactic acid bacteria (LAB) which are occur naturally as an indigenous micro flora in raw milk and milk products such as cheese, vegetables, meat and beverages13, 14. The LAB are belongs to the group of Gram-positive, non-sporulating, facultative aerobic or anaerobic rods or cocci, which produce lactic acid as the most important fermentation products of the metabolism of carbohydrates15, 16.
The arid type of LAB, that can produce antimicrobial substance such as hydrogen peroxide, bacteriocins, D-isomers of amino acids, acetaldehyde, CO2, reuterin and diacetyl already have been isolated from different fermented products17. Many researchers have been reported that bacteriocins are produced by various Lactobacilli, Leuconostoc, Lactococci, Enterococci and Pediococci18.
Lactobacillus sp. is a one of probiotics which is considered as a biological therapeutics and it is consider as a generally recognized as safe (GRAS) organisms19. Some studies have been confirmed that numerous antimicrobial mechanisms of Lactobacillus sp. such as production of inhibitory compounds, immune-stimulation, nutrient competition and competition for binding sites. Moreover these bacteria can also produce certain antimicrobial molecules viz, bacteriocins, hydrogen peroxide, ethanol and fatty acid which lead to inhibit the growth of pathogenic bacteria20, 21, 22.
According to previous research the Lactobacillus sp. has the capability to inhibit numerous bacterial pathogens including Escherichia coli23, Streptococcus mutans24, Pseudomonas aeruginosa25, Staphylococcus aureus26, Shigella spp.27, and Clostridium difficile28. Moreover recently some studies have been reported that probiotics are developing the symptoms of diabetes by regulating the increasing insulin sensitivity, intestinal microbiota composition, and mitigating autoimmune responses29. It has been reported that the favorable gut bacteria can able to decrease the blood glucose levels by regulating the release of hormones and enzymes30. Therefore, the present work aims to study the isolation and purification of Lactobacillus sp. from cow and goat milks, extraction of secondary metabolites and its antibacterial activity and alpha amylase inhibition activity.
Materials and Method
The two raw samples of cow and goat milk were collected from Koyambedu farm house, Chennai, Tamil Nadu, India. The samples were collected in the winter season of February 2022 only. The samples were carefully stored in the laboratory for further extraction and activities.
Isolation of bacterial culture
The collected two animals milk (cow and goat) were subjected to isolation and purification of Lactobacillus sp. An aliquot of 0.1 mL of each sample was introduced onto the nutrient broth and incubated for 24 hours at 37°C. The incubated tubes were examined for turbidity that indicating the bacterial growth. The turbidity samples were then inoculated onto LBS (Lactobacillus Selection) agar plates and incubated for 24 hours at 37°C and subculture onto LBS agar plates till to obtain pure culture. The subculture technique was followed up to obtaining pure culture. The isolates were subjected to Gram staining procedure. The gram stain was used for morphological identification of Lactobacillus sp.
Extraction and preparation of samples
The bacterial cultures were grown separately in large quantity in LBS broth medium and incubated for three days at 37oC. The broth was centrifuged to separate the cells at 8000 rpm for 20 minutes. The clear supernatant containing the metabolites was collected. The pH of the supernatant was adjusted to 8.0 with 2 N of NaOH and extracted with an equal volume of ethyl acetate. The organic phase has dried over anhydrous sodium sulfate and concentrated in vacuo at 40°C using rotary evaporator. The resulting crude extract was dissolved in methanol to 28 mg/ml and stored at 4°C.
Anti bacterial activity
The preliminary screening for the antibacterial activity was carried out by the following procedure described by Elumalai et al.31 in 2011. The antibacterial activity was investigated using the Pseudomonas aeruginosa (Gram Negative), Escherichia coli (Gram Negative), Bacillus subtilis (Gram Positive) and Staphytococcus aureus (Gram Positive) bacteria.
Disc-diffusion method
The disc diffusion method was used to determine antibacterial activity in the test samples. Mueller Hinton broth was used to produce the target organisms, in which they were kept for 24 hours. The diluted bacterial strains were cultured in the Mueller Hinton agar (MHA) medium containing petri dishes. On the culture medium, the prepared discs were placed. The sterilisation disc had been injected with test samples 500, 1000 and 2000µg. In order to determine the sensitivity of the microbial species tested, Streptomycin 20µg was used as a positive reference standard. Then, the inoculated petri dishes were incubated to 37 C for 24 hours. Measures of the diameter of the disc’s Clear Zone were made and as far as its antibacterial activity is concerned, it expresses in millimeters.
Assay of Alpha-amylase inhibition
The α-amylase inhibitory activity of two sample extracts (from cow’s milk and goat’s milk) was performed according to standard methods with minor modifications32. In this the α-amylase solution of 100 μL (0.1 mg/ml) was mixed with test samples at different concentrations, namely 10, 20, 40, 80, 160 and 320 μg/ml. The standard (Acarbose) and control (no standard/test sample) was used for reference and it is kept for incubation at 37 °C for 15 min. Then, 100 µL of the starch solution was added to start the reaction and incubated at 37 °C for 60 min. After that 10 µL of 1 M HCl and 100 µL of the iodine reagent were added to the test tube. The absorbance of these mixtures were measured at 565 nm and the activity of α-amylase inhibition was measured using the following formula,
% Inhibition = [(OD of test – OD of control)/OD of test] x 100
Results
In this study the sample of cow and goat raw milk were collected from Koyambedu farm house in Chennai. These samples were undergone to isolation, purification of Lactobacillus sp., extraction of secondary metabolites, antibacterial activity and alpha amylase inhibition activities.
Isolation and purification of Lactobacillus sp. from cow and goat milks
A total of two purified Lactobacillus sp. cultures were isolated from cow and goat milk using deMan Rogosa Sharpe medium. On the basis of colony morphology (viz. colour, shapes of the colony) the gram staining test were analyzed.
Morphological and cultural characterization of isolates
Cultural characterization
The isolated bacterial cultures were observed for colony morphology on MRS agar plates and the isolated both colonies were found Gram +ve rods, creamish white in colour and circular in shape (Table 1 and Figure: 1 and 2). The cultures were subjected to further confirmation using the analysis of Gram staining.
Table: 1. Characterization of isolates from cow and goat milk
| S.No. | Collection area | Source | Gram’s Reaction | Colony Morphology
(Colour, Shape) |
| 1 | Koyambedu farm house | Cow milk | Gram +ve rods | Creamish white, circular |
| 2 | Goat milk | Gram +ve rods | Creamish white, circular |
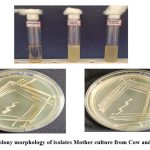 |
Figure 1: Colony morphology of isolates Mother culture from Cow and Goat milk Click here to view Figure |
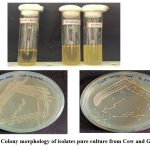 |
Figure 2: Colony morphology of isolates pure culture from Cow and Goat milk Click here to view Figure |
Gram staining
The Gram staining characteristics and cellular morphology of isolated bacterial cultures were examined using standard staining procedures. The Gram-positive bacteria retained the crystal violet colour after gram staining. The isolated bacterial culture of cow milk was found purple coloured and rod shaped of varying sizes, under 40x are shown in Figure 3 (A) , and the isolated bacterial culture of goat milk was also found purple coloured and rod shaped, under 40x Figure 3 (B) which confirms the presence of Lactobacillus sp.
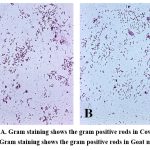 |
Figure 3: A. Gram staining shows the gram positive rods in Cow milk and B. Gram staining shows the gram positive rods in Goat milk. Click here to view Figure |
Extraction of secondary metabolites from Lactobacillus sp.
The two isolated Lactobacillus sp. from goat and cow milk samples were subjected to the extraction of secondary metabolites using the solvent ethyl acetate. The yield of the secondary metabolites from cow milk Lactobacillus sp was found138 mg and the goat milk Lactobacillus sp was found 96 mg respectively (Table 2 and Figure 4). The high yield of secondary metabolites was found from cow milk Lactobacillus sp. when compared to the goat milk Lactobacillus sp.
Table 2: Extraction of secondary metabolites from lactobacillus sp.
| S.No. | Sample | Culture volume (mL) | Extract (mg) |
| 1 | Cow milk isolate | 1000 | 138 |
| 2 | Goat milk isolate | 1000 | 96 |
 |
Figure 4: Extraction of secondary metabolites from the isolated Lactobacillus sp. from Cow and Goat milks using Ethyl acetate. Click here to view Figure |
Antibacterial activity
Three different dosages i.e., 500, 1000 and 2000 µg of each extract of the four species were tested for the antibacterial activity. Two Gram-positive and two Gram-negative strains were selected for this study (Bacillus subtilis, Staphylococcus aureus, Escherichia coli and Pseudomonas aeruginosa). The results were measured zone of inhibition in mm by each extract at each dose. Streptomycin was used as the antibacterial standard drug. DimethylSulphoxide (DMSO) used as the control of antibacterial activity. The data are summarized and presented in Table 3 and 4 and Figure 5 and 6. In this investigation, the antibacterial activity were observed at 1000 and 2000 µg in cow milks extractions against only the Bacillus subtilis and Pseudomonas aeruginosa (Figure 5) but in goat milk extractions was observed at 2000 µg against all the four species viz, Bacillus subtilis, Staphylococcus aureus, Escherichia coli and Pseudomonas aeruginosa (Figure 6) but no activities were found in 500 µg in cow milk extractions and 500 and 1000 µg in goat milk extractions.
Table 3: Antibacterial activity of the cow milk Lactobacillus sp. extract
| Species | The Zone of inhibition (mm) | ||||
| 500 µg | 1000 µg | 2000 µg | Standard 20 µg | DMSO | |
| Bacillus subtilis | 0 | 8 | 10 | 19 | 0 |
| Staphylococcus aureus | 0 | 0 | 0 | 23 | 0 |
| Escherichia coli | 0 | 0 | 0 | 22 | 0 |
| Pseudomonas aeruginosa | 0 | 10 | 16 | 19 | 0 |
Table 4: Antibacterial activity of the goat milk Lactobacillus sp. extract sp.
| Species | Zone of inhibition (mm) | ||||
| 500 µg | 1000 µg | 2000 µg | Standard 20 µg | DMSO | |
| Bacillus subtilis | 0 | 0 | 11 | 19 | 0 |
| Staphylococcus aureus | 0 | 0 | 10 | 23 | 0 |
| Escherichia coli | 0 | 0 | 8 | 22 | 0 |
| Pseudomonas aeruginosa | 0 | 0 | 9 | 19 | 0 |
 |
Figure 5: Image shows the Zone of inhibition of ethyl acetate extract of cow milk’s isolate. Click here to view Figure |
 |
Figure 6: Picture shows Zone of inhibition of ethyl acetate extract of goat milk’s isolate. Click here to view Figure |
Antidiabetic activity
The alpha amylase inhibition assay was performed using Unuofin et al., 2018. The result showed the percentage inhibition of ethyl acetate extract of cow milk Lactobacillus sp. was found 19.89% in 10 µg/mL concentration and 97.51% in 320 µg/mL concentration and the IC50 value was found 36.85 µg/mL (Table 5). Similarly, the percentage inhibition of ethyl acetate extract of goat milk Lactobacillus sp. was found 19.77% in 10 µg/mL and 97.45% in 320 µg/mL and the IC50 was found 33.07µg/mL (Table 6). The percentage inhibition of standard acarbose was found 60.32% in 10 µg/mL and 95.82% in 320 µg/mL and the IC50 was found 1.35µg/mL (Table 7).
The overall results of both cow milk and goat milk Lactobacillus sp. were showed good results of antidiabetic activity (Figure 7). Although the IC50 value were showed moderate activity in the two extractions when compared to acarbose standard.
Table 5: Antidiabetic activity of the ethyl acetate extract of cow milk’s Lactobacillus sp.
| Sample | Different Concentrations (µg/mL) | % of inhibition | IC50 value (µg/mL) |
| Ethyl acetate extract of cow milk isolate | 10 | 19.89 | 36.85 |
| 20 | 25.62 | ||
| 40 | 44.36 | ||
| 80 | 84.05 | ||
| 160 | 96.34 | ||
| 320 | 97.51 |
Table 6: Antidiabetic activity of ethyl acetate extract of goat milk’s Lactobacillus sp.
| Sample | Different Concentrations (µg/mL) | % of inhibition | IC50 value (µg/mL) |
| Ethyl acetate extract of goat milk’s isolate | 10 | 19.77 | 33.07 |
| 20 | 30.77 | ||
| 40 | 53.06 | ||
| 80 | 80.73 | ||
| 160 | 96.92 | ||
| 320 | 97.45 |
Table 7: Antidiabetic activity of the standard acarbose.
| Sample | Different Concentration (µg/mL) | % of inhibition | IC50 value (µg/mL) |
| Standard acarbose | 10 | 60.32 | 1.35 |
| 20 | 80.54 | ||
| 40 | 85.21 | ||
| 80 | 90.63 | ||
| 160 | 94.30 | ||
| 320 | 95.82 |
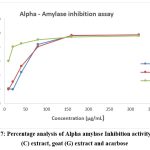 |
Figure 7: Percentage analysis of Alpha amylase Inhibition activity of cow (C) extract, goat (G) extract and acarbose Click here to view Figure |
Discussion
Khedid et al.33in 2009 reported that the presence of high amounts of LAB in cow and goat milks has useful microbiota which indicates that the source for explorations of biological materials of significant public health importance and vast applications in dairy industries. Therefore, this research cow and goat milk samples were collected from Koyambedu farm house, Chennai, Tamil Nadu, India. Based on previous reports, bacteria were grouped based on their gram stain, cell shape, cell organization, acid production from glucose and lactose, gas production from glucose, catalase activity and classify LAB strains34, 35. This research the gram stain was used for morphological identification of Lactobacillus sp. from cow and goat milk and which results confirmed the presence of Lactobacillus sp. The metabolites produced from lactic acid bacteria such as bacteriocin, hydrogen peroxide, acetic acid, lactic acid and low molecular weight proteins which results antimicrobial activities36, 37, 38, 39. The yield of metabolites from cow milk’s Lactobacillus sp. was found to be high when compared to the metabolites from goat milk’s Lactobacillus sp. These metabolites were used for the antibacterial activity. The Microbial peptides with pronounced antimicrobial activity are isolated from microbes, plants, animals, and in fermented food. Among these antimicrobial metabolites, the bacteriocins are proteins or peptides with bacteriostatic action which especially against to the closely-related species40, 41. Elegado et al.42 in 2004 reported that the metabolite bacteriocin produced from Lactobacillus plantarum which is against to Staphylococcus aureus. In this study the antibacterial activity was carried out using the two extracted metabolites. The good results of zones were observed at 1000 and 2000 µg in cow milk Lactobacillus sp. extraction but goat milk Lactobacillus sp. extractions were observed only at 2000 µg. Therefore, in this investigation the Lactobacillus sp. producing secondary metabolites was good source for the activity of antibacterial.Various researchers have been reported that some of the health-associated benefits of consuming probiotics include immune stimulatory,43, 44 and antidiabetic effects45. According to Zhang et al., 46 in 2016 found that probiotics also have the ability to exert antidiabetic effects on insulin resistance in patients with type 2 diabetes. Based on the previous report in this study also proved that the extracted secondary metabolites from the Lactobacillus sp. were showed good antidiabetic activity.
Conclusion
The natural raw cow and goat milk were contains the lactic acid producing bacteria such as Lactobacillus spp. These bacteria contain various beneficial metabolites compounds and which is used in the field of pharmaceuticals. In this study the secondary metabolites from the Lactobacillus sp. isolated from cow and goat milk were showed good results to treat microbial disease and diabetics. Moreover the Lactobacillus sp. is also use in many food industries. These are eco-friendly and cost effective at present and future.
Acknowledgement
Authors acknowledge sincere thanks to the Chairman, Vice-Chairman, Secretary, Principal, Vice-Principal and the Department of Biotechnology, Apollo Arts and Science College, Poonamallee, Chennai – 602 105, Tamil Nadu, India. Authors also express their sincere thanks to the Chancellor, Vice-Chancellor, Secretary, Principal, Vice-Principal and Department of Biotechnology, Dr. M.G.R. Education and Research Institute University, Maduravoyal, Chennai, Tamil Nadu, India.
Conflict of Interest
There is no conflict of interest.
Funding Sources
there is no funding sources
References
- Lowy, F.D. Staphylococcus aureus New England Journal of Medicine. 1998; 339: 520–532.
- Savadogo, A., Ouattara Cheik, A.T., Bassole Imael, H.N., Alfred, T. Antimicrobial activities of lactic acid bacteria strain from Burkina Faso fermented milk. Pakistan Journal of Nutrition. 2004; 3: 174-179.
- Gálvez, A., Abriouel, H., López, R.L., Omar, B.N. Bacteriocin-Based Strategies for Food Biopreservation. International Journal of Food Microbiology. 2007; 12: 51-70.
- Lipsitch, M., Bergstrom, C.T., Levin, B.R. The epidemiology of antibiotic resistance in hospitals: paradoxes and prescriptions. National Academy of Science, USA. 2000; 97: 1938-1943.
- Classification of diabetes mellitus 2019. https://www.who.int/health-topics/diabetes
- Cheng, A.Y.Y., Fantus, I.G. Oral antihyperglycemic therapy for type 2 diabetes mellitus. Canadian Medicinal Association Journal. 2005, 172(2):213-226.
- Tarling, C.A., Woods, K., Zhang, R., Brastianos, H.C., Brayer, G.D., Andersen, R.J., Withers, S.G. The Search for Novel Human Pancreatic α-Amylase Inhibitors: High-Throughput Screening of Terrestrial and Marine Natural Product Extracts. ChemBioChem 2008, 9:433-438.
- FAO/WHO. “WHO working group report on drafting guidelines for the evaluation of probiotics in food,” in Guidelines for the Evaluation of probiotics in food WHO, Ontario, Canada. Vol. 30.
- Fesseha, H. “Probiotics and its potential role in poultry production: a review,” Veterinary Medicine – Open Journal. 2019. vol. 4(2), pp. 69–76.
- Gareau, M.G., Sherman, P.M., Walker, W.A. “Probiotics and the gut microbiota in intestinal health and disease,” Nature Reviews Gastroenterology & Hepatology, 2010. vol. 7(9), pp. 503–514.
- V´elez, M.P., Hermans, K., Verhoeven, T.L.A., Lebeer, S.E., Vanderleyden, J., De Keersmaecker, S.C.J. “Identification and characterization of starter lactic acid bacteria and probiotics from Columbian dairy products,” Journal of Applied Microbiology, 2007. vol. 103(3), pp. 666–674,
- Wegh, C.A., Geerlings, S.Y., Knol, J., Roeselers, G., Belzer, C. Postbiotics and their potential applications in early life nutrition and beyond. Int J Mol Sci, 2019. 20: 4673.
- Tserovska, L., Stefanova, S., Yordanovu, T. Identification of lactic acid bacteria isolated from katyk, goat Milk and cheese. Culture Collections, 2002: 3: 48-52.
- Chen, Y.S., Yangida, F., Shinahara, T. Isolation and identification of lactic acid bacteria, from soil using enrichment procedure. Applied Microbiol., 2005:40: 195-200.
- Axelsson, L. Lactic Acid Bacteria: Classification and physiology. In: Lactic Acid Bacteria, Microbiological and Functional Aspects. Salminen, A.V. and A.O. Wright (Eds.) ouwehand. Marcel Dekker, New York, 2004: pp: 1-66.
- Hayek, S.A., Ibrahim, S.A. Current Limitations and Challenges with Lactic Acid Bacteria: A Review. Food and Nutrition Sciences, 2013:4, 73-87.
- Aslim, B., Onal, D., Beyatli, Y. Factors influencing auto aggregation and aggregation of Lactobacillus delbrueckii bulgaricus isolated from handmade yogurt. Journal of Food Protection. 2007; 70: 223-227
- Morea, M., Baruzzi, F., Cocconcelli, P.S. Molecular and physiological characterization of dominant bacterial populations in traditional Mozzarella cheese processing. Journal of Applied Microbiology. 1999; 87: 574-582
- Sieladie, D.V., Zambou, N.F., Kaktcham, P.M., Cresci, A., Fonteh, F. Probiotic properties of Lactobacilli strains isolated from raw cow milk in the western highlands of Cameroon. Roman. Food Biotechnol. 2011. 9(12): 12- 28.
- Georgieva, R., Yocheva, L., Tserovska, L., Zhelezova, G., Stefanova, N., Atanasova, A. Antimicrobial activity and antibiotic susceptibility of Lactobacillus and Bifidobacterium intended for use as starter and probiotic cultures. Biotechnol. Biotechnol. Equip 2015. 29, 84–91.
- Inglin, R.C., Stevens, M.J., Meile, L., Lacroix, C., Meile, L. High throughput screening assays for antibacterial and antifungal activities of Lactobacillus J. Microbiol. Methods, 2015. 114, 26–29.
- Kolida, S., Saulnier, D.M., Gibson, G.R. Gastrointestinal microflora: Probiotics. Appl. Microbiol., 2006. 59: 187-219.
- Kumar, M., Dhaka, P., Vijay, D., Vergis, J., Mohan, V., Kumar, A. Antimicrobial effects of Lactobacillus plantarum and Lactobacillus acidophilus against multidrug-resistant enter aggregative Escherichia coli. J. Antimicrob. Agents, 2016. 48, 265–270.
- Ahn, K.B., Baik, J.E., Park, O.J., Yun, C.H., Han, S.H. Lactobacillus plantarum lipoteichoic acid inhibits biofilm formation of Streptococcus mutans. PLoS One, 2018. 13:e0192694.
- Jamalifar, H., Rahimi, H., Samadi, N., Shahverdi, A., Sharifian, Z., Hosseini, F. Antimicrobial activity of different Lactobacillus species against multidrug resistant clinical isolates of Pseudomonas aeruginosa. Iran J. Microbiol., 2011. 3, 21–25.
- Kang, M. S., Lim, H.S., Oh, J.S., Lim, Y.J., Wuertz-Kozak, K., Harro, J.M., et al. Antimicrobial activity of Lactobacillus salivarius and Lactobacillus fermentum against Staphylococcus aureus. Dis., 2017. 75:ftx009.
- Mirnejad, R., Vahdati, A.R., Rashidiani, J., Erfani, M., Piranfar, V. The antimicrobial effect of Lactobacillus casei culture supernatant against multiple drug resistant clinical isolates of Shigella sonnei and Shigella flexneri in vitro. Iran Red Crescent Med. J 15, 122–126.
- McFarland, L.V. Probiotics for the primary and secondary prevention of difficile infections: a meta-analysis and systematic review. Antibiotics 2015. 4, 160–178.
- Tabrizi, R., Moosazadeh, M., Lankarani, K.B., Akbari, M., Heydari, S.T., Kolahdooz, F., Asemi, Z. The effects of synbiotic supplementation on glucose metabolism and lipid profiles in patients with diabetes: a systematic review and meta-analysis of randomized controlled trials. Probiotics Antimicro, 2018. 10:329–342.
- Gérard, C., Vidal, H. Impact of gut microbiota on host glycemic control. Front Endocrinol, 2019. 10:29.
- Elumalai, E.K., Ramachandran, M., Thirumalai, T., Vinothkumar, P. Antibacterial activity of various leaf extracts of Merremia emarginata. Asian Pac J Trop Biomed. 2011. (5):406-8.
- Unuofin, J.O., Otunola, G.A., Afolayan, A.J. In vitro α-amylase, α-glucosidase, lipase inhibitory and cytotoxic activities of tuber extracts of Kedrostis africana (L.) Cogn, Heliyon, 2018. 4(9), p.e00810.
- Khedid, K., Faid, M., Mokhtari, A., Soulaymani, A., Zinedine, A. Characterization of lactic acid bacteria isolated from the one humped camel milk produced in Morocco. Microbiol. Res., 2009. 164:81–91.
- Abd Gawad, E.l., Abd El Fatah A., Al Rubayyi, K. “Identification and characterization of dominant lactic acid bacteria isolated from traditional rayeb milk in Egypt,” Journal of American Science, 2010. vol. 6, pp. 728–735.
- El-Shenawy, M., Dawoud, E.I., Amin, G.A., El- S.K., Fouad, M.T., Soriano, J.M. “Antimicrobial activity of some lactic acid bacteria isolated from local environment in Egypt,” African Journal of Microbiology Research, 2017. vol. 11, no. 8, pp. 327–334.
- Barnby –Smith, F.M., Roller, S.D., Woods, L.F.J., Barker, M., Nightingale, M., Gibbs, P.A. Production of antimicrobial compounds by lactic acid bacteria. The British Food Manufacturing Industries Research Association. Research Reports. No.662.Leatherhead Food RA, 1989.
- Larsen, A. G., Vogensen, F.K., Josephsen, J. Antimicrobial activity of lactic acid bacteria isolated from sour doughs: Purification and characterization of bavaricin A, a bacteriocin produced by Lactobacillus bavaricus J.Appl. Bacterio, 1993. 75:113-122.
- Daeschel, M.A. Antimicrobial substances from lactic acid bacteria for use as food preservatives. Food Technol, 1989. 43: 164-167.
- Suchita Kholia. Isolation of Lactic Acid Bacteria and Detection of their Antimicrobial. J. on Emerging Technologies, 2017. 8(1): 260-266.
- Klaenhammer, T.R. Bacteriocins of lactic acid bacteria, Biochimie, 1988. 70: 337-349.
- Garneau, S., Martin, N.I., Vederas, J.C. Two peptide bacteriocin produced by lactic acid bacteria. Biochimie, 84; 577-592.
- Elegado, F.B., Guerra, M.A.R.V., Macayan, R.A., Mendozab, H.A., Lirazan, M.B. Spectrum of bacteriocin activity of Lactobacillus plantarum BS and fingerprinting by RAPD-PCR. J.Food Microbiol., 2004. 95:11-18.
- Nagao, F., Nakayama, M., Muto, T., Okumura, K. “Effects of a fermented milk drink containing Lactobacillus casei strain shirota on the immune system in healthy human subjects”, Biosci Biotechnol Biochem, 2000. Vol. 64 No. 12, pp. 2706-2708.
- Gill, H.S. “Stimulation of the immune system by lactic cultures”, International Dairy Journal, 1998. Vol. 8 Nos 5/6, pp. 535-544.
- Zhang, Y., Zhang, H. “Microbiota associated with type 2 diabetes and its related complications”, Food Science and Human Wellness, 2013.Vol. 2 Nos 3/4, pp. 167-172.
- Zhang, Q., Wu, Y., Fei, X. “Effect of probiotics on glucose metabolism in patient with type 2 diabetes mellitus: a meta-analysis of randomized controlled trial”, Medicina, 2016. Vol. 52 No. 1, pp. 28-34.

This work is licensed under a Creative Commons Attribution 4.0 International License.





