Manuscript accepted on : 08-04-2023
Published online on: 20-06-2023
Plagiarism Check: Yes
Reviewed by: Dr. Nagham Aljamali
Second Review by: Dr. Daya Shankar Gautam
Final Approval by: Dr. Chateen Izaddin Ali Pambuk
Pathogenesis, Updates on Current Treatment Options and Alvimopan for Postoperative Ileus
Satish Patil * , Swapnil Sharma
, Swapnil Sharma and Sarvesh Paliwal
and Sarvesh Paliwal
Department of Pharmacy, Banasthali University, Banasthali, Rajasthan, India.
Corresponding Author E-mail: ssp6878@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/3119
ABSTRACT: Postoperative Ileus (POI) is a recurrent incident following intestinal as well as other types of surgery that causes aggregation of gases and inner secretions in patients, resulting in significant costs to health care providers and morbidity. The pathophysiology of the POI is multifactorial, and treatment duration of the POI associated with the degree of surgical trauma. Exogenous opioids, neurohormonal dysfunction, fluid overload, inflammation, and gastrointestinal strain are the main pathophysiological factors underlying POI. Different treatment options currently available to reduce duration of POI. Recent studies have shown that the effective approaches in reducing patient morbidity with early return of gut functions are Enhanced Recovery After Surgery (ERAS) pathway and laparoscopic surgery. Alvimopan (ALV) is a peripherally acting antagonist of the µ opioid receptor in postoperative ileus. Alvimopan (Entereg®), the FDA-approved product for the fastest recovery of bowel (large and small) resection with primary anastomosis, shows potential advances for the treatment of POI. It has limited bioavailability through the oral route due to solubility limitations. ALV prevents binding of opioid agonists to the μ-opioid receptor and assists in stopping constipation in the GI tract; it is also not able to cross the blood-brain barrier, so it does not obstruct with centrally mediated opioid analgesia. The safety & efficacy studies of Alvimopan showed that the patients who go through segmental bowel surgeries along with primary anastomosis and given ALV reduces the duration of stay and overall direct costs compared with control group. The objectives of this systematic review were to give an update of categorization systems, pathogenesis mechanisms, current treatment for established POI, and updates on Alvimopan for POI.
KEYWORDS: Abdominal surgery; Enhanced recovery after surgery Alvimopan; Postoperative ileus
Download this article as:| Copy the following to cite this article: Patil S, Sharma S, Paliwal S. Pathogenesis, Updates on Current Treatment Options and Alvimopan for Postoperative Ileus. Biosci Biotech Res Asia 2023;20(2). |
| Copy the following to cite this URL: Patil S, Sharma S, Paliwal S. Pathogenesis, Updates on Current Treatment Options and Alvimopan for Postoperative Ileus. Biosci Biotech Res Asia 2023;20(2). Available from: https://bit.ly/3Xi4zbb |
Introduction
A prolonged functionally propulsive obstruction of the intestinal bowel function after surgery is known as a postoperative ileus (POI). The post-operative ileus can occur with other surgeries (not only intestinal) as well. This condition is characterised by the accumulation of secretions and gas, which results in abdominal distension, pain, nausea, and vomiting 1, 2. After surgical manipulation, the recovery rate of gastrointestinal tract motility is different. Ileus, which ranges from mild to severe, can last anywhere from a few hours to more than three days. The postoperative ileus lasting more than 3 days is known as paralytic postoperative ileus 3. In this case, stomach function returns within 48 hours, while colonic function returns within 72 hours. The pathophysiology and aetiology of the cause behind the cause of ileus are not fully understood, but over the last many years, numerous factors have been identified as its cause, and accordingly, new active moieties have been identified.
The mechanical bowel obstruction caused by structural abnormalities is not the same as postoperative ileus. This impairs the patients’ digestive abilities, resulting in the accumulation of gases and fluid secretion, which causes vomiting and abdominal pain. The economic impact of this on hospital charges for patients suffering from POI is estimated to be doubled in comparison to those who have a standard return of bowel function (median total hospital costs of $ 21,046 vs $ 10,945) 4. The end point of the POI is explained by various methods, such as bowel sounds, Flauts, and bowel movements, but this is debatable due to the limitations of each method. The most reliable method used to identify end point is bowl movements 5.
Fig. 1 summarises the risk factors for POI and potential causes. The following risk factors are evaluated: the health of the patient, lengthy operating periods, unintentional surgery, systemic infections, blood loss and the requirement to convey the blood through veins, prior abdominal surgery, and heavy painkiller usage. Also, each risk factor’s potential processes are described, including the following: a decrease in the body’s general capability following surgery; an inflammatory response; and pain escalation in men, which causes an increase in catecholamine release. These are the most likely causes of the patient’s present health issues. An increase in surgery’s operating duration leads to higher bowel handling and opiate effects. Accidental surgeries led to an increase in the inflammatory and catecholamine response. Systemic or pre-existing infections that result in a decreased physiological reserve. Oedema is brought on by blood loss and transfusion requirements brought on by increased crystalloid administration. Adhesiolysis and bowel handling have become more necessary in cases of prior abdominal surgery. Both acute and chronic opioid use stimulate the µ opioid receptor, which improves peristalsis. All of them, together with the potential mechanisms are risk factors for POI6,8,9,10.
Approaches to enhance rates of POI have transformed over time. Chewing gum, adequate fluid resuscitation, laxative and prokinetic drugs are all traditional approaches to POI prevention. Nonetheless, some of these approaches are combined with unproven efficacy or additional risks 6. The duration of postoperative ileus has been reduced by various modern treatment options. The treatment options briefly explained in this review article are ambulation, early postoperative feeding, nasogastric tube placement, laparoscopic surgery, and pharmacologic agents. Several experimental models have been developed to assess POI in relation to bowel motility 7.
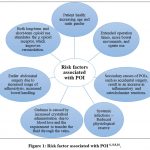 |
Figure 1: Risk factor associated with POI 6, 8,9,10.
|
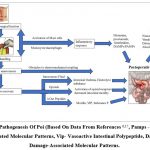
The major pathophysiological factors that cause POI are exogenous (anesthetics, pharmacological agents) or endogenous (neurohumoral response), which cause POI. The degree of abdominal manipulation can influence the severity and duration of postoperative ileus recovery 13. The levels of inflammatory mediators and cytokines have increased in the gut and impacted the reduction of gastrointestinal (GI) motility after surgery. POI development is influenced by a complex interaction of inflammatory, neurogenic, intravenous fluid and electrolytes, and pharmacological elements (Fig. 2). The POI can be developed in all kinds of GI surgery, and the basic pathogenesis is explained as follows:
Parasympathetic stimulation is inhibited by sympathetic stimulation, and it is nearly dependent on peristalsis. Neural reflexes are activated immediately and during surgical procedures, and this is the first phase of the reaction to surgery. Acute abdominal paralysis is caused by an incision into the skin, which induces a rise in adrenergic motor neuronal activity mediated by corticotropin-releasing factor. Moreover, other factors like the noradrenergic pathway play a critical role in the arrest of peristalsis.
Inflammation is mediated when the second phase begins after surgical procedures. Pro-inflammatory cytokines and chemokines are released by the endothelium, resulting in an increase in intracellular adhesion molecules 13. Migration of leukocytes to the muscularis externa occurred due to the phagocytes situated throughout the activated gut. These phagocytes block peristalsis by reducing smooth muscle contraction by releasing nitric oxide and prostaglandins directly. Since the release of acetylcholine decreases cytokines through intestinal mucosa, methods such as modulation of vagal afferents have been suggested for this inflammatory response.
The most likely cause of increased gastrointestinal inflammation is bowel manipulation during surgery, which increases the duration of POI. Although bowel manipulation cannot completely eliminate POI, minimal access procedures such as laparoscopy appear to reduce the magnitude of the systemic inflammatory response and the duration of POI.
The effect of anastomoses on enteric neural continuity is caused by surgical procedures such as visceral resection. The interruption of neural continuity caused by visceral resection, which directly impairs intestinal motility by posing a physical barrier to electro-mechanical coupling. This explanation has been examined in a murine model of small bowel resection, which gives slow waves and phasic contractions due to acute disruption of the interstitial cell of Cajal (ICC) networks 14.
The lack of early intake after surgery and surgical procedures modulates the levels of neuropeptides and gastrointestinal hormones. The critical factors of interest are substance P (SP), motilin, and vasoactive intestinal peptide (VIP), all of which are involved in normal gut motility. The restriction of the release of enteric neurotransmitters SP and VIP in preclinical models has shown to accelerate the recovery of postoperative gut functions. These conclusions are somewhat contradictory when considering that SP is a potent tachykinin known to accelerate gastrointestinal motility via direct action on smooth muscle and neuronal excitation within the ENS. Moreover, SP plays a role in mediating the neuro-immuno-humoral inflammatory response to tissue injury and is involved in the excitatory neurotransmission of visceral afferents. VIP is a smooth muscle relaxant and also acts as a major anti-inflammatory agent with its role as an excitatory secretomotor neurotransmitter within the ENS 15.
Electrolyte disturbances play a central role in the aetiology of an ileus. This hypothesis is supported by the effect of electrolyte variation on gut motility, which was observed to occur frequently during prolonged episodes of POI 16.
Endogenous (Neurohumoral Response)
Neural Reflexes
The nervous system of the body controls gastrointestinal motility, and basically three nervous systems are included in it: sympathetic, parasympathetic, and intrinsic. Reduction in intestinal motility for the sympathetic nervous system and enhancement in intestinal motility in the case of the parasympathetic nervous system 9,13,15,16. The intrinsic nervous system in the colon differs from that of the intestine due to the absence of gap junctions in the smooth muscles and the absence of peristaltic movements. 17,18,19. The surgery causes intestinal stimulation, which causes an obstructive non-adrenergic vagally mediated process that results in POI neural reflexes.
Inflammatory Factors
During surgery, the levels of gut-related humoral factors, including vasoactive intestinal peptide, endogenous opioids, calcitonin gene–related peptide, substance P, and nitric oxide, change due to the stressful stimulus, which leads to reduced gut motility. The presence of inflammatory mediators and cytokines raises the level of COX-2, which reduces jejunal muscle contractility in in-vitro experiments 20,21. The first phase of impairment of muscle due to surgery impacts contractility in the first 90 min, the influx of chemokines (e.g., TNF-α and interleukin) and pro-inflammatory cytokines. The second phase is initiated when neutrophils and monocytes enter the muscle within 24 hours, triggering IL-1 and CCL2. The trigger of the inflammatory reactions increases chemokines, cytokines, and prostaglandins (PGs). On the third day, cytokine and muscle functions returned to normal, implying that the second phase was suppressed by incoming leukocytes 22.
Exogenous (Anaesthetics, Pharmacological Agents)
Anaesthetics and opioids are most known pharmacological agents to cause and prolong postoperative ileus26,27. Anaesthetics is stabilizing neural membrane and reduces motility of muscles; they impact on areas that depends heavily on neural integration. Due to lack of gap junctions in the colon; the colon is more susceptible to these agents unlike other part of the guts3. Opioids are causing POI by reducing gut motility and increase release of acetylcholine. Different types of peptides such as calcitonin gene-related peptide, vasoactive intestinal peptide, substance P and Nitric oxide (NO) are enabled to escape locally in the gastrointestinal tract and may contribute to postoperative ileus.
Based on the available studies, three phases like smooth muscle inhibition in early and late phase and neuron dysfunction phase are responsible for POI.
Inflammatory Mechanism in Poi
Macrophages plays important role in developing POI, the surgical manipulation activates it and followed proinflammatory cytokines and chemokines, activator of activator of transcription 3 and signal transducer, early growth response protein 1 nuclear factor kB. Activation of systemic inflammatory response triggers inward flow of neutrophiles followed by monocytes in the muscularis 28. The reduction in pharmacological agents and genetic depletion reduces level of inflammatory mediators and leukocytes concentration in muscularis.
The activation pathway of macrophages is still unclear, however, one of the expected pathways is connected by activation of damage associated molecular pattern (DAMP) receptors due to damage to cells. The activation of pro-inflammatory reaction only occurs when the normal cells being affected by any kind of damage or stressful condition 29. The exposure to external environment during surgery may cause dehydration and leading to reduction in temperature, it may cause cellular damage and activation of inflammatory mediators (ATP, HMGB1, or IL-1α). These inflammatory mediators are known for activation of macrophages. The intensity and duration of POI or colonal damage depends on the surgical manipulation and exposure to external environment29.
The extreme damage to muscular tissue affects nerve fibres that impacts neurogenic inflammation through local release of calcitonin gene-related peptide (CGRP) and substance P known as pro-inflammatory neuropeptides30. The activation of interleukins was determined to bring out the release of CGRP from visceral afferents, creating connection between neurogenic inflammation and tissue damage 31. The escape of CGRP from visceral afferents in recent studies shows that it is due to bowel manipulation and capsaicin depleted CGRP from muscular nerve fibres. Capsaicin and the CGRP antagonist BIBN4096BS decreased interleukin-1β and interleukin-6 mRNA expression between the muscularis after surgical manipulation 32. The afferent nerve involves in the activation of macrophages that triggers inflammatory cascade. In few studies, it showed that mast cells involve in the neurogenic inflammatory reactions and development of POI in pre-clinical studies and humans33.
The interaction of bacterial cell wall and bacterial translocation activates toll like receptors and triggers macrophage activation. Macrophage activation triggers further cycles as mentioned above for development of POI 34.
Management of POI
Over the last few years, different strategies have been introduced to minimize postoperative consequences and improve patient care in hospitals. With short-term treatment, the use of pharmaceutical therapies and modern formulation approaches has improved management of POI. Also, the recent advance in surgical techniques reduces the stress and exposure to external factors throughout the perioperative period.
POI would be expected, and efforts to shorten its duration should begin preoperatively, incorporating many of the principles of enhanced recovery programmes aimed at limiting the strain response to surgery. Principles for management of POI are precise measurement of fluid input and output, improved physiology, incorporation of the nasogastric tube, elimination and treatment of secondary causes, and nutritional team counselling 6. Preventive and therapeutic management options for POI are summarized in Table 1.
Table 1: Preventive and Therapeutic Management Options for POI 6
|
Non-Pharmacological Options |
Pharmacological Options |
|
Enhanced recovery after surgery (ERAS) Rapid recovery: ERAS pathways implemented before (preoperative day: carbohydrate-rich diet before anaesthesia induction), during (intra-operative: sufficient perfusion of organs with flowing fluids), and after (post-operative interventions – oral diet soon after surgery and fluid reduction to recover faster). surgical manipulation. |
Epidural (anaesthesia and analgesics): It reduces inflammatory responses, sympathetic stimulation, and opioid requirement. It is effective at promoting insulin sensitivity and may decrease perioperative cytokine expression. Furthermore, epidural anaesthesia with local anaesthetic has been shown to reduce the duration of POI due to its effect on inhibiting sympathetic nerve afferents to the gastrointestinal tract. |
|
Nasogastric Tubes: Prophylactic drainage of the stomach when vomiting and abdominal distension predominate. |
NSAIDs reduce COX-mediated prostaglandin synthesis, and their use is part of a multimodal postoperative multimodal analgesic strategy for reducing opioid consumption. |
|
Early feeding, including sham feeding, stimulates GI motility and the release of hormonal factors, which have a beneficial effect on reducing the length of stay. |
Metoclopramide and erythromycin should be reserved for patients with gastroesophageal reflux associated with diabetic gastroparesis. These agents have not been used due to their effectiveness in the treatment of postoperative ileus. |
|
Early ambulation – It stimulates mechanical & intestinal function. |
|
|
Laparoscopic surgery – It reduces opioids need, less pain, less intestinal manipulation by decreasing tissue trauma & inflammatory reactions |
Laxatives: They help stimulate bowel movement but, in randomized clinical trials, did not improve gastrointestinal recovery. |
|
|
Peripherally selective opioid receptors: antagonise receptors and minimise the opioid effect on GI function without impacting CNS-mediated analgesia. |
Non-Pharmacological Treatments
Enhanced Recovery after Surgery (Eras) – Speedy Recovery
Different hospitals developed the protocol to expedite recovery and shorten the duration of POI and hospital stay. This programme is also known as the “enhanced recovery programme (ERP). These programmes (ERAS or ERP) are multimodal rehabilitation that improves surgical outcomes and speeds the patient’s 35. The ERAS pathway comprises various stages and is implemented before (preoperative day), during (intra-operative) and after (post-operative interventions) surgical manipulation. The post-operative recovery is much more effective and reduces the complications in POI 36. Initially, the ERAS protocol was adopted in colorectal surgeries, but later all other surgical specialties adopted it 37. The primary ERAS factors (epidural analgesics, avoidance of opiates, use of prokinetic vehicles, and avoidance of nasogastric tubes) promote rapid recovery after surgical manipulations 38.
Various comparative studies have been performed between conventional and ERAS, showing shorter duration with ERAS for the first flatus, time to the first stool, and duration for oral intake. Based on this study outcome, it was concluded that ERAS takes a shorter time duration for the postoperative recovery of gastrointestinal function 39. Later, other studies were conducted and found the same outcome: ERAS recovers faster than conventional techniques 40,38. Patients receiving medicines in conjunction with ERAS therapy recover faster than those receiving only ERAS protocol-based therapy 41. Fluid management is an important part of ERAS that emphasises the preoperative, intraoperative, and postoperative regularisation of liquids. During surgical manipulations, fluid shifts and complications create major problems for patients, which are taken care of by fluid management in ERAS 36. According to pre-operative guidelines, patients should drink carbohydrate-rich fluids before being sedated. Intraoperative therapy targets sufficient perfusion of organs with flowing fluids. The post-operative therapy recommended an oral diet soon after the surgery, reducing the fluids to recover faster, and minimising the length of stay in hospitals 42. Hypovolemia (reduced organ perfusion, sepsis, and multiple organ failure) and hypervolemia are associated with complications and show an increase in POI 42.
The chewing of gum is another element of ERAS that initiates vagal stimulation that leads to gastric fluid stimulation, inhibits sympathetic triggers, and increases gut motility. In gastric fluid secretion, it is mainly increased gastric and salivary secretions that reduce postoperative ileus43. According to a recent study, chewing gum could save the healthcare system $118 million. This component could be the saviour for the patients in the ERAS protocol because it reduces time and money for the patient.
Laparoscopic Surgery
Over the conventional surgery, Laparoscopic surgery has minimum invasion and potential advantages. Due to minimum invasion, less pain and inflammation that leads to minimum complications in POI that leads to less stay at hospital. Recent study of 4,614 patient shows that laparoscopic surgery takes minimum time on an average 1 day for recovery of bowel function due to less invasive surgery 45. In few preclinical studies, laparoscopic surgery even not causing intestinal inflammation and POI as compared to standard open end surgical manipulation 46.
Pharmacological Strategies
Pharmacological strategies are emerging and being widely explored over many years as they could play a very crucial role in the prevention and treatment of POI. Between many drugs, ALV, which is mu-opioid receptor antagonist and an oral peripherally acting active, has demonstrated the most encouraging results 47. However, the active was limited for use because of significant incidence of cardiovascular complications in patients on chronic ALV (0.5 mg/1 mg twice daily) for varying period of 6 to 12 weeks 48. Later, in 2008 USFDA approved the drug for 15 doses which is a short duration use in a following manner i.e., Before operative procedure 12 mg and then followed by 12 twice daily for utmost period of 7 days. It is only approved indicated for prohibition of post-operative opioid following bowel surgeries 49. ALV is only available in hospitals which are registered in EASE (Entereg Access Support and Education) & purchased by REMS (Risk Evaluation and Mitigation strategy) of USFDA.
The pharmacological agents used for quick recovery in POI are specified in Table 1, and observations of latest studies about the use of several pharmacological agents and their correlation with POI are mentioned in Table 2.
Table 2: Pharmacological Agents used in Treatment of POI.
|
Pharmacological Therapy |
Mechanism of Action (MOA) |
|
μ-opioid receptor antagonists (e.g., ALV) |
Opioids lower gastrointestinal motility by acting on µ, delta, and kappa and give additional time for water and electrolyte absorption. It is also active as an anti-diarrheal. μ-opioid receptors are the primary mediators of opioid analgesic effects in the CNS, and the source of gastrointestinal side effects 6. Peripherally acting µ opioid receptor antagonists cause blockage of µ opioid receptor. |
|
Intravenous Lidocaine |
Lidocaine has demonstrated to conquer the inflammatory action and is stated to have analgesic effects, increase the rate of GI recovery, and weaken plasma concentrations of cytokines, interleukin IL-1, IL-6 & IL-8. These effects have not been shown in extra abdominal surgical procedure 50.
|
|
Serotonin receptor-5HT4 agonists (e.g., Prucalopride) |
5-HT4 receptors act on enteric neurons, hence accelerating cholinergic, non-adrenergic, and non-cholinergic neurotransmission. These agonists (e.g., Prucalopride) are very selective, high affinity for the 5-HT4 receptor 47. |
|
Calcitonin gene related peptide (CGRP) receptor antagonist |
CGRP receptor antagonists obstruct the CGRP receptor and enhance gut motility. CGRP is discharged from myenteric nerves and triggers resident leukocytes, making it supportive for POI. |
|
5-HT3 receptor antagonists |
The 5-HT3 receptor is identified on macrophages in the gastrointestinal tract. The 5-HT3 receptor antagonists decrease intestinal motility-induced infiltration of inflammatory CD68-positive macrophages and myeloperoxidase-stained neutrophils. To enhance delayed gastrointestinal transit, anti-inflammatory activity is attributed.
|
|
Cholinergic agonist |
It is a reversible inhibitor of acetylcholinesterase and has been very successful in the management of POI. The neurotransmitter acetylcholine is discharged at synapses and is key for initiation of the gut wall’s muscle contraction. Enzyme acetylcholinesterase causes the prohibition of hormone.
|
|
Prokinetic agents (cisapride, metoclopramide, erythromycin, cholecystokinin, and dopamine) |
Metoclopramide is proven cholinergic agonist and dopaminergic antagonist. Erythromycin shows its activity of motilin receptor agonist and causes increases in release of migrating motor complexes.
|
|
Coffee 1 |
It boosts colonic motility within 4 minutes of intake. There are different mechanisms of action of coffee on ileus, but, one of them is the rise in gastrin secretion, colonic spike, and motor activity. Exorphins in coffee play an important role in increasing colonic motility through opiate receptors that are present in the brain and intestinal wall. Antagonism of adenosine receptors stimulates the motor activity of the colon, which is also one of the mechanisms of action for coffee. Patients who drink coffee also have shorter hospital stays 51. |
Postoperative ileus has gained importance in recent years due to morbidity and financial burdens, and its pharmacological therapies are still evolving and extensively researched as they play a critical role in POI prevention. Current clinical practice guidelines for ERAS after rectal and colon surgeries from the American Society of Colon and Rectal Surgeons (ASCRS) and the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) recommend a minimally invasive surgical approach over open colectomy and laparoscopic colectomy 52.Also, from this study, it was concluded that the normal bowel function in the left colectomy was faster than that in the right colectomy, as well as that the incidence of prolonged POI is relatively lower. As a result, caution should be exercised during early feeding of ERAS protocol patients who have had laparoscopic right colectomy53.The ERAS pathway and laparoscopic strategies are generally efficacious in reducing the patient’s morbidity and promoting early recovery of normal gut function, as proved in the recent studies 54.
Prokinetic medicines such as metoclopramide and erythromycin are not proven efficacious therapies and should not be used to treat postoperative ileus. Out of many clinical studies, newer opioid receptor antagonist medications, such as alvimopan and methylnaltrexone, have been effective in reducing the time to the first bowel movement and the duration of patients stays in hospitals 53.
Also, over the past few years, the effectiveness of acupuncture in POI has been evaluated in a meta-analysis report. In these reports, intestinal surgery patients were included, and it was found that electropuncture is effective for POI. In addition, a few previous studies evaluated the effectiveness of acupuncture in cancer patients and concluded that acupuncture and other related therapies improve GI function recovery 5556.All of this reference information is used in studying the safety and effectiveness of acupuncture for postoperative ileus following gastrointestinal surgery. Eighteen randomized clinical trials involving 1413 participants were included in this study, and the meta-analysis studies found that acupuncture could reduce the time for first flatus (TFF), time to first defecation (TFD), time to bowel sounds recovery (TBSR), and length of hospital stay (LOS) when compared to standard care. This study reports that acupuncture has a significant impact on decreasing POI following gastrointestinal surgery 57
Alvimopan – Peripherally Acting µ-Opioid Receptor Antagonists in the Management Of POI
ALV (Entereg®), an oral & the only peripherally acting µ-opioid receptor (PAM-OR) antagonist, which has been observed to be beneficial to speed up the time to lower & upper gastrointestinal recovery following partial bowel resection surgery In, May 2008 ALV received approval by FDA. ALV chemically known as trans-3,4-dimethyl-4-(3-hydroxyphenyl) piperidine. ALV is highly sensitive to hydrolytic degradation while stable under photolytic, thermal & oxidative forced degradation 58. Intestinal flora causes conversion of ALV to an active primary amide metabolite. However, it is not required for efficacy in patients with POI 59. The bioavailability of ALV through oral route is 6% with range of 1% to 19% and it is rapidly absorbed 60. The formulation with improved bioavailability is much more useful in the treatment of POI. Various research has been started for improving the bioavailability thorough oral route.
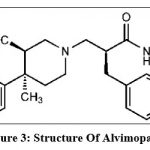 |
Figure 3: Structure of Alvimopan 61
|
ALV is zwitterionic molecule (containing both +ve & -ve charge) with molecular weight of 460.1 Da. It is having very low solubility in water & first pass metabolism after oral absorption and affects drug concentration in plasma.
The peripheral preference of ALV is because, of its zwitterionic nature, decreasing its capability to cross over blood brain barrier (BBB). In, beagle dog the bioavailability is only ~0.03% because of its low systemic absorption. Therefore, low oral bioavailability with low blood brain barrier permeability come out to synergize to make oral ALV less acceptable to reach to sufficient systemic level to have central nervous system effects 62,2. ALV is peripherally acting µ-opioid receptor antagonists because of its limited capacity to cross the BBB and reach the MORs of the central nervous system. It is approved for the treatment of POI because of its intentional blockage of MORs in the digestive tract. Speculated BA values for the MOR, KOR, NOR, and DOR -12.40, -9.50, -10.18, and -11.02 kcal/mol, respectively & potency on MOR is also confirmed on prediction. ALV is also potent on JAK1, BDKRB1, S1PR1, ACE, and SMO proteins with predicted BA values of -10.54, -10.36, -10.28, -10.07, and -10.07 kcal/mol respectively 63.
In gastrointestinal tract, ALV stops the binding of opioid agonists to the μ-opioid receptor and assists to inhibit constipation (bottom panel) Fig. 4. ALV does not block μ-opioid receptors in the central nervous system or only intervene with centrally mediated opioid analgesia because, ALV do not have the capability to cross the BBB 64.
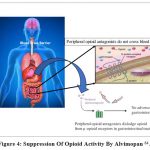 |
Figure 4: Suppression of Opioid Activity by Alvimopan 64.
|
The safety & efficacy of ALV assessed in patients undergoing colonic surgeries, including hysterectomy, radical cystectomy & bowel resection 65,66. In, previous studies duration to recovery of GI functions is revealed the primary endpoint as GI3. But GI3 not showed statistically significant differences as compared placebo 66,67. Secondary endpoint, GI2 was considered as more objective after Post hoc analysis study. Both, GI2 & GI3 endpoint is identical but removes flatus as a marker for less GI recovery. Comprehensively, ALV reduces the time for GI recovery & readiness for discharge.
39 randomised controlled trials involving 15 prokinetic drugs from different groups, as well as 10 studies involving prokinetic medicine comparisons, were examined. Regularly used medicines like vasopressin, propranolol, cisapride, cholecystokinin-like drugs, erythromycin, etc. had inadequate evidence to show any advantage. However, 6 RCTs sustained the use of ALV 68. Also, a retrospective cohort equivalent study included 480 patients who received ALV compared to 960 equivalent control patients. The authors observed after analysing the data that the recovery time from ileus and overall cost of hospitalisation were superior in patients who received ALV.
Recently, A Retrospective Study of Patients go through GI Surgery with or without giving ALV was conducted to Compare Total Direct Cost and duration of Stay. In, this study 64 patients reviewed from which 33 (51.5 %) patients met inclusion criteria & received ALV. ALV treatment was incorporated with a decreased duration for hospitalization (4.48 vs 7.39 days, P < 0.01) comparing without ALV treatment. Additionally, total of treatment cost is significantly reduced of the average total direct financial cost for therapeutic group ($7,965 vs $9,100), a difference of 12.4% 70.
Because of the high cost of the drug, frequent use of the molecule is limited. The cost of metoclopramide injection is around 250 times lower when used four times for a 10 mg dose than the cost of 15 doses of ALV. Also, ALV might not be effective once ileus is set in. Because of this, the FDA demands that its use be started preoperatively. Other supportive measures such as ambulation, mobilisation, electrolyte optimisation, early enteral feeds, and laxatives should be maintained. Naloxone, which is Nonselective opioid antagonists allow systemic opioids to cross the blood brain barrier by reversing constipation, but at the expense of antagonising systemic analgesia, which is not recommended post-operatively 49.
Conclusion
The most common problem following surgical manipulation is postoperative ileus. After abdominal surgeries, paralytic postoperative ileus remains a significant problem. The pathophysiology of postoperative ileus is multifactorial and best treated with a combination of different approaches. At the moment, the major factors that may affect postoperative ileus recovery and duration include limiting narcotic use, using substitute nonsteroidal medications, and positioning a thoracic epidural with local anaesthetics. The selective use of Enhanced Recovery After Surgery (ERAS) with correction of electrolyte imbalances and nasogastric decompression is important. These interpretations are all based on the fact that ileus was reviewed as morbidity and anticipated morbidity rather than as a protective postoperative purpose, as some theories suggest. Combination therapy is always preferable to a single treatment. Considerable progress has been made in combating POIs, but much work remains to be done. On the formulation development front, novel formulation technology will help to improve bioavailability and safety, which are preferred for a better and faster treatment response in the POI. Currently, ALV is the only opioid receptor antagonist approved for POI that improves gastrointestinal tract recovery after surgery and shortens hospital stays. However, unresolved long-term safety issues, a limited indication, and admittance schemes are likely to prevent its widespread use in the surgical population.
Conflicts of Interest
The authors report no financial or any other conflicts of interest in this work.
Funding Sources
There is no funding to report.
References
- Güngördük K, Gülseren V, Özdemir İA. The use of coffee for the prevention of ileus following abdominal surgery: A review of the current evidence. Pelviperineology. 2022;41(3):189-193. doi:10.34057/PPJ.2022.41.03.2022-3-2
- Baig MK, Wexner SD. Postoperative Ileus: A Review. Dis Colon Rectum. 2004;47(4):516-526. doi:10.1007/S10350-003-0067-9
- Livingston EH, Passaro EP. Postoperative ileus. Dig Dis Sci. 1990;35(1):121-132. doi:10.1007/BF01537233
- Chamie K, Golla V, Lenis AT, Lec PM, Rahman S, Viscusi ER. Peripherally Acting μ-Opioid Receptor Antagonists in the Management of Postoperative Ileus: a Clinical Review. Journal of Gastrointestinal Surgery. 2021;25(1):293-302. doi:10.1007/S11605-020-04671-X
- Holte K, Kehlet H. Postoperative ileus: A preventable event. British Journal of Surgery. 2000;87(11):1480-1493. doi:10.1046/J.1365-2168.2000.01595.X
- Bragg D, El-Sharkawy AM, Psaltis E, Maxwell-Armstrong CA, Lobo DN. Postoperative ileus: recent developments in pathophysiology and management. Elsevier. 2015;34:367-376. doi:10.1016/j.clnu.2015.01.016
- Zittel, Tilman T., S. Narasimha Reddy, Victor Plourde, and Helen E. Raybould. “Role of spinal afferents and calcitonin gene-related peptide in the postoperative gastric ileus in anesthetized rats.” Annals of surgery219, no. 1 (1994): 79.
- Senagore, Anthony J., Joel J. Bauer, Wei Du, and Lee Techner. “Alvimopan accelerates gastrointestinal recovery after bowel resection regardless of age, gender, race, or concomitant medication use.” Surgery142, no. 4 (2007): 478-486.
- Millan M, Biondo S, Fraccalvieri D, Frago R, Golda T, Kreisler E. Risk factors for prolonged postoperative ileus after colorectal cancer surgery. World J Surg. 2012;36(1):179-185. doi:10.1007/S00268-011-1339-5
- Artinyan A, Nunoo-Mensah JW, Balasubramaniam S, et al. Prolonged postoperative ileus – Definition, risk factors, and predictors after surgery. World J Surg. 2008;32(7):1495-1500. doi:10.1007/S00268-008-9491-2
- Huge, A., M. E. Kreis, E. C. Jehle, H. J. Ehrlein, M. Starlinger, H. D. Becker, and T. T. Zittel. “A model to investigate postoperative ileus with strain gauge transducers in awake rats.” Journal of Surgical Research74, no. 2 (1998): 112-118.
- Bauer AJ, Boeckxstaens GE. Mechanisms of postoperative ileus. Neurogastroenterology and Motility. 2004;16(SUPPL. 2):54-60. doi:10.1111/J.1743-3150.2004.00558.X
- Boeckxstaens, G. E., and W. J. De Jonge. “Neuroimmune mechanisms in postoperative ileus.” Gut58, no. 9 (2009): 1300-1311.
- Martini S, Corvaglia L. Premature Infants. Frailty in Children. Published online 2023:11-32. doi:10.1007/978-3-031-24307-3_2
- De Winter BY, Robberecht P, Boeckxstaens GE, et al. Role of VIP1/PACAP receptors in postoperative ileus in rats. ncbi.nlm.nih.gov. Published online 1998. Accessed March 21, 2023. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1565509/
- Daudova GM, Repin IS. [Thermoregulation activity of the muscle after awakening of hibernating animals]. Patol Fiziol Eksp Ter. 1993;9(2):73-74. Accessed March 21, 2023. http://www.ncbi.nlm.nih.gov/pubmed/24352104
- Vather, Ryash, Greg O’Grady, Ian P. Bissett, and Phil G. Dinning. “Pathophysiologic, translational and clinical aspects of postoperative ileus-A review.” In Proceedings of the Australian Physiological Society, vol. 44, pp. 85-99. AuPS, 2013.
- Holte K, Kehlet H. Postoperative ileus: A preventable event. British Journal of Surgery. 2000;87(11):1480-1493. doi:10.1046/J.1365-2168.2000.01595.X
- Davidson ED, Hersh T, Brinner RA, Barnett SM, Boyle LP. The effects of metoclopramide on postoperative ileus. A randomized double-blind study. ncbi.nlm.nih.gov. Accessed February 28, 2023. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1344451/
- Longo WE, Vernava AM. Prokinetic agents for lower gastrointestinal motility disorders. Dis Colon Rectum. 1993;36(7):696-708. doi:10.1007/BF02238599
- Tollesson PO, Cassuto J, Rimbäck G, Faxén A, Bergman L, Mattsson E. Treatment of postoperative paralytic ileus with cisapride. Scand J Gastroenterol. 1991;26(5):477-482. doi:10.3109/00365529108998569
- Boeckxstaens, G. E., and W. J. De Jonge. “Neuroimmune mechanisms in postoperative ileus.” Gut58, no. 9 (2009): 1300-1311.
- Schwarz, Nicolas T., Donna Beer-Stolz, Richard L. Simmons, and Anthony J. Bauer. “Pathogenesis of paralytic ileus: intestinal manipulation opens a transient pathway between the intestinal lumen and the leukocytic infiltrate of the jejunal muscularis.” Annals of surgery235, no. 1 (2002): 31.
- de Winter BY, Robberecht P, Boeckxstaens GE, et al. Role of VIP1/PACAP receptors in postoperative ileus in rats. ncbi.nlm.nih.gov. Published online 1998. Accessed March 1, 2023. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1565509/
- Farro G, Gomez-Pinilla PJ, di Giovangiulio M, et al. Smooth muscle and neural dysfunction contribute to different phases of murine postoperative ileus. Neurogastroenterology and Motility. 2016;28(6):934-947. doi:10.1111/NMO.12796
- Bult, Hetal, G. E. Boeckxstaens, P. A. Pelckmans, F. H. Jordaens, YM Van Maercke, and A. G. Herman. “Nitric oxide as an inhibitory non-adrenergic non-cholinergic neurotransmitter.” Nature345 (1990): 346-347.
- Ogilvy, A. J., and G. Smith. “The gastrointestinal tract after anaesthesia.” European journal of anaesthesiology. Supplement10 (1995): 35-42.
- Kalff, Jörg C., Wolfgang H. Schraut, Timothy R. Billiar, Richard L. Simmons, and Anthony J. Bauer. “Role of inducible nitric oxide synthase in postoperative intestinal smooth muscle dysfunction in rodents.” Gastroenterology118, no. 2 (2000): 316-327.
- Chen, Grace Y., and Gabriel Nuñez. “Sterile inflammation: sensing and reacting to damage.” Nature Reviews Immunology10, no. 12 (2010): 826-837.
- Bueno, Lionel, Jean Fioramonti, Michel Delvaux, and Jacques Frexinos. “Mediators and pharmacology of visceral sensitivity: from basic to clinical investigations.” Gastroenterology112, no. 5 (1997): 1714-1743.
- Coimbra CR, Plourde V. Abdominal surgery-induced inhibition of gastric emptying is mediated in part by interleukin-1β. Am J Physiol Regul Integr Comp Physiol. 1996;270(3 39-3). doi:10.1152/AJPREGU.1996.270.3.R556
- Glowka TR, Steinebach A, Stein K, et al. The novel CGRP receptor antagonist BIBN4096BS alleviates a postoperative intestinal inflammation and prevents postoperative ileus. Neurogastroenterology and Motility. 2015;27(7):1038-1049. doi:10.1111/NMO.12584
- Snoek SA, Dhawan S, van Bree SH, et al. Mast cells trigger epithelial barrier dysfunction, bacterial translocation and postoperative ileus in a mouse model. Neurogastroenterology and Motility. 2012;24(2). doi:10.1111/J.1365-2982.2011.01820.X
- Schwarz, Nicolas T., Donna Beer-Stolz, Richard L. Simmons, and Anthony J. Bauer. “Pathogenesis of paralytic ileus: intestinal manipulation opens a transient pathway between the intestinal lumen and the leukocytic infiltrate of the jejunal muscularis.” Annals of surgery235, no. 1 (2002): 31.
- Kalff, Jorg C., Wolfgang H. Schraut, Richard L. Simmons, and Anthony J. Bauer. “Surgical manipulation of the gut elicits an intestinal muscularis inflammatory response resulting in postsurgical ileus.” Annals of surgery228, no. 5 (1998): 652.
- Kehlet, Henrik, and Douglas W. Wilmore. “Evidence-based surgical care and the evolution of fast-track surgery.” Annals of surgery248, no. 2 (2008): 189-198.
- Luckey, Andrew, Edward Livingston, and Yvette Taché. “Mechanisms and treatment of postoperative ileus.” Archives of Surgery138, no. 2 (2003): 206-214.
- Boitano, Teresa KL, Haller J. Smith, Tullia Rushton, Mary C. Johnston, Prentiss Lawson, Charles A. Leath III, Anisa Xhaja, Meredith P. Guthrie, and J. Michael Straughn Jr. “Impact of enhanced recovery after surgery (ERAS) protocol on gastrointestinal function in gynecologic oncology patients undergoing laparotomy.” Gynecologic Oncology151, no. 2 (2018): 282-286.
- Zeng WG, Liu MJ, Zhou ZX, Wang ZJ. Enhanced recovery programme following laparoscopic colorectal resection for elderly patients. ANZ J Surg. 2018;88(6):582-586. doi:10.1111/ANS.14074
- Shetiwy, Mosab, Tamer Fady, Fayez Shahatto, and Ahmed Setit. “Standardizing the protocols for enhanced recovery from colorectal cancer surgery: are we a step closer to ideal recovery?.” Annals of coloproctology33, no. 3 (2017): 86.
- Kennedy, Gregory T., Christine M. Hill, Ye Huang, Alycia So, Joshua Fosnot, Liza Wu, John T. Farrar, and Julia Tchou. “Enhanced recovery after surgery (ERAS) protocol reduces perioperative narcotic requirement and length of stay in patients undergoing mastectomy with implant-based reconstruction.” The American Journal of Surgery220, no. 1 (2020): 147-152.
- Nisanevich, Vadim, Itamar Felsenstein, Gidon Almogy, Charles Weissman, Sharon Einav, and Idit Matot. “Effect of intraoperative fluid management on outcome after intraabdominal surgery.” The Journal of the American Society of Anesthesiologists103, no. 1 (2005): 25-32.
- Tandeter, H. “Hypothesis: hexitols in chewing gum may play a role in reducing postoperative ileus.” Medical hypotheses72, no. 1 (2009): 39-40.
- Schuster, Rob, Nina Grewal, Gregory C. Greaney, and Kenneth Waxman. “Gum chewing reduces ileus after elective open sigmoid colectomy.” Archives of Surgery141, no. 2 (2006): 174-176.Ohtani H, Tamamori Y, Azuma T, et al. A Meta-analysis of the Short- and Long-Term Results of Randomized Controlled Trials That Compared Laparoscopy-Assisted and Conventional Open Surgery for Rectal Cancer. Journal of Gastrointestinal Surgery. 2011;15(8):1375-1385. doi:10.1007/S11605-011-1547-1
- Gomez-Pinilla PJ, Farro G, di Giovangiulio M, et al. Mast cells play no role in the pathogenesis of postoperative ileus induced by intestinal manipulation. PLoS One. 2014;9(1). doi:10.1371/JOURNAL.PONE.0085304
- Webster, Lynn, Jan Peter Jansen, John Peppin, Ben Lasko, Gordon Irving, Bart Morlion, Jerry Snidow et al. “Alvimopan, a peripherally acting mu-opioid receptor (PAM-OR) antagonist for the treatment of opioid-induced bowel dysfunction: results from a randomized, double-blind, placebo-controlled, dose-finding study in subjects taking opioids for chronic non-cancer pain.” PAIN®137, no. 2 (2008): 428-440.
- Nair, Abhijit. “Alvimopan for post-operative ileus: what we should know?.” Acta Anaesthesiologica Taiwanica54, no. 3 (2016): 97-98
- McCarthy, Grace C., Sohair A. Megalla, and Ashraf S. Habib. “Impact of intravenous lidocaine infusion on postoperative analgesia and recovery from surgery: a systematic review of randomized controlled trials.” Drugs70 (2010): 1149-1163.
- Boekema PJ, Samsom M, van Berge Henegouwen GP, Smout AJPM. Coffee and gastrointestinal function: Facts and fiction: A review. Scand J Gastroenterol Suppl. 1999;33(34):35-39. doi:10.1080/003655299750025525
- Liang W, Li J, Zhang W, et al. Prolonged postoperative ileus in gastric surgery: Is there any difference between laparoscopic and open surgery? Wiley Online Library. 2019;8(12):5515-5523. doi:10.1002/cam4.2459
- Lin Z, Yang C, Wang Y, Yan M, Zheng H. Comparison of prolonged postoperative ileus between laparoscopic right and left colectomy under enhanced recovery after surgery: a propensity score matching analysis. World J Surg Oncol. 2022;20(1). doi:10.1186/S12957-022-02504-6
- Khawaja ZH, Gendia A, Adnan N, Ahmed J. Prevention and Management of Postoperative Ileus: a review of current practice. Cureus. 2022;14(2).
- Liu YH, May BH, Zhang AL, Guo XF, Lu CJ, Xue CC, et al. Acupuncture and Related Therapies for Treatment of Postoperative Ileus in Colorectal Cancer: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Evid Based Complement Alternat Med. 2018; 2018:3178472. https://doi.org/ 10.1155/2018/3178472.
- Liu YH, Dong GT, Ye Y, Zheng JB, Zhang Y, Lin HS, et al. Effectiveness of Acupuncture for Early Recovery of Bowel Function in Cancer: A Systematic Review and Meta-Analysis. Evid Based Complement Alternat Med. 2017; 2017:2504021. https://doi.org/10.1155/2017/2504021.
- Ye, Zi, Xuqiang Wei, Shouquan Feng, Qunhao Gu, Jing Li, Le Kuai, Yue Luo, Ziqi Xi, Ke Wang, and Jia Zhou. “Effectiveness and safety of acupuncture for postoperative ileus following gastrointestinal surgery: A systematic review and meta-analysis.” Plos one17, no. 7 (2022): e0271580.
- Baira, Shandilya Mahamuni, R. Srinivas, and MVN Kumar Talluri. “Identification and structural characterization of hydrolytic degradation products of alvimopan by LC/QTOF/MS/MS and NMR studies.” Journal of Pharmaceutical and Biomedical Analysis165 (2019): 399-409.
- Kraft, Michael, Robert MacLaren, Wei Du, and Gay Owens. “Alvimopan (entereg) for the management of postoperative ileus in patients undergoing bowel resection.” Pharmacy and Therapeutics35, no. 1 (2010): 44.
- FOSS J, SCHMITH V, WALLIN B, DU W, MELIKIAN A. Alvimopan (entereg?), a novel opioid antagonist, achieves active systemic concentrations. Clin Pharmacol Ther. 2005;77(2):P74-P74. doi:10.1016/J.CLPT.2004.12.175
- Leslie JB. Alvimopan for the management of postoperative ileus. Annals of Pharmacotherapy. 2005;39(9):1502-1510. doi:10.1345/APH.1E615
- Thomas, Jay. “Opioid-induced bowel dysfunction.” Journal of pain and symptom management35, no. 1 (2008): 103-113.
- Feng H, Elladki R, Jiang J, Wei GW. Machine-learning Analysis of Opioid Use Disorder Informed by MOR, DOR, KOR, NOR and ZOR-Based Interactome Networks. Published online January 12, 2023. Accessed March 1, 2023. http://arxiv.org/abs/2301.04815
- Bream-Rouwenhorst, Heather R., and Matthew A. Cantrell. “Alvimopan for postoperative ileus.” American Journal of Health-System Pharmacy66, no. 14 (2009): 1267-1277.
- Ludwig, Kirk, Warren E. Enker, Conor P. Delaney, Bruce G. Wolff, Wei Du, John G. Fort, Maryann Cherubini, James Cucinotta, and Lee Techner. “Gastrointestinal tract recovery in patients undergoing bowel resection: results of a randomized trial of alvimopan and placebo with a standardized accelerated postoperative care pathway.” Archives of Surgery143, no. 11 (2008): 1098-1105.
- Viscusi ER, Goldstein S, Witkowski T, et al. Alvimopan, a peripherally acting mu-opioid receptor antagonist, compared with placebo in postoperative ileus after major abdominal surgery: Results of a randomized, double-blind, controlled study. Surgical Endoscopy and Other Interventional Techniques. 2006;20(1):64-70. doi:10.1007/S00464-005-0104-Y
- Delaney CP, Weese JL, Hyman NH, et al. Phase III trial of alvimopan, a novel, peripherally acting, Mu opioid antagonist, for postoperative ileus after major abdominal surgery. Dis Colon Rectum. 2005;48(6):1114-1129. doi:10.1007/S10350-005-0035-7
- Traut U, Brügger L, Kunz R, et al. Systemic prokinetic pharmacologic treatment for postoperative adynamic ileus following abdominal surgery in adults. Cochrane Database of Systematic Reviews. 2008;(1). doi:10.1002/14651858.CD004930.PUB3/ABSTRACT
- Poston, Sara, Michael S. Broder, Melinda Maggard Gibbons, Robert MacLaren, Eunice Chang, Christine J. VandePol, Suzanne F. Cook, and Lee Techner. “Impact of alvimopan (entereg) on hospital costs after bowel resection: results from a large inpatient database.” Pharmacy and Therapeutics36, no. 4 (2011): 209.
- Lister, Yenisey Rodriguez, Raul Mederos, and Nestor Veciana. “A Retrospective Study of Patients Undergoing Gastrointestinal Surgery with or without Receiving Alvimopan: Comparing Length of Stay and Total Direct Cost.” Medical Research Archives10, no. 7 (2022).

This work is licensed under a Creative Commons Attribution 4.0 International License.