Manuscript accepted on : 21-02-2023
Published online on: 26-05-2023
Plagiarism Check: Yes
Reviewed by: Dr. Vishal Patel
Second Review by: Dr. Vinayaka K.S
Final Approval by: Dr. Chateen Izaddin Ali Pambuk and Dr. Eugene A. Silow
Shweta Avhad1*, Vidya Morkar1, Sagar Shinde1, Chaitanya patki1, Hemant Chikhale2 and Laxmikant Borse3
1Department of Post Graduate Study in Quality Assurance, Sandip Institute of Pharmaceutical Sciences, Affiliated to Savitribai Phule Pune University Mahiravani, Nashik-422213, MS, India
2Department of Pharmaceutical Chemistry, Sandip Institute of Pharmaceutical Sciences, Affiliated to Savitribai Phule Pune University, Mahiravani, Nashik-422213, MS, India.
3Department of Pharmacology, Sandip Institute of Pharmaceutical Sciences, Affiliated to Savitribai Phule Pune University, Mahiravani, Nashik-422213, MS, India
Corresponding Author E-mail:avhad.shwetaa@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/3099
ABSTRACT: Many questions have been raised regarding the management of acquired immunodeficiency syndrome (AIDS) which is caused by a retrovirus called as HIV, (human immunodeficiency virus) is what causes AIDS. Infection caused by HIV is particularly the world's most serious health and development challenges. Although there is no known complete cure for HIV, several drugs can help you stay healthy by lowering the amount of HIV in your body. When treating HIV infection, antiretroviral therapy is used, and a variety of medications are available from this category. Tenofovir and its salt versions, both by themselves and in combination with emtricitabine, are the most often utilized medications. HIV levels should be lowered so that your immune system can function more effectively. This article offers a summary and evaluation of several analytical techniques used on the antiretroviral medication tenofovir over the previous five years. It covers forced degradation, HPLC and RP-HPLC, HPTLC, UPLC and RP-UPLC, LC-MS.
KEYWORDS: AIDS; Analytical techniques; Antiretroviral; HIV; HPLC; Tenofovir
Download this article as:| Copy the following to cite this article: Avhad S, Morkar V, Shinde S, Patki C, Chikhale H, Borse L. Recent Advances in Analytical Method Development and Validation Techniques for Anti-HIV Pharmaceuticals of Tenofovir. Biosci Biotech Res Asia 2023;20(2). |
| Copy the following to cite this URL: Avhad S, Morkar V, Shinde S, Patki C, Chikhale H, Borse L. Recent Advances in Analytical Method Development and Validation Techniques for Anti-HIV Pharmaceuticals of Tenofovir. Biosci Biotech Res Asia 2023;20(2). Available from: https://bit.ly/3oxhbON |
Introduction
Viral infection caused by HIV weakens the immune system, by destroying CD4 T cells which plays major role against infection. The last stage of human immunodeficiency virus (HIV) infection is identified as acquired immunodeficiency syndrome (AIDS). At the end of 2021, there were 38.4 million [33.9-43.8 million] HIV-positive folks worldwide. The majority of research on the frequency of ocular problems in HIV/AIDS has been done in affluent nations.1 AIDS is not a well-known, old disease that has been redefined; a lone HIV case discovered long before AIDS was recognised baffled the medical professionals.2 Clarifying the progression of contamination and the virus-host connection in the years previous to incurable disease is essential to understanding its pathophysiology. AIDS has always been associated with a reduction in CD4C, T helper cells in the blood since it was first described.3
In 2001, the Food and Drug Administration (FDA) permitted the tenofovir disoproxil fumarate (TDF) aimed at HIV, further changing disease management and becoming a vital part of backbone therapy for many HIV-positive patients.4 Tenofovir is an ester prodrug of the nucleotide analogue of adenosine 5′-monophosphate and it have its place to the sub class of nucleotide reverse transcriptase inhibitor which inhibits the HIV replication.5 According to current US clinical guidelines, all individuals with HIV illness should start their treatment through two NRTIs (nucleoside or nucleotide analogue reverse transcriptase inhibitors) and one NNRTI.6 There are two different salt from of tenofovir i.e. tenofovir alafenamide fumarate and tenofovir disoproxil fumarate.7 In vitro testing of the disoproxil prodrug revealed significantly increased cell permeability and anti-HIV efficacy.8 despite its effectiveness and lack of side effects, it is associated with skeletal and kidney damage.
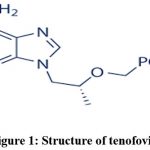 |
Figure 1: Structure of tenofovir. |
Table 1: Drug profile of tenofovir9.
|
Sr. no |
Parameter |
Tenofovir |
|
1 |
Molecular mass |
287.21 g/mol |
|
2 |
Molecular formula |
C9H14N5O4P |
|
3 |
Melting point |
117-120⁰c |
|
4 |
pKa |
3.8 |
|
5 |
IUPAC name |
[(2R)-1-(6-aminopurin-9-yl) propan-2-yl] oxymethylphosphonic acid |
|
6 |
Storage |
20-25⁰c |
Analytical methods
In particular, analytical chemistry has an essential part in the production process of medications, ensuring the quality, safety, and efficacy of novel medications. The invention of a drug particle which has demonstrated healing value to fight, regulate, check, or treat illnesses is the initial stage of medication development process. Identification of drug candidates for more extensive research requires the synthesis, characterisation, and analysis of these particles, which are similarly known as active pharmaceutical ingredients (APIs), as well as their examination to produce initial protection and therapeutic effectiveness information.10
Types of analytical techniques
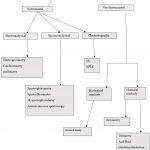 |
Graph 1: Types of analytical techniques |
Table 2: percentage of various analytical techniques recommended in order to test bulk medicinal materials in USP XXVII and Ph. Eur.11,12
|
Method |
Ph. Eur. % |
USP 27 % |
|
HPLC |
15.5 |
44 |
|
GC |
2 |
2.5 |
|
Titration |
69.5 |
40.5 |
|
Acid-Base |
57.5 |
29.5 |
|
Aqueous combinations |
21 |
5.5 |
|
Indicators |
6.5 |
4.5 |
|
Potentiometric |
14.5 |
1 |
|
Non-aqueous |
36.5 |
24 |
|
Indicator |
9.5 |
14 |
|
Potentiometric |
27 |
10 |
|
Redox |
6.5 |
5.5 |
|
Complexometry, argentometry, etc. |
5.5 |
5.5 |
|
UV-vis spectrophotometry |
9.5 |
8.5 |
|
Microbial assay |
3 |
2.5 |
|
Other (Atomic absorption spectroscopy, IR, NMR, polarimetry, fluorimetry, polarography, and gravimetry etc. |
0.5 |
2 |
RP-HPLC and HPLC
Reversed-phase high performance liquid chromatography (RP-HPLC) is the commonly performed and often employed HPLC mode and, as the name suggests implies that this method is simply NP-HPLC in reverse, If the stationary phase has a greater non-polarity than the solvent that elutes. RP-HPLC typically does have a stationary nonpolar phase, for instance C18 silica, and a mobile phase that is mildly polar.13,14 Surface-modified silica is a stationary phase that is most often employed in RP-HPLC method. They move more slowly along the column due to the silica, RMe2SiCl, where R is an alkyl group solvent with a straight chain. This results in a prolonged retention time. Polar compounds travel more swiftly through the column in RP-HPLC. When analysing, attempting to separate, and identifying compounds from a complicated mixture, RP-HPLC is frequently the most advantageous option because it permits purification of the majority of chemical classes, as well as those included in various herbal products.15
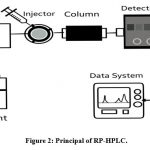 |
Figure 2: Principal of RP-HPLC. |
I have reviwed around 200 papers on system development and authentication of anti- retroviral drug tenofovir by using RP-HPLC and HPLC method from various examination engines like pubmed, scopus, google scholar, research gate, science direct, and elesevier. In this article I have included selected papers from 2017 onwards and their summary.
Kalpana Nekkala et. al. in 2017: The chromatographic separation was accomplished use a moveable phase with 0.1% v/v TFA by water and acetonitrile using a straightforward incline programme on a column of Luna C8 150*4.6mm on perticular temperature. 1 ml per min was indeed the movement rate, then the UV detector stayed to 260 nm. Tenofovir Disoproxil Fumarate discovered to have an average retention duration of 5.330 minutes. The proposed method’s selectivity, precision, linearity, and accuracy were all verified. There were no validation parameters outside of the permitted range. Tenofovir DF testing techniques were discovered to be linear from 75.0 to 225.0g/ml. They came to the conclusion from this experiment that the newly created approach for simultaneously estimating the TENOFOVIR Disoproxil Fumarate discovered towards straightforward, accurate, exact, high resolution, with time-efficient which made this technique extra palatable and economical.16
Yogesh Pawar et. al. in 2017: Tenofovir Disoproxil Fumarate RP-HPLC method was created by means of column C-18 (4.6 x 250 mm) as immobile phase, 0.1% OPA in Methanol: Water (85:15 v/v) as mobile phase. During the solvent system, kept flowing on a rate of 0.7 ml/min, the temperature was ambient, and the HPLC system used an Autochro 3000 system with a UV detector with the Younglin (S.K) Gradient System. ICH guidelines were followed in the method’s validation. The newly created HPLC technique was easy, quick, accurate, and precise. Therefore, the technique can be successfully used for the regular analysis of pharmaceutical sector use of tenofovir disoproxil fumarate.17
Bhushan Badgujar et. al. in 2017
UV detector for the Younglin (S.K) Gradient System on C18 (4.6X250 mm, 5) used to conduct the column chromatography, and During the moveable phase, made up of methanol: 1 ml/min of distillate water (60:40 v/v) flow rate Ortho phosphoric acid (0.05%) was used to raise the pH of mobile (pH-3). A UV detector operating at 260 nm was used for detection. On the basis of ICH Q2(R1) criteria, parameters like linearity, precision, correctness, ruggedness, LOD, and LOQ were investigated. Tenofovir had a retention time of 7.38 minutes. Tenofovir alafenamide is having a linearity series of 5-30 g/ml. Tenofovir’s association coefficients were discovered to be 0.999. The developed approach for simultaneously estimating tenofovir alafenamide and emtricitabine in pharmaceutical dose forms was proven to be exact, precise, selective, and quick. The suggested technique can be helpful for pharmaceutical dosage forms and bulk production quality control.18
Venkateswara Rao et.al. in 2017
Using a [Agilent TC-C18 (2) column with a C18 column 5mm, 4.6’250 mm] held on room temperature and a moving phase made up of phosphate buffer and methanol (30:70 v/v, pH 4), estimation of the medicines in this combination was accomplished. There was a stream of 1 ml/min, and a variable wavelength UV sensor was used towards examine the effluents by 261 nm. Tenofovir disoproxil fumarate had a 2.81-minute retention time. The technique’s authentication was passed in keeping through the ICH recommendations aimed at several analytical parameters. For Tenofovir disoproxil fumarate, the technique designed toward being linear throughout a series of 40-80mg/ml. With an a % RSD value of lesser than 2 and robustness and accuracy was found to be within the given bounds, the established procedure was demonstrated to be repeatable. Tenofovir disoproxil fumarate was assayed and found to contain 97.58% of the commercial formulation. When the concerned samples were examined, it was discovered that the suggested approach was both specific and stable, suggesting that there were no interfering peaks of excipient and degradation chemical concentrations. successfully used for commercial formulation estimate.19
Arun Ramaswamy et. al. 2018
The Zorbax SB CN, column (250 4.6 mm and 5 m) was employed. At 260 nm, UV detection was carried out. Methanol (A) and buffer with a pH of 4.5 (B) were used in the mobile phase, with the gradient being 0–10 min: 90% B; 10–22 min: 35% B; 22–25 min: 90% B. 1.5 ml/min of flow occurred at ambient temperature. There sample injection volume was 20 l. The technique demonstrated linearity (r2 > 0.999), precision (RSD 0.76%), accuracy (retrieval of 99.88% for Tenofovir), specificity, and robustness. Tablets containing emtricitabine, tenofovir, and efavirenz were tested by means of the approved technique on three different batches. Tenofovir concentration ranged from 99.13 to 101.81% in the pill samples. The created method shown to be a quick, exact, accurateness, and acceptable technique for quantifying antiretrovirals. It has the capacity to be used for quality assurance research and future research in additional media, like plasma.20
Aggarwal N et.al 2018
An effective experimental design was created using stress studies and systematic scouting of each essential RP-HPLC technique component. Partings were passed out utilizing an inverted phase column of C-18 (Inertsil ODS, 100 x 4.6 mm, 5 ) employing a movable phase made up of a pH 6.0 ammonium acetate buffer and an ACN:THF solvent blend (30:70) in relation to of (Mobile Phase A) 990:10 and 500:500 (mobile phase B). The mode of isocratic elution was used, then the temperature of the columnar oven was held on 45°C and detecting wavelength used as 260 nm. According to ICH Q2 (R1) recommendations, analytical validation factors like selectivity, linearity, accuracy, precision, and robustness were assessed. Therefore, the suggested approach the ability to regular analysis of Tenofovir Alafenamide Fumarate.21
Sumant Kamatham et. al. in 2018
The HPLC technique was created using methanol: 35:65 v/v 20mM phosphate buffer (pH 5.0) at 271 nm. A SHISEIDO (250 x 4.6 mm i.d., 5, C18 column). TDF retention times were discovered to be 4.91 minutes. For TDF, linearity was shown to exist between 0.75 and 2.0 g/ml. The approach had %RSD 2 for within a day and interday precision, indicating that it was accurate. The recovery remained in the region of 98-102%, and the accurateness of the procedure had tested over three concentration stages. The medications were each put through a seven-day forced degradation study. In the basic medium, TDF degraded the most. The technique was successfully used to measure the dose amounts of the medications in commercial dosage forms.22
Karunakranth d. et. al. in 2018
These medicines were parted through chromatography by means of an INERTSIL column with a C18 (150×4.6 ID) 5 m stationary phase and a solvent system with a 40:60 phosphate buffer: acetonitrile ratio. The technique was verified in accordance by the parameters of ICH i.e. the International Conference on Harmonization. For Tenofovir disoproxil fumarate, the, calibration curves were observed to be linear. In the (r2 -0.995) concentration series of 30-70 g/ml. Tenofovir disoproxil fumarate showed a technique accuracy of 99.70% and a percentage assay of 102.14%. Conferring to the ICH criteria, the projected technique discovered to stand precise, specific, robust, and stable based on percentage relative standard deviation results (2%) for both the precision and robustness studies.23
Saidulu p. et. al. in 2018
Agilent C18 column with (250 4.6 mm, i.d., 5 m) was used and 0.1% formic acid mobile phase: acetonitrile (65:35, v/v), with a 1 mL/min movement rate of isocratic elution, and injection of 20 L sample into the chromatographic system were used to establish the method. The detector used was PDA detector with a detection of 250 nm of wavelength and a temperature of 30 °C, the eluted chemicals were discovered. Tenofovir alafenamide retention periods were discovered to be 6.58 minutes. In the TAF concentration range of 1–5 g/mL. It was discovered that the TAF recoveries were and 99.18%. The developed approach was put through forced degradation tests under predetermined circumstances, which satisfies the necessary requirements. The current approach was precise, sensitive, repeatable, quick, and easy.24
Tej Kumar Kokkirala et. al. in 2019
Chromatogram was conducted within a Denali C18 column (150 mm 4.6 mm, 5 m) with movable phase comprising buffer and acetonitrile in a 50:50 relation [buffer: 0.1 percent at 2.2 pH and 30 °C] being pumped through the column at flow rate of 1 ml/min. The best wavelength used at 272 nm. Tenofovir alafenamide retention periods were discovered to be 3.754 minutes. Tenofovir alafenamide’s %RSD was discovered to be 0.4. Tenofovir alafenamide had a recovery rate of 100.38%. Since this approach was sensitive, exact, and accurate, it has the capacity to be used for monotonous quality control parameter of tenofovir alafenamide in medicinal manufacturing and drug challenging facilities.25
Chaitali Kalamkar et. al. 2019
At room temperature, a Cosmosil C18 column (250mm x 4.6ID x 5m) was used to produce the chromatographic separation. Methanol:water (80:20 v/v) makes up using the mobile phase in the separation process. At the flow rate of 0.8 ml/min and the UV detector was set at 252 nm. Tenofovir Alafenamide Fumarate was found to have an average retention time of 5.293 minutes. Tenofovir Alafenamide Fumarate testing techniques were shown to be linear from 15 to 75 g/ml. The proposed method’s selectivity, precision, linearity, and accuracy were all verified. There were no validation parameters outside of the permitted range.26
Razaei M. et. al. in 2019
It was discovered that the HPLC test technique for TDF was linear in the 15–150 g/mL concentration range. With the use of Phenomenex® C8 column (250 mm x 4.6 mm, 5 m) gradient elution is performed, mixed medicines were successfully separated. Having 1 mL/ min of flow rate, the movable phase used was made up o potassium dihydrogen phosphate, acetonitrile, and methanol (40:40:20 v/v) make up the buffer’s pH, which is 7.0 0.05. The column oven was set to a temperature of about 25 °C. Recovery studies supported the analytic findings. Every validation parameter fell in the permissible range. This approach was created with the goal of simultaneously estimating the amounts of TDF in bulk and commercial dose forms.27
Rajan Rele et. al. 2019
On the isocratic system, a reverse phase HPLC analysis was performed. The column was made of Hypersil BDS C18 and measured 150 mm by 4.6 mm by 5 m. The movable phase was composed of methanol and buffer in a 90:10% (v/v) ratio. The stream was kept at 0.8 ml per minute. Using 260 nm the detection was performed. According to ICH criteria, the technique was verified for system compatibility, linearity, accuracy, and precision. The linearity ranges of tenofovir disoproxil fumarate were 50-150 g/ml. It was determined that the accurateness and precision are substantially inside the permissible series. Tenofovir disoproxil fumarate in dosage form was effectively determined using this approach, with acceptable recoveries.28
Nachiket Dighe et. al. in 2019
On column C18 Cosmosil (250mm x 4.6ID) having the particle size of 5 micron. The process of chromatographic parting was performed using methanol: water (70:30 v/v) was movable phase at 0.8 ml per minute of movement. At a wavelength of 266 nm, the UV-300-M detector was used to carry out the detection. Tenofovir Disoproxil Fumarate’s retention times were discovered to be 5.478 minutes. Run time was 7.05 minutes for Tenofovir Disoproxil Fumarate. Accuracy, precision, ruggedness, specificity, robustness, linearity, and LOD, LOQ of the validation were all discovered to be within standard limits as according ICH requirements. The projected process is used for repetitive quality monitoring of pharmaceutical dosage forms such bulk form and tablet form was proven to remain precise and focused.29
Manojkumar I. et. al. in 2020
The chromatogram was created using a column (Hyper ODS2 C18), 260 nm UV detection, 1.2 ml per min movement rate, and a movable phase having methanol and phosphate buffer (90:10). According to ICH criteria, the technique was verified. using a variety of validation factors, including accuracy, precision, linearity, and specificity. Tenofovir was discovered to have a linearity series of 20-110 g per ml and a retention duration of 2.1 min, respectively. Tenofovir was shown to have a 0.7 percent RSD. For standard and tablets, the recovery percentages were 99.7% and 96.32%, respectively. This approach was straightforward, precise, accurate, and sensitive. As a result, Tenofovir in the pharmaceutical dose form was routinely analyzed using the devised approach.30
Gowri Gollu et. al. in 2020: The Bridge C18X phenyl column (150 4.6 mm, particle size 3 m) was used aimed at the chromatographic parting. It was eluted with acetonitrile and hexane-1-sulfonic acid (2.5 pH; 50:50, v/v) when the movement rate is 0.8 mL per min, and it was run for 12 minutes. The TDF retention times were discovered to be 7.3 min. For TDF, the technique was linear (r2 = 0.999) between 5 and 100 g/mL. The percentage of drug recoveries fell between the permitted ranges (98 – 102%). Without any outside interference excipients, the suggested RP-HPLC method can be utilized to quantify TDF in API and tablets.31
Imran A. et. al. in 2020: Sunfire column C18 (4.6 x 150 mm, particle size 5 m), movement rate of 0.6 ml per min, and mobile phase having the ratio of (60: 25: 15v/v) were used for the chromatography. 0.02M Acetonitrile Dihydrogen potassium phosphate ortho HPLC grade water is the buffer. The wavelength that was detected was 260 nm. Labsolution software was utilized, and the instrument was a Shimadzu LC-20AR. Tenofovir Disoproxil Fumarate retention time was discovered to be 3.835 minutes. Tenofovir Disoproxil Fumarate was discovered to have a purity of 98.99%. Tenofovir Disoproxil Fumarate’s linearity research revealed that it was linear in the range of concentrations of 15-75 g/ml, accompanied by a correlation coefficient (r2) of 0.999, accuracy of 100.2%, and all other validation parameters within acceptable levels according to ICH Guidelines.32
Deeksha Agrawal et. al. in 2020
Without relying on the parameters of temperature and pH, a C18 column was employed to increase the method’s effectiveness, retention, and repeatability. With isocratic elution, the buffer (0.1% trifluoroacetic acid): ACN (65:35 ratio in v/v) mixture was utilized as the movable phase. Having a movement of 1 mL per min, a C18 column (Syncronis C18 250 x 4.6 mm) with particle size of 5 m. According to the provided approach, the linearity between concentration and absorbance ranges from 30-80 g/mL, and the correlation coefficient was 0.997. The plate count and tailing factor were discovered to be 1.03 and 12110, respectively, below the permitted standards. All metrics were validated according to ICH Q2 (R1) standards, with the exception of LOD and LOQ. Temperature and pH were not used in the process of a straightforward, effective, and affordable approach. The provided approach should have ability to analyze the market for TAF in tablet form.33
Dr. Kuna Mangamma et. al. in 2020
The active compounds have been separated using a reverse phase gradient procedure. Using pH 3.2 0.05-adjusted KH2PO4 with 1-octane sulfonic acid solution with trifluoroacetic acid, ACN, and methanol as the movable phase, the substances were present in various quantities and chromatographic behavior. On a reverse phase kinetex biphenyl (2504.6 mm, 5) an average movement rate of 1 mL per min, measured at 260nm, a gradient programming has been carried out. The mean retention time were discovered to be 26.5 minutes of the drug tenofovir. The projected method was effectively used to estimate the amount of tenofovir in mixed tablet dosage forms after being measured for accuracy, range, linearity, precision, specificity, robustness, and stability tests.34
Jaybhave A. et. al. in 2021
The development of a thorough science- and risk-based RP-HPLC method was performed for the quality-by-design examination of Tenofovir Disoproxil Fumarate active pharmaceutical ingredient i.e. API and tablet is described in the current study. The presentation of an effective experimental planning based on meticulous research of the main elements (mobile phase and columns) of the RP-HPLC method. The methodology was linear. (r2=0.99). Additionally, the values for precision, ruggedness, and robustness were all within the permitted ranges. Tenofovir Disoproxil Fumarate can be routinely analyzed in quality control labs using the suggested method.35
Nagaraju Pappula et. al. in 2021
Tenofovir was successfully separated by chromatography using an 4.6 x 150 mm Agilent C18, 5 and a movable phase made up of ACN and 0.01N Na2 HPO4 (50:50, v/v) having movement rate of 1.00 mL per minute. A wavelength used was 272 nm to quantify the medicines. According to International Conference on Harmonization Technical Requirements for Pharmaceutical Registration for Medical Purpose (ICH), the reversed-phase HPLC method has been verified. Under ideal circumstances, the suggested approach demonstrated satisfactory linearity for the concentration series of 3.125–18.75 g per mL for tenofovir. Regarding the RSD values of validation criteria such as linearity, system precision, method precision, robustness, ruggedness, etc., the suggested HPLC technique were notably satisfactory. Tenofovir in tablet form was effectively quantified using the established method.36
Bhushan Bhairav et. al. in 2021
In this method, the valuation of (Tenofovir disoproxil fumarate) was assessed on a Cosmosil C-18 column with dimensions of (250mm, 4.6ID, and 5-micron particles) using Methanol: Water in the ratio of (60:40), 0.9 ml/min of movement rate, recognition wavelength of 260 nm, while the period of retention detected being approximately 4.63 mins by the assay value of 99.07%. In accordance with ICH requirements, the HPLC technique was also validated, and linearity remained in the range of 10–50 g/ml with a regression coefficient of 0.999. The Precision, robustness and accuracy had an RSD of less than 2%. The results of this study allow us to draw the conclusion that proposed procedures for (TDF) drug estimate in medicinal preparation stand straightforward, accurate, and precise, and may applied in regular examination for the measurement of the medication in a dose form.37
Ramreddy Godela et. al in 2021
The Eclipse of the Zorbax XDB-Phenyl column, by using the fluid phase of methanol: buffer (73:27 v/v formic acid, 0.1% v/v in water) with a stream of 1 mL/min also gradient elution by employing detection wavelength of 260 nm, was successful in efficiently and appropriately separating the three analytes. As a diluent, water and acetonitrile were employed in an equal ratio. Tenofovir disoproxil fumarate had retention times of 4.4 min. Tenofovir disoproxil fumarate had a linear response that ranged from 15.0 to 45.0 g/ml. The technique can simultaneously remove tenofovir disoproxil fumarate from its combination tablet and blended powder form. As a result, there is a great likelihood that the proposed method will be used in the pharmaceutical industry.38
Nageswara Rao et. al. in 2021
Performed using a movable isocratic phase containing 0.1% triethylamine buffer, phosphoric acid, ortho, and the ratio of ACN (55%: 45%) was used where the stream rate used was 1.0 mLmin-1, and a chromatographic partition column of YMC Pack ODS-AQ (250mm x 4.6mm x 5m particle size) having the temperature of 300C. The sample cooler temperature was 5°C, and the diluent is a 95:5v/v acetonitrile and water combination. The injection volume was 10 L, and ultra violet and PDA detector systems were used to detect UV at 260 nm. It is determined that the % RSD, Range, precision, linearity, toughness, and accuracy (Intermediate Precision), and Robustness are sufficient.The recently invented RP-HPLC process was quick, easy, and selective. As a result, the technique is valid and acceptable for performing assay of tenofovir disoproxil otate. It was determined that the current investigation was precise, specific, robust, linear and accurate. The validated parameters might be utilized aimed at the quality control analysis of drug samples because they met the ICH guidelines’ prerequisites.39
Akbar Basha et. al. in 2022
Tenofovir disoproxil fumarate and emtricitabine were separated chromatographically using the gradient elution mode, reverse phase, room temperature, and UV recognition at 261 nm of the Thermo scientificTM HypersilTM BDS 5 C18 120A (250 4.60 mm i.d) column. The mobile phase contained methyl and (65:35% v/v) phosphate buffer at pH 2.5. One milliliter per minute of flow was used to elute the chemicals. Emtricitabine and tenofovir disoproxil fumarate had retention durations of 3.718 min. and 2.589 min. accordingly. According to the requirements of ICH, the aforementioned technique was verified in relation of System applicability, linearity, accuracy, precision, limit of detection, and limit of quantification. The technique was quick, easy, affordable, and appropriate for standard quality control analysis.40
Challamalla Pavani et. al. in 2022
With acetonitrile and 0.1% of orthophosphoric acid in a 70:30 volume ratio as the movable phase, the Separation took place. using a DIKMA Spursil, C18, ODS, analytical column at pH 3. Using a PDA detector, the eluents were located at 254.0 nm. Tenofovir alafenamide, Darunavir, Emtricitabine, and Cobicistat reportedly extracted at 2.287 minutes, 2.507 minutes, 4.062 minutes, and 6.011 minutes, individually, at these optimized circumstances. Tenofovir alafenamide scored 99.21%, Darunavir 99.80%, Emtricitabine 99.80%, and Cobicistat 99.84% on the percentage assay. Tenofovir alafenamide was shown to be linear for cobicistat in range of 30.0-150.0 g/mL, darunavir in range of 160.0-800.0 g/mL, and emtricitabine in range of 40.0-200.0 g/mL. For each API, the correlation coefficient discovered to be 0.999. Tenofovir alafenamide’s detection limit was determined to remain 0.14 g/ml, Darunavir’s was 2.14 g/ml, Emtricitabine’s was 0.6 g/mL, and cobicistat’s was 7.32 g/ml. The quantitation limits for tenofovir alafenamide, darunavir, emtricitabine, and cobicistat were 0.47 g per mL, 7.12 g/mL, 2.10 g per mL, and 24.42 g/mL, individually, in the LOQ study. All of the investigated APIs have been determined to have incredibly appropriate for determination in equally the bulk form and the marketed dosage form under the optimized conditions.41
Puja Ramesh Rathod et. al. in 2022
At room temperature, a Cosmosil C18 column (250mm x 4.6ID x 5m) was used to produce the chromatographic separation. Methanol: water in ratio of (80:20 v/v) makes up by the mobile phase in the separation process. There was a 0.8 ml per min flow. and the UV detector remained at 252 nm. Emtricitabine and Tenofovir Alafenamide Fumarate were shown to have average retention times of 4.277 and 5.293 minutes, respectively. The proposed method’s selectivity, precision, linearity, and accuracy were all verified. There were no validation parameters outside of the permitted range. Emtricitabine’s assay methods were discovered to be linear between 10 and 50 mg/ml, and between 15 and 75 mg/ml for tenofovir alafenamide fumarate. The acceptable limit discovered to be fulfilled by every one of the validation parameters. The method’s assessment of its robustness and ruggedness shows that it is unaffected by minor modifications to the chromatographic settings.42
Aakanksha Wankhade et. al in 2022
Using a C18 Inertsil column and an ACN: Ammonium Formate Buffer of pH 5(40:60) mobile phase, the process of chromatographic separation was used. in isocratic mode with Photo Diode Array (PDA) detection at 261nm. For the optimization investigation, a Box-Behnken three-way statistical design components and three levels was chosen. Crucial quality attributes were investigated for the interaction impacts of critical quality parameters (Buffer pH, Organic Phase-% acetonitrile, and flow rate) (Retention time, NTP and symmetry factor). Conferring to the most recent International Conference Harmonization i.e. ICH Q2 R1 criteria, the optimized approach was authenticated. The method was discovered to be linear, with a precision of less than 2% and the correlation coefficient r2 is 0.9996 encompassing the series of 1.5 g/mL to 4.5 g/mL. It was discovered that the Limit of Quantification and Limit of Detection were 1 g per mL and 1.2 g per mL, accordingly. The accuracy extended from 99 to 102 percentage. The technique was effectively used to find the medicine in a commercial dose form. Tenofovir alafenamide fumarate routine analysis was found to benefit from this comprehensive stability indicating chromatographic method with a QbD approach since it ensured reproducibility.43
Rahul S. Solunke et. al. in 2022
1.0 ml per min of a movable phase with 0.1 percent trifluoroacetic acid and acetonitrile (30:70%, v/v) is used in the recommended method. Agilent Zorbax Bonus-RP Column at 265 nm was used for this work (250 x 4.6 mm, 5). The (LOD) and (LOQ) for rilpivirine (4.99 g/ml), tenofovir (13.36 g/ml), and emtricitabine (1.16 g/ml), respectively, were discovered to be satisfactory when this approach was validated in line with ICH Q2(R1) criteria. The method was remained to be accurate, exact, and consistent. When applying the Method, all three concentration ranges examined (Rilpivirine 20–30 g/ml, Tenofovir 240–360 g/ml, and Emtricitabine 160–240 g/ml) were linear. In terms of successful recoveries, all three drugs had an average range of 98% to 101%. The suggested method is straightforward and reasonably priced for instantaneous estimate of rilpivirine, tenofovir, and emtricitabine in the form of bulk and pharmaceutical dose form.44
HPTLC
Most pharmacopoeial monographs lists that the studies of thin layer chromatography as one of the essential identity tests. Industry often bases its compliance with QC criteria and modern good manufacturing practices on pharmacopoeial standards (cGMPs). High-performance thin layer chromatography (HPTLC), a development of TLC, is a reliable, straightforward, quick, and effective technique for quantitative compound analysis.45 HPTLC is still one of the most adaptable, trustworthy, and economical separation techniques, making it perfect for the examination of botanicals and herbal medicines.46 Rapid examination of several chemicals is the goal of high-throughput analysis using HPLTC.47
Sample preparation of HPTLC
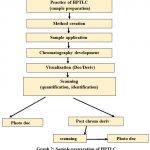 |
Graph 2: Sample preparation of HPTLC |
Arun Kashid et. al. in 2021
Merck TLC 60 F254 aluminum plates having silica gel were the immobile phase. Ethyl acetate: n-hexane: methanol: ammonia solution in the ratio of (4:4:2:0.2, V/V) was the mobile phase utilized. At 260 nm of wavelength EMT, and TAF, densitometric analysis was performed. For the study, the concentration ranges for EMT and TAF were 400–2000 ng per band and 50–250 ng per band, respectively. At retardation factors of 0.43 and 0.56, respectively, EMT and TAF both produced strong, well-defined peaks. According to the stability investigation, samples that were deteriorated by hydrogen peroxide, a base, an acid, and light produced well-separated EMT and TAF crests in addition to a few other peaks with various retardation factor values. The suggested approach complies with ICH principles and is straightforward, appropriate, accurate, and stable.48
Vijaykumar Morea et. al in 2021
Phase II mobility Sharp and symmetrical peaks were achieved in the range of 1.5:5.5:1.5:0.1 v/v/v/v of methanol, toluene, ethyl acetate, and ammonia. It was discovered that good repeatability and peak shape of three pharmaceuticals were guaranteed by prewashing HPTLC plates methanol (drying and activation to follow) and pre-saturating the 20 min HPTLC chamber with mobile phase (the ideal chamber saturation period). The outcomes of the retrieval studies conducted validate the projected process’s high degree of accuracy. The devised chromatographic approach can therefore be effectively used for estimation tenofovir and emtricitabine in its bulk and formulation. It is accurate, precise, and selective.49
Noha S Said et. al. in 2021
The second approach relied on HPTLC and was completed on HPTLC plates that had been covered by silica gel 60 F254 beforehand. It used a movable phase made of acetic acid with n-butanol (7:3, v/v), and 260 nm was used for detection. Tenofovir alafenamide was put to pressure, which included acidic and alkaline deterioration. Pertaining to the micellar UPLC and HPTLC techniques, Beer’s law was seen across the intensity spectrum of 1-18 g mL-1 and 0.1-4 g/spot, respectively. Both approaches have been authenticated conferring to ICH standards and effectively used to analyze the medicine included in its tablets. Additionally, their greenness was evaluated using three separate methodologies, each of which indicated their least harmful environmental impact.50
UPLC and RP-UPLC
The chromatographic system for Ultra Performance Liquid Chromatography (UPLC) uses 1.7-m Packaging materials for reverse phase and operates in the 6000–15,000 psi pressure range.51 For tiny particles under 2 mm in diameter, UPLC is used. With enhanced resolution, it is a highly sensitive, dynamic, and efficient technology. Because it uses less solvents and produces results more quickly, UPLC is both economical and environmentally beneficial.52 Additionally, it provides resolved peaks in the chromatogram and enables reliable simultaneous analysis of a diverse array of analytes.53
I have reviewed around 100 papers on method creation and approval of anti- retroviral drug tenofovir by using UPLC and RP-UPLC method from various search engines like pubmed, scopus, research gate, science direct, google scholar and elesevier. In this article I have included selected papers from 2017 onwards and their summary.
|
Sr. no. |
Author and year |
Description |
Ref no |
|
1 |
Imam pasha in 2017 |
Column: Column with Thermosil Octa Decyl (4.6 x 50 mm dimension, 1.7 mm) Movable phase: KH2PO4, methanol and water Detector: PDA detector Retention times: TAF- 1.528 min EMT- 0.965 min Flow rate: 0.3 ml/min
|
54 |
|
2 |
Jacob jane in 2017 |
Column: UPLC BEH C18 (dimension 100 x 2.1 mm, 1.7mm) Movable phase: A- of buffer (trifluro acetic acid in water) B- methanol Wavelength: 262nm Stream rate: 0.4ml/min Retention time: EMT- 0.6 min TAF- 1.88 min EFV- 3.23 min
|
55 |
|
3 |
Juluri Krishna Dutta Tejaswi in 2017 |
Column: (50 2.1 nm, 1.8 m) Endoversilo C18 Detector: PDA detector Movable phase: acetonitrile, ortho phosphoric acid (70:30 w/v) Stream rate: 0.3 ml per min Retention time: TAF- 4min EMT- 1.4 min Linearity: (r2 =0.999) Wavelength: 252 nm
|
56 |
|
4 |
Bhupatsinh Vihol in 2017 |
Column: (dimension 50 mm x 2.1 mm, 1.7 m) UPLC HSS Movable phase: 20 mM Potassium dihydrogen phosphate buffer: Acetonitrile Stream rate: 0.640 ml per min Wavelength: 262nm Retention time: TAF- 2.239 EMT- 0.715 Linearity: EMT- 20.14- 120.86 mg/ml TAF- 30.01 -180.04 mg/ml
|
57 |
|
5 |
Murali Krishna in 2019 |
Column: BEH Phenyl (100 mm 2.1 mm dimension), 1.7 μ Mobile phase: A- 0.1% water in Trifluoro Acetic acid B- acetonitrile Stream rate: 0.4 mL per min Wavelength: 260nm Detector: PDA
|
58 |
|
6 |
Sravanthi T in 2020 |
Column: HSS C18 (100 × 3 mm, 1.7 μ) Movable phase: 0.01 N buffer of Acetonitrile with potassium dihydrogen phosphate (60:40, vol/vol) Stream Rate: at 0.4 ml per min Wavelength: 265 nm Linearity: TAF- 75 to 450 μg per ml EMT- 50 to 300 μg per ml EVR- 150 to 900 μg per ml
|
59 |
|
7 |
Prasanthi Chengalva in 2020 |
Column: Phenyl Bridged with Ethylene (dimension 50 mm, 2.1 mm, 1.7 m) Movable phase: water and acetonitrile (50:50 v/v) Stream rate: 0.4 ml per min Wavelength: 238 nm Retention times: TDF- 1.233 min LOM- 1.012 min DOR- 1.428 min EFA- 1.666 min
|
60 |
|
8 |
Zhiyuan Ma in 2020 |
Column: UPLC HSS T3 Movable phase: methanol and 0.01% ammonia in 10 mM ammonium acetate/water Retention time: 4 min Linearity: 1–500 ng/mL Precision: −4.35% Accuracy: 6.92% |
61 |
|
9 |
Sudha, P. D in 2020 |
Column: HSS C18, 100 mm × 2.1 mm Detector: PDA Wavelength: 280 nm Movable phase: phosphate buffer and acetonitrile (70:30%V/V) Stream rate: 0.3 min Retaining time: TNF- 1.680 min EMT- 1.403 min COB- 1.921 min ELV- 1.921 min |
62 |
|
10 |
M. Satya Venkata Sakuntala in 2021 |
Column: SB C18 (50 × 2.1 × 1.8 μ) Movable phase: 60:40 ratio of 0.01 N potassium dihydrogen ortho phosphate and ACN Wavelength: 267 nm Stream rate: 0.3 ml per min Retaining time: TAF- 1.031 minutes COB- 1.341 minutes EMT- 1.630 minutes DAR- 2.153 minutes |
63 |
|
11 |
Swetha Addanki in 2021 |
Column: HSS C18 column (dimension 100 × 2.1 mm, 1.8 μ) Mobile phase: 0.01 N Potassium dihydrogen orthophosphate buffer and acetonitrile 60:40 v/v Stream rate: 0.3 mL/min Wavelength: 260 nm Retaining time: TAF- 1.8 min DOR- 1.2 min LAM-1.5 min |
64 |
|
12 |
Kishore konam in 2021 |
Column: BEH C18 125A° (100 × 2.1 mm, particle size 1.7μ) Movable phase: Buffer, Methanol and Acetonitrile 50:40:10 Flow rate: 0.6ml/min Wavelength: 274 nm Retaining time: TAF- 6.34 min EMT- 3.77 min DOL- 7.67 min |
65 |
|
13 |
Prasanthi T. in 2022 |
Column: Phenyl X-Bridge (100 x 2.1 mm, particle size 1.7) Movable phase: Methanol: 0.01N KH2PO4 (70:30 v/v) stream rate: 0.5 ml per min Wavelength: 240 nm Retaining time: TNF- 3.136 min LAM- 1.52 min DOR- 3.775 min |
66 |
|
14 |
Satish Kumar Konidala in 2022 |
Column: BEH C18 Mobile phase: buffer of methanol and phosphate 65:35 v/v stream rate: 0.3 ml per min Wavelength: 260 nm Retaining time: TNF- 0.671 min EMT- 0.432 min EFA- 2.772 min |
67 |
|
15 |
Balaji Thakare in 2022 |
Column: UPLC BEH C18 (150mm 2.1mm) 1.7 µm Movable phase:(40:60% v/v)acetonitrile: water Wavelength: 462 nm Retaining time: DLT- 1.35min LVD- 0.69min TDF- 2.36min |
68 |
LC-MS
Target compounds (or analytes) are physically separated in the LC-MS analytical process before being identified by mass spectrometry. Despite being a fairly new technology, its sensitivity, selectivity, and accurateness have prooved the method is optimal for spotting microgram and also nanogram levels of a diversity of substances. It has become clear that liquid chromatography-mass LC-MS spectrometry is a popular instrument used to detect the tiny molecule parts of cells metabolism.69 For LC-MS, the time required for sample preparation is typically greatly decreased. Additionally, sample run times for LC-MS drug confirmation tests may be shortened. These characteristics of LC-MS techniques are advantageous if quick results are required for medical care.70 because MS is more sensitive and highly selective than other chromatographic detectors, coupling it to chromatographic procedures has always been desirable.71
 |
Figure 3: liquid chromatography- mass spectroscopy process72 |
Catriona Waitt et. al. in 2017
DBS and DBMS was created using 50 and 30 L of drug-infused human breast milk and whole blood, respectively. After acetonitrile and water were used for extraction, for chromatographic partiton, Synergi polar column was utilized. Using a gradient movable phase program that contained formic acid of 0.1percent in formic acid and water of 0.1% in ACN. A weight spectrometer with three quadrupoles called the TSQ Quantum Ultra was used for detection and quantification. DBS and DBMS for 3TC, FTC, and TFV, the assay’s validity was tested at concentrations between 16.6-5000 ng/mL; however, TFV in DBMS had linearity recognized by range of 4.2-1250 ng/mL. All analytes in both matrices had accuracy within 15% and ranged from 3.5-8.7 for inter-day as well as intra-day precision (%CV). For all three analytes, the mean retrieval in DBS carried out by >61% while in DBMS it was >43%. The matrix effect was inconsequential. In pregnant DBS and DBMS, the median AUC0-8 values for 3TC, FTC, and TFV were 4683 (4165-6057) and 6050 (5217-6417) ng h/mL, 3312 (2259-4312) and 4853 (4124-6691) ng h/mL, and 1559 (930-1915) and 56 (45-80) ng h/mL, respectively. While TFV was not found in all children, the measurement of 3TC and FTC found to be (>16.6 ng per mL) from 2/6 and 1/6 in DBS newborns, separately. DBS as well as DBMS sampling 3TC, FTC, also TFV bioanalysis is simple, reliable, correct, and exact, making it the perfect choice use in low-resource areas environments.73
Bingchen Ouyang et. al. in 2017
Tenofovir alafenamide i.e. TAF was chosen as a representative of TFV prodrugs in our effort to build a practical general analytical approach. TAF, TFV, and On an XSelect HSS T3 column, TFV-DP were separated. (4.6 mm 150 mm, 3.5 m, Waters) through isocratic elution following utilizing a to precipitate proteins sensitive LC-MS/MS technique that was designed. By means of correlation coefficients (r) better than 0.999, the approach demonstrated upright linearity for entirely the substances (20-5000 nM for TFV-DP; 2-500 nM for TFV and TAF). The intra- and intraday precision and accuracy met the validation criterion with relative errors (RE) of 10.4% and coefficients of variation (CV) of 14.1%, respectively. The stability, recovery, and matrix effect all met acceptable standards. Finally, using this approach, we examined the pharmacokinetics of TAF within cells and the energetic metabolites in HepG2.2.15 cells.74
Pavan Kumar Prathipati in 2017: Elvitegravir and tenofovir alafenamide (TAF) were administered subcutaneously (SubQ) to mice in the form of free pharmaceuticals (EVG solution + TAF) otherwise drug-loaded NP formulations (EVG NP + TAF). Tenofovir (TFV) and EVG plasma and tissue concentrations measured with LC-MS/MS. Utilizing WinNonlin, non-compartmental analysis was carried out. Long residence duration and publicity for both medications were produced by subQ dosing of the TAF + EVG NP combination. For TFV and EVG, the AUC(0-72h) was 14.1 2.0, 7.2 1.8 g hr per mL as of medicines in free solution, while the AUC for (0-14day) were 23.1 4.4, 39.7 6.7 g hr/mL from NPs for the same pharmaceuticals. For free and NPs TFV, the measured half-life of elimination (t1/2) was 14.2 hours, 5.1 days, while for EVG, it was 10.8 hours, 3.3 days. This method shows that there is a TAF + EVG NP offers continuous release, which might help patients who have trouble remembering to take their medication and may make it easier to estimate the right protecting medication concentration for the prevention of HIV.75
Luisa Barreiros et. al in 2017
A 3 m, 100 2.1 mm, inverted phase C18 column at 45 °C and gradient mode elution with a mixture of formic acid 0.1% (v/v) in water and 0.1% (vol/vol) in ACN at 0.35 mL min1 were used to achieve chromatographic separation. Retention times for the TFV and EFV were 2.8 and 4.1 minutes, respectively, the total run time used is of 9 minutes. For TFV detection, the MS run utilizing positive ionization (ESI+), and for EFV exposure, it was run in negative ionization mode (ESI-). For a range of 4 to 500 ng mL1 for the ARV, calibration curves were linear, through LOD and LOQ for together analytes being 0.4 and 0.7 ng mL1 in taster extracts, accordingly. All matrices showed that the approach was specific (96.0-106.0% of nominal values), accurate (96.0-106.0%), and precise (RSD 2.4%). After standing for 24 hours 20 °C, room temperature, it was discovered that TFV and EFV both remained steady in entire mediums. A high performance liquid chromatography approach combined using HPLC-MS/MS, a triple quadrupole-tandem mass spectrometry technique was created and validated in this study to measure TFV and EFV biotic mediums levels (Serum plasma, mice vaginal tissues, and vaginal lavage). Following intravaginal delivery of both ARVs, the suggested approach was effectively used in a pharmacokinetic research.76
Andrew Ocque et. al. in 2018
Concentrations in cerebral fluid and human plasma. With solid stage withdrawal, the tenofovir alafenamide also tenofovir were removed from the matrix. For LC-MS/MS analysis, the extracted materials that were dried were dissolved in water. A Phenomenex Synergi 4 m Polar-RP 80A column (50 2 mm) was used for separation, by using isocratic elution of 0.1% of the ACN and formic acid water. There were 5 minutes in the runtime. Electrospray ionization on positive mode and triple quadrupole chosen response analyzing were made to detect analytes. For the assay of plasma and the cerebrospinal fluid assay, the standard curve concentrations varied from 0.5 to 500 ng per mL and 0.1 to 50 ng per mL, individually. For both matrices, the intra- and inter-day accuracy and precision in low, medium, and high quality control samples were less than 12%. Plasma and cerebrospinal fluid samples are analyzed. from a patient receiving tenofovir therapy using Genvoya® (150 mg of elvitegravir, 150 mg of cobicistat, 200 mg of emtricitabine, and 10 mg of tenofovir alafenamide) (150 mg of elvitegravir, 150 mg of cobicistat, 200 mg of emtricitabine, and 10 mg of tenofovir alafenamide)instead of Stribild® (300 mg of tenofovir disoproxil fumarate, 150 mg of elvitegravir, 150 mg of cobicistat, 200 mg of emtricitabine) To measure TFV and TAF in human plasma and CSF, we created and validated an LC-MS/MS technique. The technique is accurate and precise enough to quantify concentrations of analyte in plasma samples down to 0.5 ng/mL and in CSF samples down to 0.1 ng/mL. The test was approved following the USFDA Guidelines for Bioanalytical Methods and is appropriate for both pre-clinical and clinical research.77
Pamela Hummert et. al. 2018
Plasma containing K2EDTA was spiked with TAF, TFV, or CMX157. Samples were analyzed using liquid chromatography-tandem mass spectrometry (LC-MS/MS) after the use of internally labeled isotope standards and solid phase extraction sample extraction (TAF and TFV) or protein precipitation (CMX157). While CMX157 was separated making use of an API4500 mass spectrometer, TAF, and TFV, and measured on a Kinetex C8, 2.1 50 mm, 2.6 m column were separated using a 2.1 50 mm, 3.5 m column of Zorbax Eclipse Plus C18 Narrow Bore RR. The FDA Bioanalytical Method Validation criteria were followed for validating the methods. In order to monitor TAF, TFV, and CMX157 multiplexed in plasma, analytical techniques were refined. TAF, TFV, and CMX157 each have lower quantification limits (LLOQs) of 0.03, 1.0, and 0.25 ng/mL. By using standards-based weighted linear regression, calibration curves were produced. Studies on precision and accuracy within and between assays revealed %CVs 14.4% and %DEVs 7.95%, correspondingly. TAF and TFV multiplexed quantification techniques have been designed and validated using sensitive, specific, and dynamic LC-MS/MS assay for CMX157 Plasma quantification. The presented techniques can handle significant research trials with enough throughput.78
Monica Gandhi et. al. in 2018
Researchers created potential immunogens based on the molecular makeup of TFV, administered the conjugated derivatives by injection to rabbits, and then bled the animals every month to test for TFV-specific antibodies using an ELISA assay. We produced curves of dose-response for ELISA quantification after purifying an antigen-specific TFV antibody. Then, using ELISA and this TFV-specific antibody, we measured the amount of TFV in human volunteers’ urine who weren’t using TDF/FTC and coming from those who were consuming 300/200/TDF/FTC mg daily with a 7-day washout period, for 7 days. Using LC-MS/MS (liquid chromatography-tandem-MS), ELISA results were linked to the most reliable method for detecting and measuring TFV. The immunoassay had 100% specificity (95% CI: 97-100%), since none of the 115 samples of urine participants who were not consuming TDF/FTC displayed ELISA-reactivity. For people taking TDF/FTC, the ELISA-immunoassay detected positive results in 67 of 70 samples, producing a 96% (95% CI 88-99%) estimated diagnostic sensitivity for samples that remained positive according to LC-MS/MS. The assay was fairly precise (15% coefficient of variance). The ranking of their relationship between these two measurements was high (r = 0.96) 70 quantitative urine TFV results levels that tested positive by LC-MS/MS throughout a long range of values between those on TDF/FTC. The development of a sensitive and precise immunoassay opens the door to real-time analysing and input on current TFV-based regimen compliance, which should improve results and understanding throughout the roll-out of PrEP and ART.79
Amanda Schauer et. al. in 2018
To measure the three intracellular metabolites at a concentration between 100–25 000 fmol/sample, researchers created and validated an assay. By isotopically tagged 13C5-TFVdp added as the internal criterion, this test uses a straightforward extraction of liquid-liquid and protein precipitation from each 3-mm DBS punch. After extraction, materials are subjected to an 8-minute runtime of Anion exchange chromatography using electrospray ionization in the positive mode on a Thermo Biobasic AX 5 m column the use of a triple quadrupole mass spectrometer. Over the full range. It was a linear assay (R2 > 0.996). The assay found to be precise (inter-assay% CV 9.8%) and accuracy was (inter-assay%bias within 3.0%). The assay was precise (inter-assay% CV 9.8%) and accurate (Inter-assay% bias is 3.0% or less.). The assay could be repeated using both punches from one blood spot and several punches inside the same site. At room temperature for three days and up to 63 days at 80 °C were used to develop stability. Clinical samples from patients taking Truvada®, Stribild®, Descovy®, and Triumeq® regimens were examined; There were intracellular metabolites found each sample as in all anticipated, demonstrating the assay’s suitability regarding all active TFV, FTC, and 3TCformulations. Following a liquid-liquid extraction and direct injection, the extract enables direct measurement of the phosphorylated compounds while bypassing the time-consuming processes required in tests for indirect analysis. The assay was accurate (inter-assay bias within 3.0%), precise (inter-assay%CV 9.8%), and linear (R2>0.996). Clinical samples that were gathered and reviewed throughout the validation process show that the calibration range chosen is suitable for the detection of these metabolites. All three metabolites were proven to be stable for up to 63 days at -80°C and for at least 3 days at room temperature.80
Narit Wiriyakosol et. al. in 2018
The current study created and validated a straightforward and affordable LC/MS/MS technique to assess the level of tenofovir in plasma sample of human. 80 l of plasma are used for the sample preparation, which is a relatively little amount. Gradient elution was used to separate the samples on Luna C18 (100 mm 2.0 mm, 3 m) in presence of movable phase containing (water and 0.1% formic acid) and ACN in the ratio of 90:10 v/v. The mass of the triple quadrupole spectrometer’s positive ionization mode and a 10-minute run period were used to monitor numerous reactions in order to make the detection. Tenofovir’s Monitoring changes were made at m/z 288.0 176.1 and 136.1, and for acyclovir it is m/z 226.1 152.0. With a 10 ng/ml is the limit of lower quantitation, this standard curve of linearity was from 10 to 640 ng/ml. The daily and intraday precision findings were lower than 12.3%, and their range of accuracy was from 84.9 to 113.1%, which is considered to be satisfactory. The investigation of tenofovir-induced kidney damage in HIV-1-infected patients receiving 300 mg once day for more than 4 weeks was effectively conducted using the validated method.81
Lizhi Zhao et. al. in 2019: Tenofovir alafenamide and its metabolite tenofovir (TFV) have been simultaneously determined in human plasma using an UHPLC-MS/MS stands for Ultra High Performance liquid chromatography-tandem mass spectrometry technique. Only 200 l of human plasma were required to extract the inter standards and the analytes, Human plasma-derived TAF-d5 and TFV-d6 using protein coagulation (PPT). On a 100 * 2.1 mm, 1.8 m Waters Acquity UHPLC HSS T3 column, chromatography separation was accomplished during the course of a 10-minute run. Positive ionization utilizing the multiple reaction monitoring (MRM) mode and an interface for electrospray ionization (ESI) was casted to do a tandem mass spectrometric detection. For TAF concentrations of 4–400 ng/ml and TFV concentrations of 0.400–40.0 ng/ml, individually, this technique was created and verified. The stability of in acidified plasma, each analyte was confirmed through sample preparation, storage, also analysis methods. The technique was used to the investigation of treatment’s pharmacokinetics within 8 healthy participants at 25 mg TAF while refuse to eat and was successful in overcoming the lack of the analytes’ stability in plasma samples.82
Sulay Patel et. al. in 2019
A quick and highly a delicate LC-MS/MS (liquid chromatography-tandem mass spectrometry) technique was created and validated in this study in cell lysates of an immortalized human brain microvascular endothelial cell line for the simultaneous detection of tenofovir, emtricitabine, and dolutegravir (hCMEC/D3). Using a C18 reverse phase column, the movable phases of water and Formic acid in acetonitrile at 0.1% were used to separate the analytes. Electrospray ionization mode that is positive while monitoring various reactions were further used as to identify the analytes (MRM). Meant for every analyte, the test was linear in the range of concentrations of 0.1-100 ng mL1. Precision and accuracy within and between assays were 13.33% and 10.53%, respectively. Tenofovir, emtricitabine, and dolutegravir uptake in hCMEC/D3 cells were determined using the process after it had successfully been verified for linearity, precision, and accuracy.83
Sphamandla Ntshangase et. al. in 2019
A only intraperitoneal dosage after (50 mg per kg), the spatial distribution and deposition of both antiretroviral medications In the brain, elvitegravir and tenofovir examined in Sprague-Dawley rats, healthy females. Quantitative matrix-assisted laser desorption/ionization mass spectrometry (MALDI) MSI and liquid chromatography/tandem mass spectrometry (LC/MS/MS) used to achieve this. Elvitegravir had higher BBB penetration, according to LC/MS/MS, by a maximum concentration of 976.5 ng/g in the brain. Tenofovir, on the other hand, showed substantially less BBB penetration, with a Cmaxbrain of 54.5 ng/g. The uneven circulation of both medications in different brain areas, the cerebral cortex included, was demonstrated by MALDI-MSI. The two common antiretroviral medications’ relative concentrations and spatial distribution were well-understood by LC/MS/MS and MALDI-MSI. The ability of MALDI-MSI for direct viewing of therapeutic medicines in situ has also been demonstrated by this work.84
Shubhra Mandal et. al. in 2019: In these trials, nanoparticles loaded with emtricitabine (FTC) and tenofovir alafenamide (TAF) (NPs) were administered subcutaneously (SubQ) to humanized (hu) mice in order to compare their long-acting (LA) PrEP potency. TAF + TAF and NPs from FTC + FTC solution (each medication at 200 mg per kg) to be delivered to mice n = 3 time points for hu-CD34-NSG for the purpose of estimating pharmacokinetic parameters in plasma and tissues using LC-MS/MS. PrEP effectiveness in hu-BLT mice with HIV-1 infection (n = 5/group), the same amount of dose was given. Days 4, 7, and 14 post-SubQ treatments, and (PT), A transmission-founder (T/F) virus was injected vaginally into the hu-BLT mice at 5 105 TCID50 injection and against infected, untreated, and control hu-BLT animals. By day 21 PT, 100% of the mice treated with TAF + FTC solution and the control group received the infection. TAF + FTC NPs, in contrast to control mice, produced a considerable (Day 4: 80%, Day 7: 60%, and Day 14: 60%, respectively) Protection from HIV-1 (p =.0002). This verification of concept research found long-acting TAF + FTC NPs as a possible PrEP mechanism by showing identifiable TAF/FTC vaginal levels across TAF + FTC NP-treated hu-BLT mice correlating with prolonged PrEP efficiency.85
Deqing Xiao et. al. in 2020
The technique created for TFV determination after TAF administration had two major differences from TFV determination after TDF administration. First, to reduce the amount of TAF hydrolysis into TFV and prevent overestimation of TFV concentrations, samples of human plasma, were given a 20% formic acid treatment. (40 L) very away after collection. Second, a number of TFV validation experiments were carried out while TAF was present to simulate the high ratio of TAF:TFV found in medical samples taken in the time of 2 h post treatment. A medical study it was a part of a CHB medication application based on TAF used the authenticated technique from the laboratory of China, which had effectively undergone cross-validation by using technique from the laboratory of US.86
Xiaoping Qian et. al. in 2021: For the simultaneous measurement of (TAF) tenofovir alafenamide and tenofovir (TNF) in human plasma, a quick and easy liquid chromatography-tandem mass spectrometry approach was developed and validated. Analytes were removed from plasma using a straightforward protein precipitation method. On an Eclipse Plus C18 column, chromatographic separation was carried out using a fast gradient elution method that started with 2% of 2 mM ammonium acetate-formic acid (100/0.1, v/v) and then increased the proportion of acetonitrile. The transitions m/z 477.2 m/z 346.1 for TAF and m/z 288.1 m/z 176.1 for TNF were used for detection using a tandem mass spectrometer equipped with an electrospray ionization source operating in the positive ionization mode. The internal standards for TAF and TNF, respectively, were TAF-d5 and TNF-d7. With satisfactory accuracy and precision, the method was validated in the concentration ranges of 1.25-500 ng/ml for TAF and 0.300-15.0 ng/ml for TNF.87
Tanuja Attaluri et. al. in 2022: The internal standard for the current investigation is naproxen (NPX). For this investigation, the “Precipitation Extraction method” is employed. In the chromatographic parting, an movable phase of an isocratic nature consisting at a stream rate of 0.15 mL/min of acetonitrile (ACN): formic acid (0.1%) in water (70:30, v/v), is employed with the Zorbax XDB C18 analytical column of dimension (2.1 X 50 5 m). The parent-production conversions using a mass spectroscopy of triple quadrupole were discovered and running while monitoring numerous reactions (MRM) positive ion mode at m/z 248.3 130.03 (EMT), m/z 477.3 270.04 (TNF), and m/z 231.12 184.82 (BIC), respectively (NPX). Based on the foregoing, it was determined that the procedure was reliable and quick, with a minimum total run time of 3.0 minutes. According to the FDA, EMA, and ICH guidelines, the current approach was effectively validated, and the stability studies were assessed as a result.88
Quality by Design
The Food and Drug Administration of the US (FDA) promotes the use of risk-based strategies and QbD principles with in development process, production, also regulation of medicinal products. Within the publication of Pharmaceutical Formulation of ICH Q8 (R2), Risk Management for Quality of ICH Q9, and ICH Q10 over time, medicinal QbD has advanced (Pharmaceutical Quality System).89 The term “Quality by Design” (QbD) refers to an approach that includes improving scientific understanding of critical process and product qualities, developing controls and tests based on the limits of scientific knowledge during the development phase, and utilizing the knowledge gained throughout the product’s life cycle to work on a continuous improvement environment. A pharmaceutical development strategy referred to as QbD focuses on design and development of a formulation as well as manufacturing procedures to uphold the required product quality.90
Design –
The product is made in a manner that pleases patient needs and performance standards.
The process is created to constantly meet the requirements for product quality.
The influence of the initial raw materials and process variables on product quality is recognized.
Critical process variability sources are located and managed.
To ensure consistent quality throughout time, the procedure is constantly reviewed and revised.91
Sharvil Patil et. al. in 2017
For batch optimization, the quality by design (QbD) method (Factorial design) was applied. When hydrated and subjected to X-ray scattering at a modest angle analysis, TDF loaded LCP spherically as shown by scanning electron microscope images indicated cubic phase development. The ratio of GMO, Pluronic F127, and lactose monohydrate were all optimized using factororial designs. When making powder precursors, several combinations of Pluronic F127 and lactose monohydrate in the range of 0–100% w/w were examined while maintaining the same amounts of GMO (1 g) and TDF (0.5 g). The levels and variables used for batch optimization are displayed in Table 1. The optimal powder precursor recipe had 3% w/w Pluronic F127, 48.50% w/w GMO, and 48.50% w/w lactose monohydrate. The current study shows the value of GMO-Pluronic F127 spray-dried LCP in the cubic phase improving TDF penetration when administered orally. The findings of the current investigation suggest that TDF’s oral bioavailability may be improved when it is synthesized as a cubic phase LCP due to the infusion increasing effect. Stable LCP dosage form can be used as an option for creating an method of oral delivery for medications having less penetrability.92
Moolchand Kurmi et. al. in 2020
Utilizing the UHPSFC platform, the DoE methodology was effectively used for the quick chiral method creation of fixed dose combo pills containing 3TC and TDF. Primary, secondary, and final optimization were implemented in the study in three stages using the Taguchi OA, face-centered CCD, and I-optimal experimental designs, correspondingly. The initial phase enabled the choice of the stationary stage and organic convertor, the subsequent phase eliminated parameters with little to not any impression, and the third phase entailed optimizing CMPs found through additional examination. This technique demonstrated the potential for combining DoE and cutting-edge equipment (UH-PSFC) to create the initial chiral technique for Six stereoisomers of two asymmetric medicines are simultaneously separated in an FDC product.93
Sulaiman Krait et. al in 2022
Following QbD methodology, a CE method was created to assess the stereochemical purity of TEN. During the exploratory investigations, QA-CD was discovered to be a chiral selector. For method optimization, the design mainly centered on a central composite face, used after V+ with a factorial design resolution. The final technique used 45 mg/mL QA—CD as BGE, a pH of 6.4, 100 mM sodium phosphate buffer, an applied voltage of 18 kV, and a capillary temperature of 22°C to allow the measurement of S-TEN at a 0.1% concentration as TEN impurities. Although the approach was resilient, cautious regulation of the experimental limitations is advised, as detailed for many related investigations. Another illustration of the applicability of CE as a method for determining the analytes’ asymmetric purity, particularly of medicines, is the enantio-separation of TEN and its (S)-enantiomer.94
Discussion
The various techniques used for development and validation of tenofovir are summarized.
Conclusion
This review article discusses the tenofovir antiretroviral drug’s physical-chemical characteristics, drug profile and pharmacological activities. The review that is being presented provides details on the many approaches that have been used to identify tenofovir and its salt variants in the literature. This review’s conclusion is that many analytical techniques, including HPTLC, HPLC, UPLC, LC-MS, and QbD, have been described for estimating tenofovir alone and in combination. Thus, these techniques were discovered to be straightforward, precise, economical, and repeatable in nature. Because RP-HPLC and UPLC offered the finest accessible dependability, repeatability, examination time, and sensitivity, these techniques were used for the majority of procedures. This review provides information on the features of the drug and will aid in the future development of analytical techniques for this novel combination.
Acknowledgement
The authors are thankful to Management of college for providing necessary facilities and Principal Dr. L. B. Borse for their constant supports, expert advice and encouragement to write this peace of review article.
Conflict of interest
There is no conflict of Interest.
Funding Source
There are no funding sources.
References
- Cunningham Jr ET, Margolis TP. Ocular manifestations of HIV infection. New England Journal of Medicine. 1998 Jul 23;339(4):236-44.https://pubmed.ncbi.nlm.nih.gov/9673303/
- Corbitt G, Bailey AS, Williams G. HIV infection in Manchester, 1959. HIV infection in Manchester, 1959. 1990;336(July 7).https://pubmed.ncbi.nlm.nih.gov/1973229/
- Gottlieb MS, Schroff R, Schanker HM, Weisman JD, Fan PT, Wolf RA, Saxon A. Pneumocystis carinii pneumonia and mucosal candidiasis in previously healthy homosexual men: evidence of a new acquired cellular immunodeficiency. New England Journal of Medicine. 1981 Dec 10;305(24):1425-31.https://pubmed.ncbi.nlm.nih.gov/6272109/
- Plosker GL. Emtricitabine/tenofovir disoproxil fumarate: a review of its use in HIV-1 pre-exposure prophylaxis. Drugs. 2013 Mar;73(3):279-91.https://europepmc.org/article/med/23444256
- Sauer F. european Agency for the evaluation of Medicinal Products: five years of experience. Bulletin et Memoires de L’academie Royale de Medecine de Belgique. 2000 Jan 1;155(5-6):254-8. https://europepmc.org/article/med/11304960
- Hammer SM, Saag MS, Schechter M, Montaner JS, Schooley RT, Jacobsen DM, Thompson MA, Carpenter CC, Fischl MA, Gazzard BG, Gatell JM. Treatment for adult HIV infection: 2006 recommendations of the International AIDS Society–USA panel. Jama. 2006 Aug 16;296(7):827-43. https://pubmed.ncbi.nlm.nih.gov/17016878/
- Ray AS, Fordyce MW, Hitchcock MJ. Tenofovir alafenamide: a novel prodrug of tenofovir for the treatment of human immunodeficiency virus. Antiviral research. 2016 Jan 1;125:63-70. https://www.sciencedirect.com/science/article/pii/S0166354215300310
- Robbins BL, Srinivas RV, Kim C, Bischofberger N, Fridland A. Anti-human immunodeficiency virus activity and cellular metabolism of a potential prodrug of the acyclic nucleoside phosphonate 9-R-(2-phosphonomethoxypropyl) adenine (PMPA), bis (isopropyloxymethylcarbonyl) PMPA. Antimicrobial agents and chemotherapy. 1998 Mar 1;42(3):612-7.https://pubmed.ncbi. nlm.nih.gov/9517941/
- https://pubchem.ncbi.nlm.nih.gov/compound/Tenofovir cited on 10 Nov 2022
- Velagaleti R, Burns PK, Gill M. Analytical Support for Drug Manufacturing in the United States-from Active Pharmaceutical Ingredient Synthesis to Drug Product Shelf Life. Therapeutic Innovation & Regulatory Science. 2003 Oct 1;37(4):407.https://journals.sagepub.com/doi/abs /10.1177/ 009286150303700407
- Siddiqui MR, AlOthman ZA, Rahman N. Analytical techniques in pharmaceutical analysis: A review. Arabian Journal of chemistry. 2017 Feb 1;10:S1409-21.https://www.sciencedirect.com/science/ article/pii/S1878535213001056
- Görög S. The sacred cow: the questionable role of assay methods in characterising the quality of bulk pharmaceuticals. Journal of Pharmaceutical and Biomedical Analysis. 2005 Jan 4;36(5):931-7. https://www.researchgate.net/publication/8109236
- Kirkland, J.J. Development of some stationary phases for reversed-phase HPLC. Journal of Chromatography A, 2004 1060(1-2), pp.9-21.https://www.researchgate.net/publication/223122467
- Moldoveanu SC, David V. Essentials in modern HPLC separations. Elsevier; 2022 Jun 24. https://www.elsevier.com/books/essentials-in-modern-hplc-separations/moldoveanu/978-0-12-385013-3
- Snyder LR, Kirkland JJ, Glajch JL. Practical HPLC method development. John Wiley & Sons; 2012 Dec 3.https://onlinelibrary.wiley.com/doi/book/10.1002/9781118592014
- Nekkala K, Kumar VS, Ramachandran D. Development and validation for the simultaneous estimation of lamivudine, tenofovir disproxil and dolutegravir in drug product by RP-HPLC. Journal of Pharmaceutical Sciences and Research. 2017 Sep 1;9(9):1505.http://www.jpsr.pharmainfo.in/ Documents/Volumes/vol9Issue09/jpsr09091719.pdf
- Pawar YP, Shaikh SN, Dabhade PS. Analytical Method Development And Validation For Determination Of Tenofovir Disoproxil Fumarate In Bulk And Dosage Form. https://www.researchgate.net/publication/319602313
- Badgujar P B, Mahajan M P, Sawant S D. Development and Validation of RP-HPLC Method for the Simultaneous Estimation of TenofovirAlafenamide and Emtricitabine in Bulk and Tablet Dosage Form. International Journal of ChemTech Research. 2017,10(5): 731-739.https://sphinxsai.com/2017/ ch_vol10_no5/abstracts/A(731-739)V10N5CT.pdf
- Rao BV, Vidyadhara S, Nagaraju B, Jhonbi SK. A novel stability indicating RP-HPLC method development and validation for the determination of tenofovir disoproxil fumarate and emtricitabine in bulk and pharmaceutical formulations. Int J Pharm Sci Res. 2017 May 1;8(5):2168-76. https://ijpsr.com/bft-article/a-novel-stability-indicating-rp-hplc-method-development-and-validation-for-the-determination-of-tenofovir-disoproxil-fumarate-and-emtricitabine-in-bulk-and-pharmaceutical-formulations/
- Ramaswamy A, Dhas AS. Development and validation of analytical method for quantitation of Emtricitabine, Tenofovir, Efavirenz based on HPLC. Arabian Journal of Chemistry. 2018 Feb 1;11(2):275-81.https://www.sciencedirect.com/science/article/pii/S1878535214001695
- Aggarwal NN, Bhat KI, Jacob JT. Stability indicating assay method development and validation for tenofovir alafenamide fumarate by RP-HPLC. Pharm Anal Acta. 2018;9(12):1-6.https://asset-pdf.scinapse.io/prod/2912031000/2912031000.pdf
- Sumanth KS, Rao AS, Shankar DG. A new stability indicating RP-HPLC method development and validation for simultaneous estimation of emtricitabine and tenofovir with degradation kinetics. Asian Journal of Research in Chemistry. 2018 Jun 30;11(3):569-79. https://ajrconline.org/ HTML_Papers/Asian%20Journal%20of%20Research%20in%20Chemistry__PID__2018-11-3-11.html
- Karunakranth D, Midha AK, Babu RS, Kishore DV. Development and Validation of HPLC Method for Simultaneous Estimation of Emtricitabine, Rilpivirine and Tenofovir Disoproxil Fumarate Tablet Dosage form. Indian Journal of Research in Pharmacy and Biotechnology. 2018;6(1):8-15.
- Saidulu P, Mastanamma SK, Suresh PV, Prameela RA. Development and validation of stability-indicating HPLC-DAD method for simultaneous determination of emtricitabine, rilpivirine, and tenofovir alafenamide in bulk and their pharmaceutical dosage forms. International Journal of ChemTech Research. 2018;11(09):329-39.
- Kokkirala TK, Suryakala D. RP-HPLC method development and validation for the estimation of Emtricitabine, Bictegravir and Tenofovir alafenamide in bulk and pharmaceutical dosage form. Journal of Taibah University for Science. 2019 Dec 11;13(1):1137-46. https://www.tandfonline.com/ doi/full/10.1080/16583655.2019.1689601
- Kalamkar CS, Bhawar SB. Development and validation of RP-HPLC method for the simultaneous estimation of tenofovir alafenamide fumarate and emtricitabine in bulk and tablet dosage form. Journal of Drug Delivery and Therapeutics. 2019 Jun 15;9(3-s):243-7.
- Rezaei M, Ramazani A, Hokmabadi F. Simultaneous estimation and validation of tenofovir disoproxil fumarate, emtricitabine and efavirenz by RP-HPLC method in combined tablet dosage form. Current Pharmaceutical Analysis. 2019 Oct 1;15(6):561-7.
- Rele RV, Patil SP. Application of RP-HPLC Technique for development of Analytical method for Validation of Tenofovir disoproxil fumarate from Bulk drug and Dosage form. Research J. Pharm. and Tech. 2019 Oct 12;12(10):4752-6.
- Dighe NS, Shinde GS, Magar SD, Deodhe AV. Development and validation of RP-HPLC method for simultaneous estimation of emtricitabine and tenofovir disoproxil fumarate in bulk and tablet dosage form. Journal of Drug Delivery and Therapeutics. 2019 Jun 20;9(3-s):693-8. http://jddtonline.info/index.php/jddt/article/view/2952
- Manojkumar I, Saravanan D, Maheswaran A, Divakar P. A new RP-HPLC method for the determination of Tenofovir Disoproxil Fumarate in pure form and pharmaceutical formulation. Int. J. Res. Pharmaceut. Sci. Technol.. 2020;2(1):17-24.https://core.ac.uk/download/pdf/337605958.pdf
- Gollu G, Gummadi S. Simultaneous quantification of lamivudine, tenofovir disoproxil fumarate and doravirine in pharmaceutical dosage form by liquid chromatography with diode array detection. Pharmaceutical Chemistry Journal. 2020 Aug;54(5):526-35.https://link.springer.com/article/ 10.1007/s11094-020-02232-9
- Imran A, Chandran SR. Method Development and Validation for Simultaneous Estimation of Emtricitabine, Tenofovir Disoproxil Fumarate And Isoniazid In Bulk And Pharmaceutical Dosage Form By RP-HPLC. Journal of Pharmaceutical Sciences and Research. 2020 Apr 1;12(4):574-9. https://www.proquest.com/openview/ 140326bae26a1b1dd9c870cf113d48b2/1?pq-origsite=gscholar&cbl=54977
- Agrawal D, Dahiya M, Wakode S, Singh GP, Shiv J. Method Development and Validation of Stability Indicating HPLC Assay for the Determination of Tenofovir Alafenamide Fumarate in Tablet Formulation. International Journal of Pharmaceutical Sciences and Nanotechnology. 2020 Nov 16;13(6):5226-33.http://www.ijpsnonline.com/index.php/ijpsn/article/view/1161
- Mangamma K, Kavya KS, Kavya KS, Priyanka KR. Development And Validation For The Simultaneous Estimation Dolutegravir, Lamivudine And Tenofovir Disoproxil Fumarate By Rp–Hplc. 9(9), 2017, 1505-1510https://wjpr.s3.ap-south-1.amazonaws.com/article_issue/1600391930.pdf
- Jaybhave AR, Shindhe M, Mogal R, Narkhede S, Jadhav A. QbD Approach to Analytical RP-HPLC Method Development and Validation of Tenofovir Disoproxil Fumarate in Dosage Form. Journal of Pharmaceutical Sciences and Research. 2021 Jul 1;13(7):381-6.https://www.proquest.com/openview/ 3a3a2a50b3f09c90a95d40dde711be88/1?pq-origsite=gscholar&cbl=54977
- Pappula N, Teja AD. Method Development And Validation For The Simultaneous Estimation Of Emtricitabine, Bictegravir And Tenofovir In Bulk And Tablet Dosage Form By Rp-Hplc Method. 10, 13, 25 Oct 2021. 1832-1846.
- Bhairav BA, Chavan MJ. Method Development and Validation for Estimation of TinofovirDisproxil Fumarate in Pharmaceutical Preparation by UV-Spectroscopy and HPLC With Force Degradation Study. Asian J. Pharm. Res. Heal. Care. 2021;13:78-94. https://www.researchgate.net/ publication/350069699
- Godela R, Gummadi S. A simple stability indicating RP-HPLC-DAD method for concurrent analysis of Tenofovir Disoproxil Fumarate, Doravirine and Lamivudine in pure blend and their combined film coated tablets. InAnnales Pharmaceutiques Françaises 2021 Nov 1 (Vol. 79, No. 6, pp. 640-651). Elsevier Masson.https://pubmed.ncbi.nlm.nih.gov/34019910/
- Rao JN, Sudhakar C, Dubey SS. Rp-hplc method validation for the assay of tenofovir disoproxil orotate. Research Journal of Pharmacy and Technology. 2021 Jul 1;14(7):3855-9. https://www.indianjournals.com/ijor.aspx?target=ijor:rjpt&volume=14&issue=7&article=065
- Basha AD, Anil SD, Talla R, Haque KA, Vasudha HB. Method Development And Validation For Simultaneous Estimation Of Tenofovir Disoproxil Fumarate And Emtricitabine In Pharmaceutical Dosage Form By Rp-Hplc Method. International Journal of Innovative Pharmaceutical Sciences and Research. 2022. 3 (10), 1537-1545
- Pavani C, Susithra E. A novel simultaneous high performance liquid chromatography-PDA method for the determination of Tenofovir AF, Darunavir, Emtricitabine and Cobicistat in bulk and its application to marketed formulation. Future Journal of Pharmaceutical Sciences. 2022 Dec;8(1):1-1. https://fjps.springeropen.com/articles/10.1186/s43094-021-00390-5
- Rathod PR, Udapurkar P, Hingane LD. RP- HPLC Method Development and Validation For the Simultaneously Estimation of Emtricitabine and Tenofovir Alafenamide Fumarate in It’s Dosage Form Using Internal Standard Method. International Journal of Pharmaceutical Research and Applications Volume 7, Issue 4 July-Aug 2022, pp: 643-645
- Wankhade AJ, Hamrapurkar PD. Development and Validation of Quality by Design based RP-HPLC Method for determination of Tenofovir Alafenamide Fumarate from Bulk drug and Pharmaceutical dosage form and its application to Forced Degradation Studies. Research Journal of Pharmacy and Technology. 2022 May 30;15(5):2127-34. https://www.proquest.com/openview/ c8fe2689f58bebfdb5bd65be2122cce0/1?pq-origsite=gscholar&cbl=1096441
- Solunke RS, Poul BN, Usnale SV, Shinde GV. Method Development And Validation Of Simultaneous Estimation Of Emtricitabine, Rilpivirine And Tenofoviralafenamide In Bulk And Formulation By Rp-Hplc Method. Harbin Gongye Daxue Xuebao/Journal of Harbin Institute of Technology. 2022 Sep 8;54(9):58-66.http://hebgydxxb.periodicales.com/index.php/JHIT/article/view/1300
- Schibli A, Reich E. Stationary Phases for Planar Separations—Plates for Modern TLC. https://www.chromatographyonline.com/ view/stationary-phases-planar-separations-plates-modern-tlc
- Arup U, Ekman S, Lindblom L, Mattsson JE. High performance thin layer chromatography (HPTLC), an improved technique for screening lichen substances. The Lichenologist. 1993 Jan;25(1):61-71.
- Morlock G, Schwack W. Determination of isopropylthioxanthone (ITX) in milk, yoghurt and fat by HPTLC-FLD, HPTLC-ESI/MS and HPTLC-DART/MS. Analytical and bioanalytical chemistry. 2006 Jun;385(3):586-95.https://link.springer.com/article/10.1007/s00216-006-0430-5
- Kashid AM, Kadam RR. Stability-indicating high-performance thin-layer chromatography method for the simultaneous estimation of emtricitabine and tenofovir alafenamide fumarate. JPC–Journal of Planar Chromatography–Modern TLC. 2021 Jun;34(3):253-61. https://link.springer.com/ article/10.1007/s00764-021-00080-1
- Morea V, Bondgeb A, Mominc K. Estimation of Emtricitabine and Tenofovir by HPTLC Method. International Journal For Innovative Research In Multidisciplinary Field.
- Said NS, Nasr ZA, Abdel-Razeq SA. Assessment of the greenness of new stability indicating micellar UPLC and HPTLC methods for determination of tenofovir alafenamide in dosage forms. Journal of Chromatographic Science. 2021 Nov;59(10):909-22.
- Trenerry VC, Rochfort SJ. Natural products research and metabolomics. 2010. https://doi.org/ 10.1016/B978-008045382-8.00211-2.
- Chawla G, Ranjan C. Principle, instrumentation, and applications of UPLC: A novel technique of liquid chromatography. Open Chemistry Journal. 2016 May 6;3(1). https://doi.org/ 10.2174/1874842201603010001
- Taleuzzaman M, Ali S, Gilani SJ, Imam SS, Hafeez A. Ultra performance liquid chromatography (UPLC)-a review. Austin J Anal Pharm Chem. 2015;2(6):1056.
- Pasha SI, Varanasi MB, Mohammed I. Stability indicating RP-UPLC-PDA method development, validation of multi drug combination of emtricitabine, tenofovir alafenamide and rilpivirine in bulk drug and its tablet formulation. Oriental Journal of Chemistry. 2017 Jan 1;33(2):925-9. https://pdfs.semanticscholar.org/2ed2/314f5888ff06a6630ea7d02401b1da88004b.pdf
- Jacob J, Nadig S. A UPLC method for simultaneous estimation of emtricitabine, tenofovir disoproxil fumarate and efavirenz in pharmaceutical dosage forms. Research Journal of Pharmacy and Technology. 2017;10(12):4463-6.https://www.indianjournals.com/ijor.aspx?target=ijor:rjpt&volume=10&issue=12&article=074
- Tejaswi JK, Rajan RG. Rp-Uplc Method Development And Validation For Simultaneous Estimation And Forced Degradation Studies Of Elvitegravir, Cobicistat, Emtricitabine And Tenofovir Disoproxil Fumarate In Solid Dosage Form. 06 Dec 2018, 2730-2738.
- Fumarate, F., International Research Journal Of Pharmacy. https://www.researchgate.net/ publication/333147704-
- Murali Krishna MV, Rao SV, Venugopal NV. Forced Degradation Study for Tenofovir DF, Lamivudine and Efavirenz in Triple Combination Anti-Retroviral Tablets and Development of Validated Stability Indicating Assay Method by UPLC. Current Pharmaceutical Analysis. 2019 Jan 1;15(1):82-94. https://www.ingentaconnect.com/content/ben/cpa/2019/00000015/00000001/art00014
- Sravanthi T, Madhavi N. Stability indicating UPLC method to quantify emtricitabine, tenofovir, and efavirenz simultaneously in tablets: method establishment. International Journal of Research in Pharmaceutical Sciences. 2020 Jan 7;11(1):120-8.https://ijrps.com/index.php/home/article/view/402
- Chengalva P, Kuchana M. Development and Validation of Ultra Performance Liquid Chromatographic Method for the Simultaneous Estimation of Lamivudine, Tenofovir Disoproxil Fumarate, Doravirine and Efavirenz in Bulk and Pharmaceutical Formulations. Indian Journal of Pharmaceutical Sciences. 2020 Dec 31;82(6):1006-14. https://www.ijpsonline.com/articles/ development-and- validation-of-ultra-performance-liquid-chromatographic-method-for-the-simultaneous-estimation-of-lamivudine-tenofo-4053.html
- Ma Z, Li S, He D, Wang Y, Jiang H, Zhou H, Jin J, Lin N. Rapid quantification of tenofovir in umbilical cord plasma and amniotic fluid in hepatitis B mono‐infected pregnant women during labor by ultra‐performance liquid chromatography/tandem mass spectrometry. Rapid Communications in Mass Spectrometry. 2020 May 15;34(9):e8728. https://analyticalsciencejournals.onlinelibrary.wiley.com/ doi/abs/10.1002/rcm.8728
- Sudha PD, Avulapati U, Sohail P. Stability-indicating reverse-phase ultra-performance liquid chromatography method for the simultaneous determination of emtricitabine, tenofovir, cobicistat, and elvitegravir. Drug Invention Today. 2020 Jun 15;14(6).
- Satya Venkata Sakuntala M, Lakshmana Rao A, William Carey M. Stability-indicating method development and validation for the concurrent determination of darunavir, cobicistat, emtricitabine and tenofovir alafenamide by UPLC in bulk and tablet dosage forms. Future Journal of Pharmaceutical Sciences. 2021 Dec;7(1):1-0.https://fjps.springeropen.com/articles/10.1186/s43094-021-00384-3
- Addanki S, Kuber BR. A new stability indicating RP-UPLC method for simultaneous estimation of Doravirine, Lamivudine and Tenofovir disoproxil fumarate in bulk and their combined pharmaceutical formulation. Future Journal of Pharmaceutical Sciences. 2021 Dec;7(1):1-4. https://link.springer.com/article/10.1186/s43094-021-00349-6
- Konam K, Kanala SR. A stability indicating method development and validation for the simultaneous estimation of emtricitabine, tenofovir alafenamide and doultegravir in pharmaceutical formulation by ultra performance liquid chromatography. Research Journal of Pharmacy and Technology. 2021 Nov 1;14(11):6017-24. https://www.proquest.com/openview/5efe82554e9570cbbe29a 5f60c664679/1?pq-origsite=gscholar&cbl=1096441
- Prasanthi T, Rajendra Prasad RY, Rao LA. A Validated Stability Indicating RP-UPLC- DAD Method Development for Simultaneous Determination of Lamivudine, Tenofovir Dispoproxil Fumarate and Doravirine in Pure and Tablet Dosage Form. 28, 8, 2022http://www.gjstx-e.cn/gallery/68-aug2022.pdf
- Konidala SK, Samineni R, Rao YV, Chimakurthy J, Kolakaluri CS, Korrapati V. Novel RP-UPLC Method Development and Validation for Simultaneous Quantification of Emtricitabine, Tenofovir and Efavirenz in Bulk and Tablet Dosage Forms. Research Journal of Pharmacy and Technology. 2022 Jul 29;15(7):3141-6.https://rjptonline.org/AbstractView.aspx?PID=2022-15-7-47
- Thakare B, Mittal A, Charde M, Umbarkar R, Kohle N, Chandra P, Kadam M. Development and Validation of Stability-indicating assay UHPLC Method for Simultaneous analysis of Dolutegravir, Lamivudine and Tenofovir disoproxil fumarate in Bulk and Pharmaceutical Formulation. Research Journal of Pharmacy and Technology. 2022 Sep 28;15(9):4061-6. https://rjptonline.org/ HTML_Papers/Research%20Journal%20of%20Pharmacy%20and%20Technology__PID__2022-15-9-41.html
- Crutchfield CA, Lu W, Melamud E, Rabinowitz JD. Mass spectrometry-based metabolomics of yeast. InMethods in enzymology 2010 Jan 1 (Vol. 470, pp. 393-426). Academic Press. https://www.sciencedirect.com/science/article/abs/pii/S0076687910700161
- Lynch KL. Toxicology: liquid chromatography mass spectrometry. InMass spectrometry for the clinical laboratory 2017 Jan 1 (pp. 109-130). Academic Press. https://www.sciencedirect.com/ science/article/abs/pii/S0076687910700161
- Fenn JB, Mann M, Meng CK, Wong SF, Whitehouse CM. Electrospray ionization for mass spectrometry of large biomolecules. Science. 1989 Oct 6;246(4926):64-71. https://www.science.org/ doi/abs/10.1126/science.2675315
- https://emerypharma.com/chemistry/lcms-services/ cited on 13 Nov 2022
- Waitt C, Penchala SD, Olagunju A, Amara A, Else L, Lamorde M, Khoo S. Development, validation and clinical application of a method for the simultaneous quantification of lamivudine, emtricitabine and tenofovir in dried blood and dried breast milk spots using LC–MS/MS. Journal of Chromatography B. 2017 Aug 15;1060:300-7.https://www.sciencedirect.com/science/article/pii/S157002321730346X
- Ouyang B, Zhou F, Zhen L, Peng Y, Sun J, Chen Q, Jin X, Wang G, Zhang J. Simultaneous determination of tenofovir alafenamide and its active metabolites tenofovir and tenofovir diphosphate in HBV-infected hepatocyte with a sensitive LC–MS/MS method. Journal of pharmaceutical and biomedical analysis. 2017 Nov 30;146:147-53. https://www.sciencedirect.com/ science/article/abs/pii/S0731708517312025
- Prathipati PK, Mandal S, Pon G, Vivekanandan R, Destache CJ. Pharmacokinetic and tissue distribution profile of long acting tenofovir alafenamide and elvitegravir loaded nanoparticles in humanized mice model. Pharmaceutical research. 2017 Dec;34(12):2749-55. https://link.springer. com/article/10.1007/s11095-017-2255-7
- Barreiros L, Cunha-Reis C, Silva EM, Carvalho JR, das Neves J, Sarmento B, Segundo MA. Development and validation of a liquid chromatography-MS/MS method for simultaneous quantification of tenofovir and efavirenz in biological tissues and fluids. Journal of Pharmaceutical and Biomedical Analysis. 2017 Mar 20;136:120-5. https://www.sciencedirect.com/science/ article/abs/pii/S0731708516308937
- Ocque AJ, Hagler CE, Morse GD, Letendre SL, Ma Q. Development and validation of an LC–MS/MS assay for tenofovir and tenofovir alafenamide in human plasma and cerebrospinal fluid. Journal of pharmaceutical and biomedical analysis. 2018 Jul 15;156:163-9. https://www.sciencedirect.com/ science/article/abs/pii/S0731708517327875
- Hummert P, Parsons TL, Ensign LM, Hoang T, Marzinke MA. Validation and implementation of liquid chromatographic-mass spectrometric (LC–MS) methods for the quantification of tenofovir prodrugs. Journal of pharmaceutical and biomedical analysis. 2018 Apr 15;152:248-56.https://www. sciencedirect.com/science/article/abs/pii/S0731708517328443
- Gandhi M, Bacchetti P, Rodrigues WC, Spinelli M, Koss CA, Drain PK, Baeten JM, Mugo NR, Ngure K, Benet LZ, Okochi H. Development and validation of an immunoassay for tenofovir in urine as a real-time metric of antiretroviral adherence. EClinicalMedicine. 2018 Aug 1;2:22-8.https://www. sciencedirect.com/ science/article/pii/S2589537018300245
- Schauer AP, Sykes C, Cottrell ML, Prince H, Kashuba AD. Validation of an LC–MS/MS assay to simultaneously monitor the intracellular active metabolites of tenofovir, emtricitabine, and lamivudine in dried blood spots. Journal of pharmaceutical and biomedical analysis. 2018 Feb 5;149:40-5.https://www.sciencedirect.com/science/article/abs/pii/S0731708517321763
- Wiriyakosol N, Puangpetch A, Manosuthi W, Tomongkon S, Sukasem C, Pinthong D. A LC/MS/MS method for determination of tenofovir in human plasma and its application to toxicity monitoring. Journal of Chromatography B. 2018 May 15;1085:89-95.https://www.sciencedirect.com/ science/article/abs/pii/S1570023217317828
- Zhao L, Li Z, Zhou Z, Kang X, Fang B, Ma H, Ge Q. Simultaneous determination of tenofovir alafenamide and tenofovir in human plasma by LC-MS/MS and its application to pharmacokinetics study in clinic. Journal of Chromatography B. 2019 Jun 1;1117:148-57. https://www.sciencedirect. com/ science/article/abs/pii/S1570023219304003
- Patel SH, Ismaiel OA, Mylott Jr WR, Yuan M, McClay JL, Paris JJ, Hauser KF, McRae M. Cell-type specific differences in antiretroviral penetration and the effects of HIV-1 Tat and morphine among primary human brain endothelial cells, astrocytes, pericytes, and microglia. Neuroscience letters. 2019 Nov 1;712:134475.https://www.sciencedirect.com/science/article/abs/pii/S0003267019300546
- Ntshangase S, Mdanda S, Naicker T, Kruger HG, Baijnath S, Govender T. Spatial distribution of elvitegravir and tenofovir in rat brain tissue: Application of matrix‐assisted laser desorption/ionization mass spectrometry imaging and liquid chromatography/tandem mass spectrometry. Rapid Communications in Mass Spectrometry. 2019 Nov 15;33(21):1643-51. https://analyticalsciencejournals.onlinelibrary.wiley.com/doi/abs/10.1002/rcm.8510
- Mandal S, Kang G, Prathipati PK, Zhou Y, Fan W, Li Q, Destache CJ. Nanoencapsulation introduces long-acting phenomenon to tenofovir alafenamide and emtricitabine drug combination: A comparative pre-exposure prophylaxis efficacy study against HIV-1 vaginal transmission. Journal of controlled release. 2019 Jan 28;294:216-25. https://www.sciencedirect.com/science/article/ abs/pii/S0168365918307302
- Xiao D, Ling KH, Tarnowski T, Majeed SR, German P, Kearney BP, Zhao Y, Chen YS, Ma L. An LC-MS/MS method for determination of tenofovir (TFV) in human plasma following tenofovir alafenamide (TAF) administration: development, validation, cross-validation, and use of formic acid as plasma TFV stabilizer. Analytical biochemistry. 2020 Mar 15;593:113611.https://www. sciencedirect.com/science/article/abs/pii/S0003269719308991
- Qian X, Chen Q, Chen Y, Ji S, Wang Y, Sun Y, Qi H, Zhong K, Jiang J, Chen X, Huang L. A simple and fast LC–MS/MS method for the simultaneous determination of tenofovir alafenamide and tenofovir in human plasma. Biomedical Chromatography. 2022 Mar;36(3):e5273 .https://analyticalsciencejournals. onlinelibrary.wiley.com/doi/abs/10.1002/bmc.5273
- Tanuja A, Ganapaty S. Bio-analytical Method Development and Validation for Simultaneous Determination of Bictegravir, Emtricitabine, and Tenofovir Alafenamide Fumarate in Human Plasma by LC-MS/MS. 2022, 56(4): 1190-1205.https://www.ijper.org/sites/default/files/IndJPhaEdRes-56-4-1190.pdf
- Yu LX, Amidon G, Khan MA, Hoag SW, Polli J, Raju GK, Woodcock J. Understanding pharmaceutical quality by design. The AAPS journal. 2014 Jul;16(4):771-83. https://www.ncbi.nlm.nih.gov/ pmc/articles/PMC4070262/#CR3
- Nadpara NP, Thumar RV, Kalola VN, Patel PB. Quality by design (QBD): A complete review. Int J Pharm Sci Rev Res. 2012;17(2):20-8.https://globalresearchonline.net/journalcontents/v17-2/04.pdf
- Lionberger RA, Lee SL, Lee L, Raw A, Yu LX. Quality by design: concepts for ANDAs. The AAPS journal. 2008 Jun;10(2):268-76.https://link.springer.com/article/10.1208/s12248-008-9026-7
- Patil S, Kadam C, Pokharkar V. QbD based approach for optimization of Tenofovir disoproxil fumarate loaded liquid crystal precursor with improved permeability. Journal of advanced research. 2017 Nov 1;8(6):607-16.https://www.sciencedirect.com/science/article/pii/S2090123217300954
- Kurmi M, Jayaraman K, Natarajan S, Kumar GS, Bhutani H, Bajpai L. Rapid and efficient chiral method development for lamivudine and tenofovir disoproxil fumarate fixed dose combination using ultra-high performance supercritical fluid chromatography: A design of experiment approach. Journal of Chromatography A. 2020 Aug 16;1625:461257. https://www.sciencedirect.com/ science/article/abs/pii/S0021967320305355
- Krait S, Schneidmadel FR, Scriba GK. Quality by design‐assisted development of a capillary electrophoresis method for the enantiomeric purity determination of tenofovir. Electrophoresis. 2022 May;43(9-10):964-9.https://analyticalsciencejournals.onlinelibrary.wiley.com/doi/full/ 10.1002/elps.202100345

This work is licensed under a Creative Commons Attribution 4.0 International License.





