Manuscript accepted on : 10-05-2023
Published online on: 23-06-2023
Plagiarism Check: Yes
Reviewed by: Dr. Vinayak KS
Second Review by: Dr. Maysaloun Abdulhameed AL-Sadoon
Final Approval by: Dr. Eugene A. Silow
Serum Protein Profiles of Rheumatoid Arthritis Samples –A Case Study
Rupal H Desai1* , Priyanka Dangar 2
, Priyanka Dangar 2 and Jayaprada Rao Chunduri1
and Jayaprada Rao Chunduri1
1Department of Biotechnology, S.V.K. M’S Mithibai College, Vile Parle (W), Mumbai, India.
2Department of Statistics, S.V.K. M’S Mithibai College, Vile Parle (W), Mumbai, India.
Corresponding Author E-mail: rupdimjas@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/3103
ABSTRACT: Rheumatoid arthritis is the 3rd autoimmune disease with a degenerative, chronic inflammatory characteristics. Diagnosis criteria suggested by American College of Rheumatology/European League Against Rheumatism based on serological blood tests and acute phase reactant measurements analyses are the key steps in the diagnosis of disease. Genetic, environmental, or hormonal factors may have contributed to the development of this illness. Characterization of Rheumatoid arthritis-related proteins can be beneficial for early diagnosis, prognosis, and therapeutic aspects. During the current study the serum samples of rheumatoid arthritis subjects were analyzed using serum electrophoresis and Orbitrap Liquid Chromatography-Mass Spectrometry along with biochemical confirmatory tests. The patterns of total protein and gamma globulin ratios, interrelationships of the different test criteria for diagnosis indicated unique pattern. The orbitrap Liquid Chromatography-Mass Spectrometry analyses indicated the presence of 20 unique proteins exclusively in the subjects with Rheumatoid arthritis. Testicular protein Li 227, uncharacterized protein Q6DHW4 and protein S 100-A7 can aid in the early confirmation of the disease. Further analyses of these specific proteins may help in the prognosis, diagnosis, and therapeutic aspect of the disease.
KEYWORDS: Characterization; Correlations; Orbitrap LC-MS; Rheumatoid arthritis; Serum electrophoresis
Download this article as:| Copy the following to cite this article: Desai R. H, Dangar P, Chunduri J. R. Serum Protein Profiles of Rheumatoid Arthritis Samples –A Case Study. Biosci Biotech Res Asia 2023;20(2). |
| Copy the following to cite this URL: Desai R. H, Dangar P, Chunduri J. R. Serum Protein Profiles of Rheumatoid Arthritis Samples –A Case Study. Biosci Biotech Res Asia 2023;20(2). Available from: https://bit.ly/3pmmAZt |
Introduction
Rheumatism covers several disorders that cause pain and inflammation at joins or in connective tissue. Rheumatoid arthritis (RA) is one such, that results due to autoimmune disorder. The development of inflammation, hyperplasia of the synovial lining, and over-activation of osteoclasts, results in pain and the development of chronic synovitis, bone destruction, restricted mobility, and increased mortality. The aetiology of the disease is not known except the possible role played by the genetic or environmental factors. Various auto-antigens and infectious agents (viruses and bacteria) can also initiate RA 1,2. The arginine to citrulline conversion at the post translation level can be evoked by tobacco and smoking. In genetically susceptible individual, this type of reaction triggers the autoimmune disorders 3. The progression of this disorder is modified by the antigens of T and B cell origin 4. The incidence ratio of RA in women is double to that of men though it its overall representation remains low in the world population (1%) 5.
There are several stages to the RA etiology, which is still not fully understood. Rheumatoid factor (RF), an antibody responsive to epitopes on the Fc region of the IgG molecule, is typically thought to be the catalyst for the RA process. “Seropositive” RA is associated with more aggressive articular disease and a high rate of extra-articular manifestations, and the intensity and activity of RA correlate with RF levels 6, 7. Polyarthritis, a form of RA, manifests mainly in the smaller joints in the hands and feet 7. Other organ manifestations, including those of the skin, eyes, lungs, heart, and sometimes even expedited atherosclerosis, are indeed a potentially significant consequence of RA, which can decrease life span 8, 9.
Seventy percent of patients have irreparable joint damage, and eighty percent of active young adults suffer from stiffness and excruciating pain 10. Due to this circumstance, there is a significant loss of productivity in everyday tasks and the workplace, which lowers quality of life 11. Early detection and treatment can lessen patients’ disability and joint damage 12. It is difficult for physicians to forecast the severity of diseases and to select the best early treatment due to the diversity of disease presentations and clinical courses 13.
The American College of Rheumatology (ACR) and the European League against Rheumatism (EULAR) have recommended anti-citrullinated peptide antibodies (anti-CCP/ACPA), C-reactive protein (CRP), and rheumatoid factor (RF) as criteria and parameters to identify early RA patients in order to provide early treatment strategies 14, 15.
An attempt made to study the rheumatoid arthritis at two levels. (1). The possible correlation between suggested ACR and EULAR criteria and biochemical parameters such as uric acid, total protein, albumin, thyroid, sugar levels and Erythrocyte sedimentation rate (2). The identification of unique protein from the globulin samples of the subjects in comparison with normal population to understand their role in the onset of disease.
Materials and Methods
Sample collection
The convenience sampling method was chosen for the sample collection of suspected cases of RA, with a sample size of 500. Healthy volunteers were selected based on criteria such as weight (>50 KG), haemoglobin (> 12 g/dL), and no past history of any illness or medicine.
Serum sample separation and Biochemical assays
Blood was collected from the anti-cubital regions following accepted practices and safety measures. After clot formation, serum was separated by centrifuging at 3000 rpm for 10 to 15 minutes. In addition to additional parameters such as uric acid, total protein, albumin, thyroid hormone levels, blood sugar levels, and erythrocyte sedimentation rate, the testing of serology biomarkers and acute phase reactants was taken into consideration. A chemiluminescent micro-particle immunoassay (CMIA) was used to test for anti-CCP (anti-citrullinated protein antibody) (sensitivity: 70.6%, specificity: 98.2%). Rheumatoid factor and C-reactive protein examinations were carried out with the immunoturbidimetric methodology (sensitivity: 70.6% and specificity: 98.2%). For representative groups of men and women an ESR (erythrocyte sedimentation rate) test was performed.
Serum gel electrophoresis
Representative samples from different score samples (> 6) based on 2010 ACR/EULAR criteria were chosen for horizontal gel electrophoresis. Test was performed with 1 percent Agar-rose gel as per standard protocol and stained with Coomassie blue stain 16.
Gel protein digestion
The Thermo Fisher scientific In-Gel Tryptic Digestion Kit protocol was used. In-gel digestion along with mass spectrometric study was carried out at 37 °C was considered as a promising approach for the identification and characterization of protein17,18. Excised gel pieces were mixed with 200 µl of distaining solution, followed by incubation at 37oc. A series of steps involving reduction, alkylation, and digestion were carried out further. Total procedure took overnight incubation at 30oC.
LC-MS/MS
Digested samples were analysed on a Thermo EASY-Nlc 1000 UPLC system. The Q Exactive TM Quadrupole-Orbitrap instrument is also another part of the system; it is manufactured by Thermo Scientific, Waltham, Massachusetts, USA. The analyses were performed using the pre-column Acclaim PepMap 100, a nanoviper, as well as the analytical column PepMap RSLC C18, 2 um; 100 A x 50 cm. The mobile phase solvents A and B (80:20 ACN in milliQ water plus 0.1% FA and 0.1% formic acid) were used. With a scan range of 350–2000 m/z, the entire MS scan was processed in orbitrap at 70,000 resolutions. The mixture has been run for 120 minutes with an inner instrument diameter of 2.303 mm and a constant flow rate of 3.0 l/min.
Protein identification based on peptide sequencing
Thermo Proteome Discoverer 2.2 software and the Homo sapiens data base were utilized to find proteins and peptides. 2 distinct peptides and a false discovery rate (FDR) of 1% are required for identification 19.
Unique protein within γ globulin
Proteins obtained from healthy control and disease subjects were analysed using Venny 2.1 software. Those proteins found exclusively in the RA subjects while absent in the control were considered as unique proteins and used for further study.
Bioinformatics Shiny Go v0.75
Gene ontology Enrichment analysis was carried out to study functions and association.
Results and Discussion
Number of disease subject
Total number of subjects screened were 500 and 233 were found having ACR/EULAR 2010 score equal to or more than 6. Further analysis per gender wise showed 191 were women and 42 were men.
Biochemical testing
Serological biomarker for RA, such as anti-CCP and RF testing, along with acute phase reactants CRP and ESR, were assessed in serum samples from subjects and controls. Additional tests such as Uric acid to rule out Gout; Total Protein and Albumin analysis to rule out Hypo and hyper proteinemia; Sugar levels to rule out Diabetes; and Thyroid Stimulating Hormone (TSH) levels to rule out hyper or Hypo thyroidism were also carried out for representative groups. The subjects above the reference ranges of anti-CCCP ( <5.0 U/ml) , CRP (0 TO 5 mg/L), RA (< 30 IU/ML & Borderline Positive between 30-50 IU/mL), Uric Acid (2.5 to 6.2 mg/ dl), Total protein (6.2 to 8.1 gm/dl) ,Albumin ( 3.2 to 4.6 gm/dl), Fasting sugar(70-99 mg/dl),TSH (0.35-4.94 ulu/ml), ESR (0-15 mm-men; women-0-20 mm) and, MMP-3 (24-120 ng/ml-women, 18-60 ng/ml –men )were considered which was represented by 46.6%. The representation Rheumatoid effected females were 81.97% while males remained 18.03 %.
The correlation between the selected parameters and the reference biomarkers indicated an extraordinary relationship during the study. Significant positive correlation observed between reference markers viz. CRP and RF (0.73, p-value 0.0), anti CCP and RF (0.54, p-value 0.0), anti-CCP and CRP (0.33, p-value 0.01), however the MMP3 remained unique and no relation with others. The biomarker representation tests also showed positive correlation with other selected biomarkers. ESR showed positive correlation with CRP (0.46, p-value 0.0002), RF (0.44, p-value0.0004) and anti-CCP (0.34, p-value 0.01). RF showed positive correlation with sugar (0.45, p-value 0.0003). MMP3 showed a negative correlation with thyroid stimulating factor (-0.2, p-value 0.13). (Figure.01).
 |
Figure 1: Correlogram representing the relationship between selected parameters and confirmatory markers. |
The serum proteins of the selected samples based on EULAR criteria with very high levels of anti CCP, RF and CRP, MMP3 representation were considered for separation using electrophoresis. All these samples indicated comparatively broad bands of gamma globulin (Figure 02) than the control sample.
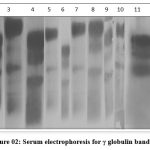 |
Figure 2: Serum electrophoresis for γ globulin band. |
LC-MS results analysis
The proteins of gamma globulin bands of the controls and subjects were further pooled and analysed using LC- MS orbitrap. Results of γ-globulin band proteins from disease subjects and controls were compared. The overall results indicated 20 unique proteins in the gamma globulin bands of Rheumatoid arthritis subjects (Table-1) which were not reported previously. These 20 unique proteins were mainly associated with functions such as apoptosis, epidermis development, and extracellular vesicles.
Table 1: List of Unique proteins observed in serum gamma globulin of arthritis patients
|
Sr.No |
Gene |
Accession number |
Description of protein |
Unique Peptides |
Amino acids |
MW [kDa] |
calc. pI |
|
1 |
KRT72 |
Q14CN4 |
Keratin, type II cytoskeletal 72 |
1 |
511 |
55.8 |
6.89 |
|
2 |
KRT77 |
Q0IIN1 |
Keratin 77 |
1 |
578 |
61.8 |
5.85 |
|
3 |
KRT31 |
Q15323 |
Keratin, type I cuticular Ha1 |
3 |
416 |
47.2 |
4.88 |
|
4 |
HEL-S-271 |
V9HW31 |
ATP synthase subunit beta |
3 |
529 |
56.5 |
5.4 |
|
5 |
gro-EL |
A0A2P9ALX2 |
Chaperone Hsp60, peptide dependent ATPase, heat shock protein |
2 |
539 |
56.8 |
5.39 |
|
6 |
RPS27A |
B2RDW1 |
Epididymis luminal protein 112 |
2 |
156 |
18 |
9.64 |
|
7 |
MC107L |
Q98274 |
MC107L |
1 |
421 |
43.7 |
5.53 |
|
8 |
|
Q6DHW4 |
Uncharacterized protein |
1 |
237 |
25.1 |
7.99 |
|
9 |
|
Q14473 |
Uncharacterized protein |
1 |
175 |
18.9 |
6.79 |
|
10 |
CAT |
P04040 |
Catalase |
1 |
527 |
59.7 |
7.39 |
|
11 |
CASP14 |
A9UFC0 |
Caspase 14 |
1 |
242 |
27.6 |
5.34 |
|
12 |
EEF1A2 |
A0A2U3TZH3 |
Elongation factor 1-alpha |
1 |
496 |
54.3 |
9.04 |
|
13 |
atpA |
A0A2P9ARL3 |
ATP synthase subunit alpha |
1 |
509 |
55 |
6.83 |
|
14 |
SPRR2E |
P22531 |
Small proline-rich protein 2E |
1 |
72 |
7.9 |
8.31 |
|
15 |
|
A0A140VK00 |
Testicular tissue protein Li 227 |
1 |
298 |
34.2 |
6.05 |
|
16 |
DIRAS2 |
Q96HU8 |
GTP-binding protein Di-Ras2 |
1 |
199 |
22.5 |
8.76 |
|
17 |
S100A7 |
P31151 |
Protein S100-A7 |
1 |
101 |
11.5 |
6.77 |
|
18 |
|
A0A1U9X8X6 |
CDSN |
1 |
529 |
51.5 |
8.35 |
|
19 |
pol |
A4GR43 |
Pol protein (Fragment) |
1 |
92 |
9.9 |
8 |
|
20 |
DLST |
P36957 |
Dihydrolipoyllysine -residue succinyl transferase component of 2-oxoglutarate dehydrogenase complex, mitochondrial |
1 |
453 |
48.7 |
8.95 |
Gene ontology Enrichment analysis using Shiny Go v0.75 of the unique proteins results enabled to understand the functions, associations and networking of the proteins. The proteins indicated their involvement in different biological process and their clustering pattern (fig 3, 3a) as well as their presence as cellular components and clusters in the tree forming the major areas of their involvement (Fig.4 and 4a). The biological processes of cornification, keratinization, keratinocyte differentiation, and epidermal cell differentiation are significant. All processes associated with the skin disrupt the corneocyte layer’s functionality, increasing the risk of infection. The intermediate filament cytoskeleton and intermediate filament play a significant role as cellular components. Consequently, transportation functions between cells are impacted.
The presence of 2 unique proteins which are represented as uncharacterized proteins found to represent Ig like domain containing protein (Q6DHW4) and globin family profile domain containing protein (Q14473) as per UNIPROT database.
 |
Figure 3: and 03a. Biological process and the tree indicating the clusters. |
 |
Figure 4: and 04a: Cellular component and the tree associated interaction tree. |
Of these, Q14473 present cytosol component and take a role in the positive regulation of cell death and seem to be associated molecular carrier activity. The other uncharacterized protein Q6DHW4 was represented by 237 amino acids, and an Ig like domain containing protein. This Alpha folded protein has two Ig like domains 5-110(105 aa) Amino acids peptide and 138-232 amino acids peptide (94 aa). Among the animals’ species the Ig like domain is most wide spread, heterogenous group with a common fold. These proteins may show different biological role, amino acid composition depending the site where they present. Being a membrane protein Q6DHW4, with the domain of 94 aa acids, involved in binding and the immune system components such as Immunoglobulins (Ig), Major Histocompatibility Complex (MHC) molecules and T-cell receptors (TcR).
The biological Process network showed groups of pathways sharing many genes together. Bigger dots indicated more significant pathway and the lighter ones representing little significant. Aerial, cellular respiration and energy derivation for the respiration are grouped together into one closed network and distantly connected to the bigger major group of cell development, differentiation and program cell death-based protein/ gene components (Fig.5).
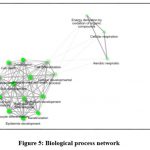 |
Figure 5: Biological process network |
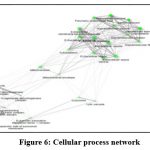 |
Figure 6: Cellular process network |
At the cellular level the component interactions represented by the genes, proteins are similar to that of the network (Fig.6) also showed 5 clusters. The 2 major clusters represented by keratin filament-intermediate filament complex with 6 cellular actions, extracellular vesicle complex; 3 minor complexes of enzyme complex, proton transport complex and lysosomal complex.
A crucial part of the RA disease is played by B-cells, T-cells, and macrophages. Either the synovium or the blood can contain it. Various cytokines and chemokines are produced by them in response to inflammation 20,21. As the symptoms worsen in RA, the synovial lining becomes inflamed, and the neighbouring bones and cartilage get damaged. The pathophysiology of this disease depends upon synovial macrophages, fibroblasts, and lymphocytes, whereby apoptosis may play a number of different roles 22. Apoptosis of the synovial layer cells gets altered due to presence of inflammatory cells and associated with the pathogenesis of rheumatoid arthritis 23.
Several apoptotic cells are evident in the synovial fluid of active RA patients, and experimental study indicates that accelerating apoptosis within in the joint may also have therapeutic effects 23. Apoptosis regulates tissue composition and homeostasis. A change in this function can lead to the pathogenesis of RA, contributing to chronic inflammation and hyperplasia. Synovial fibroblasts become resistant to apoptosis, leading to the progressive destruction of articular cartilage. Fibroblasts are lacking of having mechanism which can prevent programmed cell death function 22,24,2. Thus, detection of these proteins and an in-depth understanding of how they affect apoptosis function can help in early targeted therapy and prevent a more aggressive form of RA.
The stratified squamous epithelium of animals’ outer skin protects them from dehydration, mechanical stress, and infections. The hair follicle (HF), sebaceous gland (SG), sweat gland, and touch dome are a couple of the appendages that constitute the epidermis and are necessary for thermoregulation, environment sensing, and modulating social behaviour. The epithelial tissue initiates a high turnover, and distinct stem cells (SCs) are able to account for the homeostasis of the different epidermal compartments. Dysregulation of the regulating proteins can elevate a human’s vulnerability to infections, which might work as a trigger for an autoimmune disease like RA 25.
Mesenchymal stem cells generate extracellular vehicles (EVs), which have immunomodulatory properties and accelerate cartilage remodelling. These EVs have shown potential therapeutic outcomes in animal models of RA. EVs from neutrophils, myeloid-derived suppressor cells, and dendritic cells could influence the biological activity of the immune system and joint cells 26. In the aetiology of rheumatoid arthritis (RA), cell-derived extracellular vehicles (EVs) are essential in antigen presentation, inflammation, angiogenesis, signal transduction, thrombosis, and the decomposition of articular cartilage extracellular matrix. In-depth understanding of EV functions can help in evaluating RA activity. Monitoring its activity can also express its function as anti-inflammatory or immunosuppressive 27.
Joint deformities, malfunctions, or even loss were eventually brought on by the degradation of joints and the tissues that surround them in RA. With even more than 20 members, the S100 protein family is one of the larger subtribes in the calcium-binding protein family. The aetiology of rheumatoid arthritis is intimately correlated with the overexpression of the majority of S100 proteins. Therefore, keeping track of its presence and functionality can help researchers discover new rheumatoid arthritis treatment targets and clinical diagnostic markers 28.
RA is a condition that induces oxidative stress, and under this condition, testicular protein gets affected and is presented as overexpressed, harming male fertility. It causes decreased production of testosterone. Testosterone induces anti-inflammatory effects by suppressing the cellular and humoral immune systems. The current study indicated the presence of the testicular tissue protein Li 22729. Thus, RA can lead to infertility. These unique proteins were expressed under severe inflammatory conditions in RA.
Conclusion
A distinct corelation exist among the biomarkers, a moderate relation between the biomarkers and a least correlation between biochemical analysis parameters was observed. In contrast, ESR showed positive correlation with anti-CCP, CRP and RF as well as between sugar levels. Protein profiling of RA subjects serum sample showed twenty unique proteins which are exclusively expressed in RA subjects and not expressed in control. These proteins are found to be associated with apoptotic process, the growth of the epidermis, and extracellular vesicles. Thus, all these factors resulted into a complicated, chronic auto immune illness such as rheumatoid arthritis. The uncharacterized protein Q6DHW4 is identified to possess an Ig-like domain and to adhere to T-cell receptors, MHC molecules, and immunoglobulins (Ig), amongst many other immune system components (TcR). Proteins such as Testicular protein Li 227, Q6DHW4 and Protein S 100 can also aid on confirmation of the disease. Further studies enable the application of these unique proteins in the early diagnosis, Prognostic and Therapeutic purpose.
Acknowledgement
We thank SVKM management for providing infrastructure to conduct research work and Dr. Kritika Desai, Principal, S.V.K.M.’s Mithibai College, for encouragement and support. A heartfelt thanks to Ms. Manali Jadhav, coordinator of the mass spectrometry department at SAIF-IIT Bombay, for providing results and guiding the interpretation. We thank Dr. Ajay Shah Pathology Laboratory, Dr. Jariwala Pathology Laboratory and Sunflower Pathology Laboratory for providing permission to collect samples.
Conflict of Interest
There is no Conflict of interest
Funding Sources
No financial support has been taken.
References
- Fang Q., Zhou C., Nandakumar K.S. Molecular and Cellular Pathways Contributing to Joint Damage in Rheumatoid Arthritis. Mediators Inflamm.2020; Mar17;3830212. doi: 10.1155/2020/3830212. PMID: 32256192; PMCID: PMC7103059.
CrossRef - Sparks J.A. Rheumatoid Arthritis. Ann Intern Med.2019 Jan 1;170(1): ITC1-ITC16. doi: 10.7326/AITC201901010. PMID: 30596879.
CrossRef - Kourilovitch M., GalarzaM.C., Ortiz P. Diagnosis and classification of rheumatoid arthritis. Journal of autoimmunity. 2014 Feb 1; 48:26-30.
CrossRef - Cope A.P., Schulze K.H., Aringer M. The central role of T cells in rheumatoid arthritis. Clin Exp Rheumatol.2007 Sep-Oct;25: S4-11. PMID: 17977483
- Handa R., Rao U.R., Lewis J.F., Rambhad G., Shiff S., Ghia C.J. Literature review of rheumatoid arthritis in India. Int J Rheum Dis.2016 May;19(5):440-51. doi: 10.1111/1756-185X.12621. Epub 2015 Jul 14. PMID: 26171649.
CrossRef - Zhang Z., and LouisS.B. Pathogenesis of rheumatoid arthritis. Role of B lymphocytes. Rheumatic diseases clinics of North America.2001;27(2) : 335-53 .
CrossRef - Ngian G.S. Rheumatoid arthritis. Aust Fam Physician. 2010 Sep;39(9):626-8. PMID: 20877764.
- Gabriel S.E., Michaud K. Epidemiological studies in incidence, prevalence, mortality, and comorbidity of the rheumatic diseases. Arthritis Res Ther. 2009;11(3):229. doi: 10.1186/ar2669. Epub 2009 May 19. PMID: 19519924; PMCID: PMC2714099.
CrossRef - Bartok B., Firestein G.S. Fibroblast-like synoviocytes: key effector cells in rheumatoid arthritis. Immunol Rev. 2010 Jan;233(1):233-55. doi: 10.1111/j.0105-2896.2009.00859.x. PMID: 20193003; PMCID: PMC2913689.
CrossRef - Filipovic I., Walker D., Forster F., Curry A.S. Quantifying the economic burden of productivity loss in rheumatoid arthritis. Rheumatology (Oxford).2011 June;50(6):1083-90. doi: 10.1093/rheumatology/keq399. Epub 2011 Jan 18. PMID: 21245074.
CrossRef - Waljee J.F., Chung K.C. Outcomes research in rheumatoid arthritis. Hand Clin. 2011 Feb;27(1):115-26. doi: 10.1016/j.hcl.2010.10.005. PMID: 21176806.
CrossRef - Shovman O., Gilburd B., Watad A., Amital H., Langevitz P., Bragazzi N..L, Adawi M., Perez D., Bornstein G., Grossman C., Lidar M. The diagnostic value of 14-3-3η protein levels in patients with rheumatoid arthritis. Best Practice & Research Clinical Rheumatology. 2018 Aug 1;32(4):610-7.
CrossRef - Khanna N., Kumar A., Pawar S.V. A Review on Rheumatoid Arthritis Interventions and Current Developments.Curr Drug Targets.2021;22(4):463-483. doi: 10.2174/1389450121999201125200558. PMID: 33243118.
CrossRef - Gupta B.M. Arthritis Research in India: A Scientometric Assessment of Publications Output.2017;16(1).
CrossRef - Aletaha D., Neogi T., Silman A.J., Funovits J., Felson D.T., Bingham III C.O., Birnbaum N.S., Burmester G.R., Bykerk V.P., Cohen M.D., Combe B. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis & rheumatism. 2010 Sep;62(9):2569-81.
CrossRef - Syaifudin M.Gel electrophoresis: The applications and its improvement with nuclear technology. AIP Conference Proceedings.2021; 2331. 050008. 10.1063/5.0042067.
CrossRef - Huynh M.L., Russell P., Walsh B. Tryptic digestion of in-gel proteins for mass spectrometry analysis. Methods Mol Biol. 2009; 519:507-13. doi: 10.1007/978-1-59745-281-6_34. PMID: 19381606.
CrossRef - Goodman J.K., Zampronio C.G., Jones A.M.E., Hernandez-Fernaud J.R. Updates of the In-Gel Digestion Method for Protein Analysis by Mass Spectrometry. Proteomics, 2018 Dec;18(23):e1800236. doi: 10.1002/pmic.201800236. Epub 2018 Nov 25. PMID: 30259661; PMCID: PMC6492177.
CrossRef - Li X., Franz T.,Atanassov I., Colby T. Step-by-Step Sample Preparation of Proteins for Mass Spectrometric Analysis. Methods Mol Biol.,2021;2261:13-23. doi: 10.1007/978-1-0716-1186-9_2. PMID: 33420981.
CrossRef - Weyand C.M., Goronzy J.J. The immunology of rheumatoid arthritis. Nat Immunol.2021(22), 10–18. https://doi.org/10.1038/s41590-020-00816-x
CrossRef - Yap H.Y., Tee S.Z., Wong M.M., Chow S.K., Peh S.C., Teow S.Y. Pathogenic Role of Immune Cells in Rheumatoid Arthritis: Implications in Clinical Treatment and Biomarker Development. Cells. 2018 Oct 9;7(10):161. doi: 10.3390/cells7100161. PMID: 30304822; PMCID: PMC6211121.
CrossRef - Baier A., Meineckel I., Gay S., Pap T. Apoptosis in rheumatoid arthritis. Curr Opin Rheumatol.2003May;15(3):274-9. doi: 10.1097/00002281-200305000-00015. PMID: 12707581.
CrossRef - Liu H., Pope R.M. The role of apoptosis in rheumatoid arthritis. Curr Opin Pharmacol.,2003 Jun;3(3):317-22. doi: 10.1016/s1471-4892(03)00037-7. PMID: 12810199.
CrossRef - Mountz J.D., Hsu H.C., Matsuki Y., Zhang H.G. Apoptosis and rheumatoid arthritis: past, present, and future directions. Curr Rheumatol Rep. 2001 Feb;3(1):70-8. doi: 10.1007/s11926-001-0053-y. PMID: 11177773.
CrossRef - Sotiropoulou P.A., Blanpain C. Development and homeostasis of the skin epidermis. Cold Spring Harb Perspect Biol.2012 Jul 1;4(7): a008383. doi: 10.1101/cshperspect.a008383. PMID: 22751151; PMCID: PMC3385954.
CrossRef - Miao H.B., Wang F., Lin S., Chen Z. Update on the role of extracellular vesicles in rheumatoid arthritis. Expert Rev Mol Med.2022 Mar 17;24: e12. doi: 10.1017/erm.2021.33. PMID: 35297366; PMCID: PMC9884793.
CrossRef - Fu H., Hu D., Zhang L., Tang P. Role of extracellular vesicles in rheumatoid arthritis. Mol Immunol. 2018 Jan; 93:125-132. doi: 10.1016/j.molimm.2017.11.016. Epub 2017 Nov 22. PMID: 29175592.
CrossRef - Wu Y.Y., Li X.F., Wu S., Niu X.N., Yin S.Q., Huang C., Li J. Role of the S100 protein family in rheumatoid arthritis. Arthritis Research & Therapy. 2022 Dec;24(1):1-9.
CrossRef - Lashkari M., Noori A., Oveisi S., Kheirkhah M. Association of serum testosterone and dehydroepiandrosterone sulfate with rheumatoid arthritis: a case control study. Electron Physician. 2018 Mar 25;10(3):6500-6505. doi: 10.19082/6500. PMID: 29765575; PMCID: PMC5942571.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





