Manuscript accepted on : 14-11-2023
Published online on: 30-11-2023
Plagiarism Check: Yes
Reviewed by: Dr. Sonal Tuljaram Kame
Second Review by: Dr. Prabin Shrestha
Final Approval by: Dr. Fernando Lidon
Samira El-Majnaoui1 , Mounia Lekrati2
, Mounia Lekrati2 , Said El Broudi3
, Said El Broudi3 , Fatiha Benkhalti3
, Fatiha Benkhalti3 , Naima Zehhar4
, Naima Zehhar4 , Ahmed Belmouden5
, Ahmed Belmouden5 , Abdellah Houari1
, Abdellah Houari1 and Nadia El-Kadmiri1*
and Nadia El-Kadmiri1*
1Molecular Engineering, Biotechnology and Innovation Team, Geo-Bio-Environment Engineering and Innovation Laboratory, Polydisciplinary Faculty of Taroudant, IBN ZOHR University, Taroudant, Morocco.
2Department of Fisheries Technologies, Higher Institute of Marine Fisheries, Agadir, Morocco.
3Laboratory of Sustainable Development and Health Research, Faculty of Sciences and Techniques, Cadi Ayyad University, Marrakech, Morocco.
4Laboratory of Biotechnology and Molecular Bioengineering, Cadi Ayyad University, Marrakech, Morocco.
5Laboratory of Cell Biology and Molecular Genetics, Faculty of Sciences, IBN ZOHR University, Agadir, Morocco.
Corresponding Author E-mail:n.elkadmiri@uiz.ac.ma
DOI : http://dx.doi.org/10.13005/bbra/3178
ABSTRACT: Extensive harvest of seaweeds in Morocco has caused overexploitation of these resources. Four Moroccan common seaweed species, wild and cultivated, were studied to assess the cultivated species' potential in comparison to their wild counterparts. The macronutrient composition, total phenolic compounds (TPC), as well as potential antioxidant activity (DPPH and FRAP), were established. Seaweeds contained a wide range of proteins (5–16% of DW), lipids (1–3% of DW), carbohydrates (20–59% of DW), and total polyphenols (3-6 mg EAG/g of DW). Red seaweeds contained high levels of polysaccharides (47–59% of DW), mainly the cultivated Gigartina pistillata, and important amounts of proteins, especially Gelidium sesquipedale (14–15% of DW), whereas the cultivated type was richer in proteins. Gracilaria gracilis and wild Gelidium sesquipedale are characterized by notable antioxidant activity, particularly the cultivated Gracilaria gracilis (TPC: 6.807 mg GAE/g of DW; low values of IC50 of DPPH: 53.863μg/mL and FRAP: 67.033μg/mL). Besides, the green seaweed Ulva lactuca is poor in carbohydrates but contains essential amounts of proteins and phenolics. This work highlights the significant potential of cultivating Moroccan seaweeds through algaculture to achieve more sustainable production with enhanced nutrient and bioactive molecule content.
KEYWORDS: Algaculture; Antioxidant activity; Macronutrients; Moroccan seaweeds; Total phenolic content
Download this article as:| Copy the following to cite this article: El-Majnaoui S, Lekrati M, Broudi S. L, Benkhalti F, Zehhar N, Belmouden A, Houari A, El-Kadmiri N. A Comparative Study on Macronutrients Content and Antioxidant Activity of Four Wild and Cultivated Seaweeds from the Moroccan Atlantic Coast. Biotech Res Asia 2023;20(4). |
| Copy the following to cite this URL: El-Majnaoui S, Lekrati M, Broudi S. L, Benkhalti F, Zehhar N, Belmouden A, Houari A, El-Kadmiri N. A Comparative Study on Macronutrients Content and Antioxidant Activity of Four Wild and Cultivated Seaweeds from the Moroccan Atlantic Coast. Biotech Res Asia 2023;20(4). Available from: https://bit.ly/3sSThzz |
Introduction
The majority of seaweed is edible and serves as an excellent source of nutrients for human diets. Algal-derived bioactive compounds have generated considerable nutraceutical interest in seaweed. Polysaccharides, proteins, pigments, phenolic compounds, vitamins (especially B, A, C, D, K, and E), vital minerals (for example, calcium, magnesium, iodine, iron, and potassium) and poly-unsaturated fatty acids (notably Omega 3) constitute an interesting group of compounds.1,2 Phenolic compounds, known as stress and defense substances, are secondary metabolites that can be found in all types of seaweed. They exhibit a wide range of chemical properties, including antioxidants, UV protection, and anti-fouling effects.3,4 Furthermore, seaweeds have the potential to exhibit antibacterial, anti-inflammatory, antiviral, antifungal, and other properties.5,6 The benefits and consequences on human health of bioactive compounds derived from algae have been confirmed by numerous studies.7,8
However, these marine resources are susceptible to overexploitation due to their numerous applications. They are harvested or cultured for a wide array of purposes, including food production, fish feed, functional food ingredients, agricultural fertilizers, pharmaceuticals, cosmetic products, materials, biofuels, fine chemicals…etc.9,10
As wild resources become increasingly limited, algae farming is emerging as a natural solution for producing additional resources. Seaweed stands out as one of the most attractive resources thanks to its remarkable adaptability, fast growth, and durability. According to FAO statistics, global seaweed production, comprising both wild and aquaculture sources, reached 358,200 tons in 2019, with 97% of this production coming from aquaculture. This thriving industry encompasses various algae types, including Rhodophyta (red), Chlorophyta (green), and Phaeophyceae.11
Furthermore, seaweed compounds varied widely depending on various factors. Therefore, there has been a rapid expansion in quantifying the natural variability of algal components due to the increase in the use of seaweeds as pharmacological and nutritional products on an industrial scale.13,10,14
In Morocco, various algal floras are known for their high potential, namely species (Gracilaria gracilis, Gigartina pisttillata and Ulva lactuca) and specifically Gelidium sesquipedale, which is the most collected due to its high-quality “agar-agar”compound12. To preserve their sustainability, Morocco has implemented various management measures, including seaweed farming. The cultivation of these species was carried out for the first time near Casablanca city, on the site of Sidi Rahal.
The current study aims to evaluate and compare the biochemical composition (polysaccharides, proteins, lipids and polyphenols), and antioxidant activity of four different groups (three red seaweed species and one green) of wild and cultivated seaweeds originating from the site of Sidi Rahal.
Materials and Methods
Sampling collection and drying process
The eight macroalgae, consisting of natural and cultivated species, were harvested by hand from the Sidi Rahal site (33°28′39′′N, 7°56′11′′W), located approximately 43 km from the city of Casablanca on the west center Atlantic coast of Morocco, at the end of August 2022. The algaculture method used on this site is horizontal rope cultivation placed between two rockery bands. The seaweeds were taxonomically classified as described in Table 1. After removing epiphytes and sand, the samples were washed with seawater and air-dried, following the traditional practice of the local community where they are sold in a dried form. Subsequently, the cleaned samples were placed in an oven at 30°C for 6 hours and then ground into a fine powder.
Table 1: Code names and origins of the studied algae species.
|
Phylum, order |
Seaweed species |
Origin/Type |
Species code |
|
Rhodophyta, Gracilariales |
Gracilaria gracilis |
Cultivated |
Gg C |
|
Rhodophyta, Gracilariales |
Gracilaria gracilis |
Wild |
Gg W |
|
Rhodophyta, Gigartinales |
Gigartina pistillata |
Cultivated |
Gp C |
|
Rhodophyta, Gigartinales |
Gigartina pistillata |
Wild |
Gp W |
|
Rhodophyta, Gelidiales |
Gelidium sesquipedale |
Cultivated |
Gs C |
|
Rhodophyta, Gelidiales |
Gelidium sesquipedale |
Wild |
Gs W |
|
Chlorophyta, Ulvales |
Ulva lactuca |
Cultivated |
Ul C |
|
Chlorophyta, Ulvales |
Ulva lactuca |
Wild |
Ul W |
Reagents and chemical Products
Folin-Ciocalteu’s reagent, Gallic Acid, Sulphuric Acid 98% DW and bovine serum-albumin (BSA) were brought from (LOBA CHEMIE PVT LTD, INDIA); 2,2-Dipyenyl-1-picrylhydrazyl-free radical (DPPH) 98% powder was brought from (ALTA AESARA, USA); Comassie Brillant Blue G 250 was purchased from (SERVA, USA); Sodium Carbonate anhydrous (Na2CO3) and phenol 5% PA-ACS were provided from (Panreac, EU); Trichloroacetic acid (TCA), Sodium hydroxide pellets (NaOH), methanol, (HCl) and ferric reducing antioxidant activity (FRAP) assay required chemicals were supplied by (Panreac, EU).
Biochemical analysis
Initial moisture
Each sample was oven-dried at 105 °C for 24 hours (Shanghai Boxum, China) to extract the moisture content from it.1,5
Total carbohydrates
The carbohydrate content in dried biomass was estimated using the Phenol-sulphuric acid method16 using a UV/visible single beam spectrophotometer (Akribis Scientific Limited, UK). The extraction was performed using 2.5N HCl,17and the absorbance of the sample was determined at 490nm.The results were identified based on the obtained glucose standard curve.
Total proteins: Proteins in seaweed were obtained via the Bradford method18 with a few modifications. Selections of dried vegetal material were digested in 1 N NaOH for 18 h with discontinued shaking.19 The protein content was determined using a standard curve of a BSA standard at 595nm.
Total lipids
The total lipids were obtained by the method of Bligh and Dyer (1959),with few modifications. The dry powder of the sample was blended with chloroform/methanol/water (4:2:1). The obtained mixture was vortexed for 5 min and centrifuged (1-6P-Sigma). The lipids were collected and evaporated from the lower layer .21 The end result is a percentage of the total dry weight.
Extracts phenolic preparation
The dried powdered samples were soaked in methanol/water (70:30 v/v) at a ratio of (1:10 w/v) mixed well using a vortex for 1 minute according to22 with slight modification. The mixtures were then placed in the centrifugation at 1400 rpm for 20 minutes and filtered through filter paper.
Total phenolic (TPC)
To measure phenol content, an aliquot of 100 µl of the extracts was blended. 500 µl of Folin–Ciocalteu reagent were added. The mixture was kept to stand for 5 minutes before adding 400 µl of aqueous solution of Na2CO3 (7.5%.).1 The solution turned blue after being incubated at room temperature for 30 minutes. The absorbance of the mixture was determined at 765 nm. A calibration curve of gallic acid was plotted to determine TPC, which was expressed as milligrams of gallic acid equivalents (GAE)/gram of extract.
Activities of antioxidants
Radical Scavenging Capacity (DPPH): 1 mL of algal methanol extract was laid in a test tube and added to 2.5 mL of 0.3 mM DPPH ( 2,2-diphenyl-1-picrylhydrazyl) solution.22 Then vortexed for 1 min and placed for 30 min in the dark. The absorbance was evaluated at 518 nm. The percentage of inhibition (I%) was measured as follows:
Ao, A1 are respectively the control and samples absorbance.
The graph of scavenging effect percentage depending on extract concentrations was established. The extract concentration providing 50% inhibition (IC50) was concluded from the graph.
Ferric Reducing Antioxidant Power (FRAP): Different concentrations of extract from each sample were added to 2.5 ml of a 0.2M phosphate buffer solution (pH = 6.6) and 2.5 ml of a 1% potassium ferricyanide solution (K3Fe(CN)6) and then left at 50°C for 20 min By adding TCA solution (10 % w/v), the reaction was stopped. The whole is centrifuged at 6000 g for 10 min. afterward, 2.5 ml of the supernatant of each mixture is then diluted with 2.5 ml of distilled water and 0.5 ml of the ferric chloride solution (0.1 % w/v). Then we read the absorbance at 700 nm and the concentration corresponding to 50% inhibition (IC50) was expressed as µg/ml of extract.23
Statistical methods
All results are calculated as the mean ± standard deviation. The data was analyzed using one-way analysis of variance (ANOVA) with SPSS statistics v.26, with a 5 % level of probability to demonstrate statistical significance. Additionally, differences between means were analyzed through pairwise multiple comparison, specifically Tukey’s honestly significant difference (HSD) test.
Results and Discussion
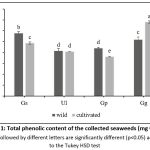
The obtained sugar contents ranged between 20.62% and 59.17% of the dry weight (Table 2). The sugar content in the studied samples varies significantly depending on the species (Gg>Gs>Ul). However, there are no notable differences in the polysaccharide content between wild and cultivated samples for those species, except for Gp, which had the highest concentration of these molecules in its cultivated sample (59.11% DW). The protein contents of these studied samples vary between 5 and 16% of DW (Table 2). The cultivated & wild Gs samples (15.75% DW; 14.37% DW) are very rich in proteins, especially the cultivated type. All samples, whether cultivated or wild, have a statistically similar low lipid fraction ranging from 1.01 to 2.35% DW (Table 2). Indicating that the origin of the seaweeds selected did not affect the lipid content. The Gg samples, particularly the cultivated one, have the highest phenolic content (6.807 mg GAE/g) as shown in Fig.1, while Gp samples had the lowest TPC (cultivated 3.617; wild 4.417 mg GAE/g). The outcomes of antioxidant activity are shown in Fig.2, where strong antioxidant activity is indicated by a low IC50 value.
The results of this research are consistent with prior studies indicating that polysaccharides derived from seaweeds, especially red ones, constitute between 4% and 76% of the algal biomass’s DW.24 The value determined for Gracilaria gracilis (58.62% DW) is similar to that of Gracilaria dura.25 Furthermore, Gelidium is known for its high percentage of polysaccharides, including agar, which can reach up to 41% DW, compared to other genus within the same species (G. Micropterum23.54% ; G. Pusillum25.23%;G. acerosa 24.5% DW).24,26 Gp is known for its high content of carrageenan about 37% DW,27 which is used in the medicinal industry for its anti-tumoral propriety.2,28,25
The protein content of Gs is equivalent to that of G. Corneum (14.61% DW)29, G. microdon (15.18% DW)30 and G. chamissoi (14.08% DW).31 For Gg and Ul, both presented a good amount of protein, and the wild samples were observed to have a higher content than the cultivated ones. The protein content of Gracilaria and Ulva was in line with other studies conducted on Gracilaria salicornia (6.0% DW), Gracilaria sp.(7.0% DW) and Ulva rigida (6.4 % DW).32,33 Besides, the Gp samples (cultivated 5.09% DW; wild 5.95% DW) have a similar, and relatively lower, protein content compared to the other samples. In general, proteins found in algal biomass can varies from 5 to 47% DW of dry weight, depending on the species, environmental conditions, habitat, maturity and extraction method.34,35 Red seaweeds, compared to green seaweeds, have a higher protein fraction.36
The lipids are typically low in seaweeds, from 1 to 7% DW for most macroalgal species.37,38 The values of lipids are like those found by Cavaco al. (2021) for Gelidium sesquipedale (2.62% DW) and byKendel et al. (2015) for Ulva armoricana. Nevertheless, the ratio of long-chained polyunsaturated fatty acids is nutritionally beneficial.40,41
The phenolic content of the collected Gracilaria gracilisis noteworthy compared to other genera of the same species (Gracilaria gracilisis edulis 3.4 ± 0.21 mg GAE/g; G. corticata 4.00 ± 0.35 mg GAE/g )42.Additionally, wild Gs has registered the highest content (5.780mg GAE/g). The diversity in values could be due to the varying production of phenolic compounds in seaweeds can be caused by environmental factors such as nutrients, UV radiation, salinity, or herbivory pressure, as well as tide emersion.43,44 Furthermore, the results reported by Ortiz-Viedma et al. (2021) for red seaweeds such as Gelidium chilense, Gracilaria chilensis, Gigartina chamissoi and others showed that the TPC ranged from 2.6 – 11.3 mg GAE/g, which is consistent with our findings.
Regarding antioxidant activity, Gg had been also shown to have low DPPH scavenging activity and FRAP, with low values of IC50 observed mainly in the cultivated type (53.863; 67.033 μg/mL). Moreover, Wild Gs have been observed to have an important inhibition power (69.520; 84.587 μg/mL), while Gp species, both the cultivated type (112.537; 109.863μg/ml) and the wild type (93.023; 125.250 μg/mL), have registered the weakest performance. In comparison to the results found by Grina et al. (2020) for the same species in another region, Gelidium sesquipedale had the highest activity value of DPPH (84.61 μg/mL) and the same value of FRAP (83.73 μg/mL), while cultivated and wild Ul had the same values of antioxidant activity. Other genus of Ulva had a low scavenger activity compared to the results found in this study of Farasat et al. (2014). Gicartina pisttillata wild was the lowest in ferric reducing antioxidant activity with IC50 (124.070 μg/mL), whereas the wild type presented more scavenging activity potential than the cultivated type (92.146; 11.476 µg/mL). So, this study shows the potential of cultivated seaweed, which can play a promising role in supplying the local and international markets. The cultivated samples presented the best results, with cultivated Gigartina pistillata for its sugar content, cultivated Gelidium sesquipedale for its protein content, and cultivated Gracilaria gracilis for its polyphenols and antioxidant activity.
Table 2: Chemical Composition (% of dry weight) of wild and cultivated seaweeds.
|
Species |
Proteins (g/100gDW) |
Polysaccharides (g/100gDW) |
Lipids (g/100g DW) |
|
Gg C |
5,95±0,21e |
56.70±0.57a |
2.19±0.29a |
|
Gg W |
8,28±0,73c |
58.27±1.46a |
2,22±0,74a |
|
Gp C |
5,09±0,31e |
59.11±1.08a |
1,85±0,29a |
|
Gp W |
5,95±0,19e |
37.10±1.74c |
1,01±0,51a |
|
Gs C |
15,75±0,18a |
50.47±0.99b |
1,51±0,51a |
|
Gs W |
14,37±0,16b |
47.87±1.02b |
2,35±0,77a |
|
Ul C |
5,90±0,12e |
21.14±2.47d |
2,35±0,77a |
|
Ul W |
7,24±0,55d |
20.23±1.46d |
1,85±0,29a |
|
|
Figure 1: Total phenolic content of the collected seaweeds (mg GAE/g).
|
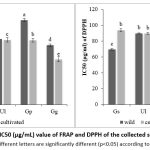 |
Figure 2: IC50 (μg/mL) value of FRAP and DPPH of the collected seaweeds.
|
Means followed by different letters in the same column are significantly different (p<0.05) according to Tukey HSD test
Conclusion
This research underscores the potential of cultivated seaweed as a sustainable and valuable resource compared to wild sources. Moroccan cultivated seaweed samples displayed superior traits, with cultivated Gigartina pistillata containing high sugar content, cultivated Gelidium sesquipedale excelling in protein content, and cultivated Gracilaria gracilis showing significant levels of polyphenols and antioxidant activity. As well, these red seaweeds are also rich in polysaccharides, making them suitable for polysaccharide extraction, and they all have low lipid contents. Ulva, with low sugar content, can serve as a dietary product. This study confirms the viability of seaweed farming as a source of essential bio compounds. The Moroccan policy, which promotes investments in seaweed cultivation, proves highly beneficial for conserving these resources.
Acknowledgements
We acknowledge ENNAHDA cooperative, responsible for the algaculture project at the Sidi Rahal site, for providing us the samples. The Laboratory of Sustainable Development and Health Research (LRDDS), Faculty of Sciences and Techniques, Cadi Ayyad University, Marrakech, is gratefully acknowledged for permitting us to use laboratory facilities.
Conflict of Interest
The authors declare that there is no conflict of interest regarding the publication of this article.
Funding Sources
This work did not receive any specific funding.
References
- Chew, Y.L., Lim, Y.Y., Omar, M., and Khoo, K.S. Antioxidant activity of three edible seaweeds from two areas in South East Asia. LWT – Food Science and Technology. 2008;41, 1067–1072. 10.1016/j.lwt.2007.06.013.
CrossRef - Cotas, J., Leandro, A., Pacheco, D., Gonçalves, A.M.M., and Pereira, L. A Comprehensive Review of the Nutraceutical and Therapeutic Applications of Red Seaweeds (Rhodophyta). Life (Basel). 2020; 10, E19. 10.3390/life10030019.
CrossRef - Singleton, V.L., Orthofer, R., and Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In Methods in Enzymology (Elsevier). 1999; pp. 152–178. 10.1016/S0076-6879(99)99017-1.
CrossRef - Stengel, D.B., Connan, S., and Popper, Z.A. Algal chemodiversity and bioactivity: Sources of natural variability and implications for commercial application. Biotechnology Advances. 2011;29, 483–501. 10.1016/j.biotechadv.2011.05.016.
CrossRef - Rosemary, T., Arulkumar, A., Paramasivam, S., Mondragon-Portocarrero, A., and Miranda, J. Biochemical, Micronutrient and Physicochemical Properties of the Dried Red Seaweeds Gracilaria edulis and Gracilariacorticata. Molecules. 2019;24, 2225. 10.3390/molecules24122225.
CrossRef - Hossain, Md.S., Sifat, S.A., Hossain, M.A., Salleh, S., Hossain, M., Akter, S., and Hossain, M.B. Comparative assessment of bioactive compounds, antioxidant capacity and nutritional quality of red seaweeds and water spinach. Regional Studies in Marine Science. 2021;46, 101878. 10.1016/j.rsma.2021.101878.
CrossRef - Brown, E.M., Allsopp, P.J., Magee, P.J., Gill, C.I., Nitecki, S., Strain, C.R., and McSorley, E.M. Seaweed and human health. Nutr Rev. 2011;72, 205–216. 10.1111/nure.12091.
CrossRef - García, V., Uribe, E., Vega-Gálvez, A., Delporte, C., Valenzuela-Barra, G., López, J., and Pastén, A. Health-promoting activities of edible seaweed extracts from Chilean coasts: assessment of antioxidant, anti-diabetic, anti-inflammatory and antimicrobial potential. Rev. chil. nutr. 2020;47, 792–800. 10.4067/s0717-75182020000500792.
CrossRef - Holdt, S.L., and Kraan, S. Bioactive compounds in seaweed: functional food applications and legislation. J Appl Phycol. 2011;23, 543–597. 10.1007/s10811-010-9632-5.
CrossRef - Tibbetts, S.M., Milley, J.E., and Lall, S.P. Nutritional quality of some wild and cultivated seaweeds: Nutrient composition, total phenolic content and in vitro digestibility. J Appl Phycol. 2016;28, 3575–3585. 10.1007/s10811-016-0863-y.
CrossRef - Zhang, L., Liao, W., Huang, Y., Wen, Y., Chu, Y., and Zhao, C. Global seaweed farming and processing in the past 20 years. Food Prod Process and Nutr. 2020;4, 23. 10.1186/s43014-022-00103-2.
CrossRef - Lebbar, S., Fanuel, M., Le Gall, S., Falourd, X., Ropartz, D., Bressollier, P., Gloaguen, V., and Faugeron-Girard, C. Agar Extraction By-Products from Gelidiumsesquipedale as a Source of Glycerol-Galactosides. Molecules. 2018;23, 3364. 10.3390/molecules23123364.
CrossRef - Fleurence, J. Seaweeds as Food. In Seaweed in Health and Disease Prevention (Elsevier). 2016; pp. 149–167. 10.1016/B978-0-12-802772-1.00005-1.
CrossRef - Roleda, M.Y., Marfaing, H., Desnica, N., Jónsdóttir, R., Skjermo, J., Rebours, C., and Nitschke, U. Variations in polyphenol and heavy metal contents of wild-harvested and cultivated seaweed bulk biomass: Health risk assessment and implication for food applications. Food Control. 2019;95, 121–134. 10.1016/j.foodcont.2018.07.031.
CrossRef - Said, E.B., Hicham, E.F., Naïma, Z., Naji, A., Hafida, B., and Fatiha, B. Effect of Drying Techniques on the Moroccan Pelargonium graveolensL’Hér. Leaves Essential Oil: Yield, Composition, Total Polyphenol Content, Antioxidant Activity, and Hygroscopic Parameters. Journal of Essential Oil Bearing Plants, 2022;25, 508–523. 10.1080/0972060X.2022.2086826.
CrossRef - DuBois, Michel., Gilles, K.A., Hamilton, J.K., Rebers, P.A., and Smith, Fred..Colorimetic Method for Determination of Sugars and Related Substances. Anal. Chem.1956;28, 350–356. 10.1021/ac60111a017.
CrossRef - El-Said, G.F., and El-Sikaily, A. Chemical composition of some seaweed from Mediterranean Sea coast, Egypt. Environ Monit Assess. 2013; 185, 6089–6099. 10.1007/s10661-012-3009-y.
CrossRef - Bradford, M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72(1-2), 248-254.
CrossRef - Foster, G.G., and Hodgson, A.N. Consumption and apparent dry matter digestibility of six intertidal macroalgae by Turbo sarmaticus (Mollusca: Vetigastropoda: Turbinidae). Aquaculture. 1998;167, 211–227. 10.1016/S0044-8486(98)00315-9.
CrossRef - Bligh, E.G., and Dyer, W.J. A RAPID METHOD OF TOTAL LIPID EXTRACTION AND PURIFICATION. Can. J. Biochem. Physiol. 1959;37, 911–917. 10.1139/o59-099.
CrossRef - Erickson, M.C. Lipid Extraction from Channel Catfish Muscle: Comparison of Solvent Systems. J Food Sci. 1993;58, 84–89. 10.1111/j.1365-2621.1993.tb03217.x.
CrossRef - Ling, A.L.M., Yasir, S., Matanjun, P., and Abu Bakar, M.F. Effect of different drying techniques on the phytochemical content and antioxidant activity of Kappaphycusalvarezii. J Appl Phycol. 2015;27, 1717–1723. 10.1007/s10811-014-0467-3.
CrossRef - Chakraborty, K., Joseph, D., and Praveen, N.K. Antioxidant activities and phenolic contents of three red seaweeds (Division: Rhodophyta) harvested from the Gulf of Mannar of Peninsular India. J Food Sci Technol. 2015;52, 1924–1935. 10.1007/s13197-013-1189-2.
CrossRef - Kraan, S. Algal Polysaccharides, Novel Applications and Outlook. In Carbohydrates – Comprehensive Studies on Glycobiology and Glycotechnology, C.-F. Chang, ed. (InTech). 2012; 10.5772/51572.
CrossRef - Kumar, M., Kumari, P., Trivedi, N., Shukla, M.K., Gupta, V., Reddy, C.R.K., and Jha, B. Minerals, PUFAs and antioxidant properties of some tropical seaweeds from Saurashtra coast of India. J Appl Phycol. 2011; 23, 797–810. 10.1007/s10811-010-9578-7.
CrossRef - Baghel, R.S., Reddy, C.R.K., and Jha, B. Characterization of agarophytic seaweeds from the biorefinery context. Bioresource Technology. 2014; 159, 280–285. 10.1016/j.biortech.2014.02.083.
CrossRef - Amimi, A., Mouradi, A., Bennasser, L., and Givernaud, T. Seasonal variations in thalli and carrageenan composition of Gigartina pistillata (Gmelin) Stackhouse (Rhodophyta, Gigartinales) harvested along the Atlantic coast of Morocco. Phycological Res. 2007;55, 143–149. 10.1111/j.1440-1835.2007.00457.x.
CrossRef - Campo, V.L., Kawano, D.F., Silva, D.B. da, and Carvalho, I. Carrageenans: Biological properties, chemical modifications and structural analysis – A review. Carbohydrate Polymers. 2009;77, 167–180. 10.1016/j.carbpol.2009.01.020.
CrossRef - Cavaco, M., Duarte, A., Freitas, M.V., Afonso, C., Bernardino, S., Pereira, L., Martins, M., and Mouga, T. Seasonal Nutritional Profile of Gelidium corneum (Rhodophyta, Gelidiaceae) from the Center of Portugal. Foods. 2021;10, 2394. 10.3390/foods10102394.
CrossRef - Patarra, R.F., Paiva, L., Neto, A.I., Lima, E., and Baptista, J. Nutritional value of selected macroalgae. J Appl Phycol. 2011;23, 205–208. 10.1007/s10811-010-9556-0.
CrossRef - Ortiz-Viedma, J., Aguilera, J.M., Flores, M., Lemus-Mondaca, R., Larrazabal, M.J., Miranda, J.M., and Aubourg, S.P. Protective Effect of Red Algae (Rhodophyta) Extracts on Essential Dietary Components of Heat-Treated Salmon. Antioxidants, 2021;10, 1108. 10.3390/antiox10071108.
CrossRef - Renaud, S.M., and Luong-Van, J.T. Seasonal Variation in the Chemical Composition of Tropical Australian Marine Macroalgae. J Appl Phycol. 2006;18, 381–387. 10.1007/s10811-006-9034-x.
CrossRef - McDermid, K.J., and Stuercke, B. Nutritional composition of edible Hawaiian seaweeds. Journal of Applied Phycology. 2003;15, 513–524. 10.1023/B:JAPH.0000004345.31686.7f.
CrossRef - Ito, K., and Hori, K. Seaweed: Chemical composition and potential food uses. Food Reviews International, 1989;5, 101–144. 10.1080/87559128909540845.
CrossRef - Černá, M. Seaweed Proteins and Amino Acids as Nutraceuticals. In Advances in Food and Nutrition Research (Elsevier). 2011; pp. 297–312. 10.1016/B978-0-12-387669-0.00024-7.
CrossRef - Cian, R., Drago, S., de Medina, F., and Martínez-Augustin, O. Proteins and Carbohydrates from Red Seaweeds: Evidence for Beneficial Effects on Gut Function and Microbiota. Marine Drugs. 2015;13, 5358–5383. 10.3390/md13085358.
CrossRef - Matanjun, P., Mohamed, S., Mustapha, N.M., and Muhammad, K. Nutrient content of tropical edible seaweeds, Eucheuma cottonii, Caulerpa lentillifera and Sargassum polycystum. J Appl Phycol. 2009;21, 75–80. 10.1007/s10811-008-9326-4.
CrossRef - Rodrigues, D., Freitas, A.C., Pereira, L., Rocha-Santos, T.A.P., Vasconcelos, M.W., Roriz, M., Rodríguez-Alcalá, L.M., Gomes, A.M.P., and Duarte, A.C. Chemical composition of red, brown and green macroalgae from Buarcos bay in Central West Coast of Portugal. Food Chemistry. 2015; 183, 197–207. 10.1016/j.foodchem.2015.03.057.
CrossRef - Kendel, M., Wielgosz-Collin, G., Bertrand, S., Roussakis, C., Bourgougnon, N., and Bedoux, G. Lipid Composition, Fatty Acids and Sterols in the Seaweeds Ulva armoricana, and Solieriachordalis from Brittany (France): An Analysis from Nutritional, Chemotaxonomic, and Antiproliferative Activity Perspectives. Marine Drugs. 2015;13, 5606–5628. 10.3390/md13095606.
CrossRef - Kumari, P., Bijo, A.J., Mantri, V.A., Reddy, C.R.K., and Jha, B. Fatty acid profiling of tropical marine macroalgae: An analysis from chemotaxonomic and nutritional perspectives. Phytochemistry. 2013;86, 44–56. 10.1016/j.phytochem.2012.10.015.
CrossRef - Øverland, M., Mydland, L.T., and Skrede, A. Marine macroalgae as sources of protein and bioactive compounds in feed for monogastric animals. J. Sci. Food Agric. 2019;99, 13–24. 10.1002/jsfa.9143.
CrossRef - Mahendran, S., Maheswari, P., Sasikala, V., Rubika, J. jaya, and Pandiarajan, J. In vitro antioxidant study of polyphenol from red seaweeds dichotomously branched gracilariaGracilaria edulis and robust sea moss Hypneavalentiae. Toxicology Reports. 2021;8, 1404–1411. 10.1016/j.toxrep.2021.07.006.
CrossRef - Lomartire, S., Cotas, J., Pacheco, D., Marques, J.C., Pereira, L., and Gonçalves, A.M.M. Environmental Impact on Seaweed Phenolic Production and Activity: An Important Step for Compound Exploitation. Marine Drugs. 2021;19, 245. 10.3390/md19050245.
CrossRef - Pliego-Cortés, H., Bedoux, G., Boulho, R., Taupin, L., Freile-Pelegrín, Y., Bourgougnon, N., and Robledo, D. Stress tolerance and photoadaptation to solar radiation in Rhodymeniapseudopalmata (Rhodophyta) through mycosporine-like amino acids, phenolic compounds, and pigments in an Integrated Multi-Trophic Aquaculture system. Algal Research. 2019;41, 101542. 10.1016/j.algal.2019.101542.
CrossRef - Grina, F., Ullah, Z., Kaplaner, E., Moujahid, A., Eddoha, R., Nasser, B., Terzioğlu, P., Yilmaz, M.A., Ertaş, A., Öztürk, M., et al. In vitro enzyme inhibitory properties, antioxidant activities, and phytochemical fingerprints of five Moroccan seaweeds. South African Journal of Botany. 2020; 128, 152–160. 10.1016/j.sajb.2019.10.021.
CrossRef - Farasat, M., Khavari-Nejad, R.-A., Nabavi, S.M.B., and Namjooyan, F. Antioxidant Activity, Total Phenolics and Flavonoid Contents of some Edible Green Seaweeds from Northern Coasts of the Persian Gulf. Iran J Pharm Res. 2014; 13, 163–170.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.