Manuscript accepted on : 23-02-2023
Published online on: 09-05-2023
Plagiarism Check: Yes
Reviewed by: Dr. Robert Susło
Second Review by: Dr. Ahmed Lafi
Final Approval by: Dr Jahwarhar Izuan Bin Abdul Rashid
Md Fahim Ansari1 , Fahad Afzal2*
, Fahad Afzal2* and Satya Mehra3
and Satya Mehra3
1Department of Electrical Engineering, Graphic Era Deemed to be University, Dehradun, Uttarakhand -248002, India.
2Institute of Health Management Research, IIHMR University, Jaipur, Rajasthan- 302029, India.
3India Health Action Trust- UPTSU, Lucknow, Uttar Pradesh- 226001 India.
Corresponding Author E-mail: syedfahadafzal@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/3118
ABSTRACT: Ensuring food and water safety has always been a health challenge globally. The present papers underscores HV-PEF (High Voltage Pulsed Electric Field), a novel approach to eliminate five kinds of microbes prevalent in milk and water in fractions of a second. These microorganisms are Enterobaracter aerogenes, Escherichia coli, Listeria monocytogene, Staphylococcus aurous, and Acetobacter. Objective was to find out the impact of HV-PEF on survival of selected species of bacteria, which are often considered as a concern for water and milk safety. A laboratory experimental setup was designed for producing HV-PEF, which was applied on sample of water and milk. The water sample was obtained from tap, and the milk sample was obtained from Bubalus bubalis. The microbial count was measured by plate method for both samples pre and post exposure to the HV-PEF. The effect was measured with combinations of varying field intensity and number of pulses, the intensity of the field having the highest value of 180 kV/cm (kilovolt-per-centimeter), and the pulse count varied between one to one hundred. Results showed, the endurance of few species was extremely low, at 40 kV filed intensity and 40 pulses. Other microbes also demonstrated low survival ratio (SR) at more than 40kV with pulses-count above 40. Complete inactivation of E. coli was achieved at around 80kV. The inactivation of microbe by HV-PEF varies with morphology and shape of the microorganisms. The annihilation of microorganisms is due to the rupturing of cell wall of microbe by the effect of HV-PEF, instead of ohmic heating (resistance induced).
KEYWORDS: Food Poisoning; Food Safety; High Voltage Pulsed Electric Fields; Microbial Inactivation; Public Health; Pasteurization
Download this article as:| Copy the following to cite this article: Ansari M. F, Afzal F, Mehra S. Eradication of Enterobaracter aerogenes, Escherichia coli, Listeria monocytogene, Staphylococcus aurous, and Acetobacter by High Voltage Pulsed Electric Field in Water and Milk Samples. Biosci Biotech Res Asia 2023;20(2). |
| Copy the following to cite this URL: Ansari M. F, Afzal F, Mehra S. Eradication of Enterobaracter aerogenes, Escherichia coli, Listeria monocytogene, Staphylococcus aurous, and Acetobacter by High Voltage Pulsed Electric Field in Water and Milk Samples. Biosci Biotech Res Asia 2023;20(2). Available from: https://bit.ly/3HRnbbr |
Introduction
Health issues related to food and drinks are highly concerning at global level. As per World Health Organization, 1 out 10 individuals become ill due to consuming contaminated food and approximately 420,000 fatal cases annually due to food poisoning1. Ensuring food and water safety has always been a challenge, and many techniques are developed to ensure food safety. This research focuses on 5 types of bacteria which are widely found in milk and water, these are Enterobacter aerogenes, Escherichia coli (also known as E. coli), Listeria monocytogenes, Staphylococcus aurous, and Acetobacter. Enterobacter aerogenes, E. coli and Acetobacter are gram-negative whereas Listeria monocytogenes, Staphylococcus aurous are gram-positive in nature.
Literature review revealed that these microorganisms are one of the leading causes of food poisoning in tropical and equatorial countries. A study conducted in India revealed that the presence of Enterobacter aerogenes in food items increases the odds of food poisoning more than twice2. A study in Iraq reported that consumption of E. coli contaminated water has caused five outbreaks and 1000 hospitalizations between 2013 and 20213. The detrimental effects of these bacteria are not limited to liquid items, but also causes mass food poisoning via solid food items. Researchers have reported that in South Africa, adulteration of beef with horsemeat (which has high Listeria monocytogenes count) leads to serious health consequences and huge economic burden on the public health system4.
A study conducted on ready-to-eat fruits available at street vendors in Nigeria revealed the presence of the antibiotic resistant Staphylococcus aurous. This research underscored that this could lead to the broad-spectrum antibiotic resistance development in the consumers5.
Next section briefly discusses plate technique and underscores the recent advancements in pasteurization, with focus on a novel technology called Pulse Electric Field (PEF) to annihilate the microorganisms.
Plate Technique
Traditional Plate Count (TPC) technique is used for quality verification of probiotic goods, which is based on the reproduction of bacterial cells on agar plates. The quantity of bacterial cells that can grow on the culture medium and their identity can be determined via culture-based analysis6. Fluorescent stains, which can detect living, damaged, and dead bacterial cells, are utilised to acquire a better understanding of the cells’ physiological status and metabolic activities7. In the present experiment for microbial inactivation, plate count technique has been utilised to calculate SR of the aforementioned five bacterial species.
Recent advancements in Pasteurization and Food Processing
Traditional pasteurization process is based on heating a liquid below its boiling point to inactivate the pathogenic and undesired microorganisms. The aim of pasteurization is to prolong the shelf life of wine, milk or any other liquid food item8. In the last couple of years, there has been some notable advancement in the field of pasteurization and food processing. Pasteurization technique is developed gradually since its inception and has variants namely thermalisation, holder vat pasteurization (low pasteurization), high pasteurization (High Temperature Short Time – HTST), sterilization and ultra-heating. These differ in temperature ranges and exposure duration9. Solar energy source-based techniques of pasteurization are also proposed. The major drawback of using solar energy based pasteurization is that it completely depends on weather and geographical location. High Intensity Ultrasound Pasteurization (HIUS) has emerged as a non-thermal technique10. It was found that the HIUS is faster and more effective in reducing the bacterial count than conventional HTST11. The thermal techniques such as ohmic heating is also gaining popularity due to its cost efficiency, but it has limitations such as alteration of food texture, complexity of operations and safety concern when operated at industrial scale12.
Various experiments and studies had been conducted analyzing microbial destruction by use of High Voltage-Pulsed Electric Field (HV-PEF) with different set-up and methodologies. In an experiment conducted by a group of researchers13, the highest applied voltage was 40 kV/cm of bipolar square pulses of width ranging between 20 to 180×10-6 s. They reported it as the most effective method for microorganism annihilation, however the species of microbes were not identified and only voltage range was specified, i.e. 12 to 40kV/cm. A laboratory study explored the microbial inactivation by different kinds of shape of PEF wave14. Another experimental research reported inactivation of microorganisms (such as E. coli prevalent in water) with constant temperature, and further the impact of pulse electric-field on viruses was discussed15. A huge reduction in bacterial count of E. coli was reported at 110kV/cm. A proposed model PSpice and Pulse forming network of PEF demonstrated a potential of significantly limiting the growth of microalgae16. This is corroborated by another research which reported that the effect of PSpice is disinfection of approximately 70% of microbes by 2-log in orange juice, where PEF was used for very short duration17.
Few other studies analyzing the effect of PEF on milk (ranging from 10-40kV/cm), argued only E. coli microbes were inactivated18. A group of researchers investigated three bacterial species inactivation in fruit juice by using Cascaded H-bridge Multilevel Inverter (CHMI). The microorganism investigated were Saccharomyces cerevisiae, Staphylococcus Aureus and E. coli, and the CHMI was reported as a power efficient method of microbial inactivation for food in liquid and semi-liquid form. The finding of this study is corroborated by another experiment in which a square wave pulsed generator for the treatment of food items were utilized by the researchers who applied CHMI integrated PEF on food items19,20. Recent studies have highlighted and compared the PEF with ohmic heating methods. PEF is reported as a significantly efficient method for preservation of nutritive components on food items as it does not involve changes in temperature21. Coban and Fidan designed a circuit schematic for the generation of HV-PEF20. This circuit design was used by past researchers for the experiments with similar calculation technique of SR of five kinds of microbes present in liquid items, namely milk and water22, the results of which are supported by other studies23. A similar methodology is adopted in present study. The past literature provides evidence that PEF effects are significant, nevertheless the impact of PEF at a high voltage is not explored enough in regard to available tap water in residential areas of northern India from borewell and commonly available untreated milk. Researchers have indicated a scaled-up HV-PEF could be developed for industrial use. However, reviewing the existing literature revealed that limited types of microbes have been studied using only a Restricted Potential-difference per unit length with maximum value of 40kV.
The present study attempts to answer the question; what is the effect of HV-PEF on five selected bacterial species in commonly available water and milk? The present study aims to find out the impact of HV-PEF on survival of selected species of bacteria, which are often considered as a concern for food safety.
Materials and methods
This experiment was conducted in November 2022 in a lab setting. The milk and water samples are poured into the PEF chamber, which is located between the anode and cathode. Five undermentioned prevalent bacteria were exposed to HV-PEF ranging from 0 to 180 kV/cm applied in water and milk, (i) Listeria monocytogene, (ii) Staphylococcus aurous, (iii) Enterobacter aerogenes, (iv) E. coli, and (v) Acetobacter.
In the experiment dependent variable is ‘Survival ratio’ of the microorganism which is the endurance of the microorganism towards HV-PEF. This is equal to the proportion of microbial count afterward application of HV-PEF and microbial count initially present in the sample.
At a time, either ‘the number of pulses’ or ‘the peak voltage’ is used as an independent variable which is manipulated. If ‘number of pulses’ is used as an independent variable then peak voltage remains constant as control variable, and vice-versa. The experiment was conducted in lab setting with controlled temperature ranging between 27°-28° Celsius.
Logarithmic graph was used to depict the results. The ratio was explored at different combinations of voltage levels and pulse counts. The sample of milk was obtained from a local dairy supplier. The milk was collected from Bubalus bubalis (breed commonly known as ‘Murrah’). The water sample was obtained from tap (200 feet deep borewell water supply) of a residential area. Both samples were crude and previously unprocessed. The TDS (Total Dissolved Solids) of water sample was approximately 205 ppm measured by TDS meter (model: HM-TDS-3). The specific gravity of the milk sample was 1.038. For both water and milk the five bacterial species were identified and extracted using microscopy, and cultured in petri dishes separately. Then five samples of both items (milk and water) were treated by HTST pasteurization and checked for presence of any remaining microbes by methylene blue reduction and phosphatase tests. After ensuring the samples are uniformly made free of bacteria, one single species of bacteria was reintroduced in the one sample of each item (milk and water). Such as E.coli in one water sample and one milk sample. After reintroducing all the bacterial species, all ten samples were incubated for 24 hours at 37° Celsius in an incubator. Then using plate technique, the initial bacterial count was measured. After exposing these sample to different combinations of pulses and peak voltage, final bacterial counts were measured. Using these initial and final counts survival ratios was calculated and superimposing graphs were plotted.
The circuit schematic for an experimental setup to produce HV-PEF is depicted in figure 1. Experimental laboratory setup is depicted in figure 2, which shows the treatment chamber for samples of water and milk, and high-voltage pulse generator.
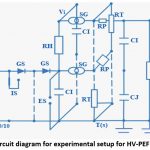 |
Figure 1: Circuit diagram for experimental setup for HV-PEF generation. |
 |
Figure 2: Laboratory setup for experiment. |
A maximum magnitude of 280kV can be obtained using this arrangement. The configuration consists of couple of multiplier circuit, electric field generator and electrodes. A resistance RL (high-value) is placed in the parallel connection with an impulse-capacitor (CI). Voltage (potential difference) Vi is generated across the circuit. The generated potential difference is regulated. When Vi is increased accompanied by closing of spear gaps (SG) with both CI connected in series, results in high voltage generation. Insulating rod (IS) is used to remove any residual surface charge. Ts denoted the zero ground potential. Two unidirectional diodes (GS) were used to unsure direct-current maintenance and the circuit safety from high voltage. Damping resistor (CJ) are linked in series with CI, and CJ is electrically charged by the damping resistors. The resistors RP and RT discharge the voltages of CI and CJ. The simultaneous breakdown of spear gaps (SG) is done by making the value of RH dramatically more in comparison to RT.
The specifications of various elements of the circuit diagram and experimental setup are enumerated in Table 1. In the present study fluid items namely water and milk were utilized, the quantity taken is 50ml of each. The concentration of microbes (105 CFU/ml) for each the sample was high, so that high concentration of microbes give a more precise SR value.
Table 1: Configuration details of experimental setup.
| S. No. | Components | Specifications |
| 1 | Electrodes (anode and cathode) | Disc plates (parallel adjusted), Contour edge,
Alignment: Horizontally Built Material: Brass Contact Area: 64 sq. cm |
| 2 | Sample Volume | 50 ml Water
50 ml Milk |
| 6 | Treatment chamber | Built Material: Perspex |
| 7 | Voltage Range (minimum to maximum) | 0.001 to 180 (kV) |
| 8 | Maximum field | 225kV/cm |
| 9 | Shape of Impulse | 1.2/50 µsec |
| Capacitors | Load capacitor: 1.20 nF
Impulse capacitor: 25.00 nF Capacitor for standard media: 5.06 µF Water capacitance: 4.03 µF Milk capacitance: 8.57 µF |
|
| 11 | Resistors | Wave resistor (front): 245 ohms
Wave resistor (tail): 2400 ohms |
For the data analysis MATLAB software (version 2014a) was utilized. Aforementioned methodology and similar setup are employed by other researchers to explore the impact of HV-PEF on fluid items (apple juice and cranberry juice)24,25.
Mechanism of Bacterial Destruction
Electric field has a tendency of polarizing the non-vegetative bacterial cell. PEF acts by hampering peptidoglycan compounds that are responsible for maintaining the structural integrity of the bacterial cell wall26.
When the bacteria are exposed to increasing PEF, when a certain level of electric field is reached formation of hydrophilic pores in the bacterial membrane (cell wall) takes place, allowing effective mass transfer. When the field intensity is increased furthermore (i.e. either number of pulses, exposure duration or amplitude), an irreparable damage to the cell membrane occurs27. This leads to complete loss of the bacterial cell’s structural integrity and cell death. In the present set-up same destruction of microorganism is based on the same phenomenon.
Results
Overall, a markedly high proportion (99.9%) of the aforementioned microbes were eliminated. The findings of the experiments are represented in two sections, as the experiment is done on milk and water samples separately. For each section finding in terms of survival ratio is depicted in graphical form.
Experimental findings for milk sample
Figures 3(A) and 3(B) show experimental results for microbial inactivation of E. coli, Listeria monocytogene, Enterobaracter aerogenes, Acetobacter and Staphylococcus aurous in the milk sample. Each bacterial species under investigation demonstrated varied endurance at different voltage levels and number of pulses imposed on the sample. Bacterial destruction is due to exposure to intensified electric fields, instead of resistance induced heating. The highest number of pulses is 100 along with highest applied voltage approximately 160kV. The number of microorganisms present in the milk before and after application of HV-PEF is counted by the plate count approach. The initial number of bacteria is Ni, while the microbial count after high voltage exposure is Nf. The calculation of endurance (SR) is done by dividing Nf by Ni.
Figure 3(A) depicts a graph of the SR against the number of pulses at a fixed highest voltage (100kV), for the milk sample. The SR of E. coli decreases by 6th power of magnitude (1×10-6) at pulse-count 40. Acetobacter demonstrated similar SR at around 80 pulse-counts. At 100 pulses, the SR for Enterobaracter aerogenes is 0.000001. At 100 pulse-count, SR for Staphylococcus aurous is 1×10-4. Listeria monocytogene SR remained unchanged till 40 pulse counts but further increase in pulse counts (at 100 pulses) led to decrease in SR (0.001).
The SR and peak voltage are presented in Figure 3(B) with the pulse number constant at 50 for the milk sample. The SR for the E. coli and rest of the microbial species showed a significant difference. In case of E. coli, the ratio is 0.0001 at 40kV, which drops remarkably to 1×10-6 at 80kV, and then remains unchanged, whereas for Acetobacter at 80kV the ratio is 0.0001 which reduces further at 120kV to 1×10-6. The biggest reduction in the SR for Staphylococcus aurous is 1×10-4, which remained constant after 80kV. Enterobaracter aerogenes has a SR of 1×10-6 which is achieved at 160kV. The data revealed that the endurance of Listeria monocytogene is unchanged till 40kV but at higher voltage (approx. 160kV), the SR drops to 0.001.
Experimental findings for water sample
Figures 3(C) and 3(D) show experimental findings for microbial inactivation of E. coli, Enterobaracter aerogenes, Listeria monocytogene, Acetobacter and Staphylococcus aurous, in the water sample. Figure 3(C) depicts the endurance of water microorganisms versus the count of pulses at an unchanged maximum voltage of 100 kV. The SR for E. coli declines rapidly, reaching 1×10-6 at 40 pulses and remaining constant up to 100 pulses. Acetobater, Enterobaracter aerogenes, Listeria monocytogenes and Staphylococcus aurous had similar SRs.
The overall findings suggest that SR of microorganisms are inversely proportional to the intensity and pulse counts.
Figure 3(D) represents the effects of changing peak voltage up to 160kV on SR in the water sample. E. coli and Acetobacter exhibit lowest SR in comparison to other species included in the experiment. Enterobaracter aerogenes, Listeria monocytogenes and Staphylococcus aurous showed lower survival rates.
 |
Figure 3: Variation of SR with change in pulse-count and peak-voltage. |
The empirical results revealed by the present experiment are corroborated by the findings of past studies, however the incorporation of plate techniques with HV-PEF is novel approach13,14. It is evident that the SR of microbes is inversely proportional to the peak voltage value and the number of pulses imposed on food sample. Findings of the present experiment revealed a dramatic change in SR (by approximately four orders of the magnitude). This experiment revealed the most significant effect of HV-PEF was on E. coli among all microbial species under investigation. E. coli was found to have least resistance towards HV-PEF, as this species of microorganism demonstrated lowest SR at lowest peak voltage and lowest number of applied pulses for both water and milk. An important finding of this experiment is that destruction of microorganisms is by the rupturing of cell wall by the effect of HV-PEF, instead of ohmic heating27. Same impact has been reported on other bacterial species by other researchers28. The inactivation of microbe by HV-PEF depends on the morphology, shape, and dimension of that microorganism. The findings are indicative of HV-PEF technology as a substitution for traditional ohmic heating as well as other preservative techniques that involve addition of chemicals to beverages, milk, and other food items29. Two most common such chemicals are sulfur dioxide and hydrogen peroxide, which acts as an antioxidant. Besides tempering with the nutritive properties of the food, these also have detrimental physiological impact on the human body29,30. It is worth mentioning, in recent years the researchers have shown the recovery of Staphylococcus aureus and other bacteria after exposure to PEF in milk and water sample31. Nevertheless, the advantages of HV-PEF over other conventional methods cannot be ignored and needs more comprehensive comparative analysis with other methods.
Conclusions
The HV-PEF does not involve change in temperature or any heating mechanism, therefore the taste of the food sample remains unaltered. Further, the researchers have argued that the components and enzymes contained in food items do not get hampered by PEF and is more efficient in preserving the majority of nutritive compounds of milk32. The physical characteristics such as color and colloidal stability also remain unchanged33. Limited number of studies are done on safety aspect of PEF processing and have reported consumption of PEF treated liquid food item is safe for human being34. These findings imply that this experimental method have industrial application for food processing and could substitute traditional pasteurization techniques. Research should be carried out to make use of above stated properties of HV-PEF in a scaled-up model for industrial use35. The cost aspect of this technology should be explored while designing a full-scale industrial model.
The research has shown that the population residing in rural and low socioeconomic regions is already underprivileged and vulnerable especially in terms of health36. Therefore, the research on portable setup (for domestic use) powered by solar energy could prove extremely efficient and technologically appropriate for rural and remote areas of underdeveloped nations. It is intriguing to point out, an Artificial Intelligence integrated system could be conceptualized to develop more advanced models, where microbial counting by plate technique and automated deactivation are amalgamated with hybrid powering options (electric and solar). At present there is lack of literature in this regard, this needs further analysis to come up with such models and prototypes.
Limitation
In this experimental study we provide evidence regarding the association of the impact of HV-PEF and morphology of bacteria. In the study we analyzed only five species, however, there are many other types of bacteria found in water and milk. The present study does not report the effect of HVPEF on bacterial spores and other viral pathogenic species. This needs to be examined in future research.
Acknowledgement
We would like to thank the Department of Biotechnology, Aligarh Muslim University, Aligarh for allowing us to conduct the experiment in laboratory and provide us required equipment.
Conflict of Interest
There is no conflict of interest.
Funding Sources
There is no funding sources.
References
- WHO International. Food safety factsheet. 2020. Retrieved on April 1, 2022. Available from: https://www.who.int/news-room/fact-sheets/detail/food-safety
- Nugrahaeni A, Pertiwi J. Studi Case Report: Kejadian Luar Biasa Keracunan Makanan di Desa Parikesit Kecamatan Kejajar Kabupaten Wonosobo Case Report Study: Food Poisoning Outbreak in Parikesit Village, Kejajar District, Wonosobo Regency. Jurnal Ilmu Kesehatan Masyarakat Berkala (JIKeMB). 2020;2:1.
CrossRef - Abbas SA, Ali IA, Shafeeq WS. Epidemiological Study of Food Poisoning Cases from 2013 to 2021, in Diyala Province, Iraq. Diyala Journal for Pure Science. 2022 Jul 20;18(3).
CrossRef - Boatemaa, S., Barney, M., Drimie, S., Harper, J., Korsten, L., & Pereira, L. (2019). Awakening from the listeriosis crisis: Food safety challenges, practices and governance in the food retail sector in South Africa. Food Control, 104, 333-342.
CrossRef - Adeoye AO, Bankole EM, Onifade AO, Arotiowa CA, Adegbola GM. Microbial Examination of Ready-to-eat Fruit Samples (Pineapple, Pawpaw and Watermelon) from Street Vendors in Ogbomoso Market, Oyo State, Nigeria. 2021;12(12):138-147.
- Naghili H., Tajik H., Mardani K., Rouhani S.M., Ehsani A., Zare P. Validation of drop plate technique for bacterial enumeration by parametric and nonparametric tests. Res. Forum. 2013;4(3):179-83.
- Davey HM. Life, death, and in-between: meanings and methods in microbiology. Environ. Microbiol. 2011;77(16):5571-6. DOI: 10.1128/AEM.00744-11
CrossRef - Suebsiri N., Kokilakanistha P., Laojaruwat T., Tumpanuvatr T., Jittanit W. The application of ohmic heating in lactose-free milk pasteurization in comparison with conventional heating, the metal contamination and the ice cream products. Food Eng. 2019;262:39-48. DOI: 10.1016/j.jfoodeng.2019.05.017
CrossRef - Sakr M., Liu S. A comprehensive review on applications of ohmic heating (OH). Sustain. Energy Rev. 2014;39:262-9. DOI: 10.1016/j.rser.2014.07.061
CrossRef - Scudino H., Silva E.K., Gomes A., Guimaraes J.T., Cunha R.L., Sant’Ana A.S., Meireles M.A., Cruz A.G. Ultrasound stabilization of raw milk: Microbial and enzymatic inactivation, physicochemical properties and kinetic stability. Sonochem. 2020;67:105185.. DOI: 10.1016/j.ultsonch.2020.105185
CrossRef - Monteiro S.H., Silva E.K., Alvarenga V.O., Moraes J., Freitas M.Q., Silva M.C., Raices R.S., Sant’Ana A.S., Meireles M.A., Cruz A.G. Effects of ultrasound energy density on the non-thermal pasteurization of chocolate milk beverage. Sonochem. 2018;42:1-10. DOI: 10.1016/j.ultsonch.2017.11.015
CrossRef - Gavahian M., Tiwari B.K., Chu Y.H., Ting Y., Farahnaky A. Food texture as affected by ohmic heating: Mechanisms involved, recent findings, benefits, and limitations. Trends Food Sci. Technol. 2019;86: 328-39. DOI: 1016/j.tifs.2019.02.022
CrossRef - Min S., Evrendilek G.A., Zhang H.Q. Pulsed electric fields: processing system, microbial and enzyme inhibition, and shelf life extension of foods. IEEE Transactions on Plasma Science. 2007;35(1):59-73. DOI: 1109/TPS.2006.889290
CrossRef - Narsetti R., Curry R.D., McDonald K.F., Clevenger T.E., Nichols L.M. Microbial inactivation in water using pulsed electric fields and magnetic pulse compressor technology. IEEE Transactions on Plasma Science. 2006;34(4):1386-93. DOI: 1109/TPS.2006.889290
CrossRef - Huang K., Jiang T., Wang W., Gai L., Wang J. A comparison of pulsed electric field resistance for three microorganisms with different biological factors in grape juice via numerical simulation. Food Bioproc. Tech. 2014;7(7): 1981-95. DOI: 1007/s11947-014-1272-3
CrossRef - Qin S., Timoshkin I., Wilson M., Maclean M., MacGregor S., Given M., Anderson J., Wang T. Pulsed electric field assisted treatment of microorganisms for lysis. In 2013 19th IEEE Pulsed Power Conference (PPC). 2013. pp. 1-5. DOI: 1109/PPC.2013.6627487
CrossRef - Singh G.L., Dudheria G., Kumar H.A., Kruthika S., Palati M., Banerjee S. Application of pulsed electric field for food preservation. In 2016 International Conference on Circuits, Controls, Communications and Computing (I4C). 2016. pp. 1-4. DOI: 10.1109/CIMCA.2016.8053256
CrossRef - Moonesan M.S., Jayaram S.H. Effect of pulsewidth on medium temperature rise and microbial inactivation under pulsed electric field food treatment. IEEE Transactions on Industry Applications. 2013;49(4):1767-72. DOI: 1109/TIA.2013.2256411
CrossRef - Yogesh K. Pulsed electric field processing of egg products: a review. Food Sci. Technol. 2016;53(2):934-45. DOI: 10.1007/s13197-015-2061-3
CrossRef - Coban M, Fidan M. High Voltage Pulse Generator for Pulsed Electric Field Treatments. In 2019 IEEE 3rd International Symposium on Multidisciplinary Studies and Innovative Technologies (ISMSIT). pp. 1-4.
CrossRef - Käferböck A., Smetana S., de Vos R., Schwarz C., Toepfl S., Parniakov O. Sustainable extraction of valuable components from Spirulina assisted by pulsed electric fields technology. Res. 2020;48: 101914. DOI: 10.1016/j.algal.2020.101914
CrossRef - Ansari M.F., Afzal A. The microbial inactivation by high voltage pulsed electric field. In 2021 IEEE 6th International Conference on Computing, Communication and Automation, ICCCA: 2021. pp 642-645. DOI: 1109/ICCCA52192.2021.9666427
CrossRef - Ansari M.F., Afzal F., Afzal A. Microbial handling of water using high voltage pulsed electric field. Materials Today: Proceedings. 2022;56:1558-61. DOI: 1016/j.matpr.2022.01.295
CrossRef - Gupta S.B., Masterson F., Magee T.R. Inactivation of E. coli K12 in apple juice by high voltage pulsed electric field. Food Res. Technol. 2003;217:434-7. DOI 10.1007/s00217-003-0756-6
CrossRef - Gupta S.B., Masterson F., Magee T.R. Inactivation of E. coli in cranberry juice by a high voltage pulsed electric field. Eng. Life Sci. 2005;5(2):148-51. DOI: 10.1002/elsc.200420064
CrossRef - Pillet F., Formosa-Dague C., Baaziz H., Dague E., Rols M.P. Cell wall as a target for bacteria inactivation by pulsed electric fields. Scientific reports. 2016;6(1):19778.
CrossRef - Perminaitė E., Zinkevičienė A., Malyško-Ptašinskė V., Radzevičiūtė E., Novickij J., Girkontaitė I., Novickij V. Killing bacteria using acetic acid and nanosecond pulsed electric fields—an in vivo superficial infection model study and immune response. Appl Sci. 2023;13(2):836. DOI: 10.3390/app13020836
CrossRef - Huang H., Gao T., Qian X., Wu W., Fan X., Shi L., Xiong G., Ding A., Li X., Qiao Y., Liao L. In vitro antibacterial mechanism of high-voltage electrostatic field against Acinetobacter johnsonii. Foods. 2022;11(7):955. DOI: 10.3390/foods11070955
CrossRef - Silva F.V., Van Wyk S. Emerging non-thermal technologies as alternative to SO2 for the production of wine. Foods. 2021;10(9):2175. DOI: 10.3390/foods10092175
CrossRef - Raturi N., Aman J., Sharma C. Study of adulteration in milk and milk products and their adverse health effects. 2022;10(1):37-50.
- Emanuel E., Dubrovin I., Pogreb R., Pinhasi G.A., Cahan R. Resuscitation of pulsed electric field-treated Staphylococcus aureus and Pseudomonas putida in a rich nutrient medium. Foods. 2021;10(3):660. DOI: 10.3390/foods10030660
CrossRef - Sharma P., Oey I., Everett D.W. Effect of pulsed electric field processing on the functional properties of bovine milk. Trends Food Sci. Technol. 2014;35(2):87-101. DOI: 10.1016/j.tifs.2013.11.004
CrossRef - Manzoor M.F., Zeng X.A., Ahmad N., Ahmed Z., Rehman A., Aadil R.M., Roobab U., Siddique R., Rahaman A. Effect of pulsed electric field and thermal treatments on the bioactive compounds, enzymes, microbial, and physical stability of almond milk during storage. Food Process Preserv. 2020;44(7):e14541. DOI: 10.1111/jfpp.14541
CrossRef - Van Wyk S, Silva FV, Farid MM. Pulsed electric field treatment of red wine: Inactivation of Brettanomyces and potential hazard caused by metal ion dissolution. Food Sci. Emerg. Technol. 2019;52:57-65. DOI: 10.1016/j.ifset.2018.11.001
CrossRef - Taha A., Casanova F., Šimonis P., Stankevič V., Gomaa M.A., Stirkė A. Pulsed Electric Field: Fundamentals and Effects on the Structural and Techno-Functional Properties of Dairy and Plant Proteins. Foods. 2022;11(11):1556. DOI: 10.3390/foods11111556
CrossRef - Afzal F., Raychaudhuri P.S., Afzal M.A., Ahmad A.A. Challenges Faced by BPL Population in Availing Public Healthcare–Analysing Government Initiatives, Technology and Cultural Barriers in Aligarh District, UP. South Asian Journal of Social Science and Humanities. 2021;2(5):1-9. DOI: 48165/sajssh.2021.2501%20
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





