Manuscript accepted on : 26-03-2023
Published online on: 30-03-2023
Plagiarism Check: Yes
Reviewed by: Dr. Oguzhan Özdemir
Second Review by: Dr. Nkoyo Nubila
Final Approval by: Dr. Haseeb Ahmad Khan
Advances in the CD40-mediated Manipulation Strategies
Department of Zoology, Burdwan Raj College, Burdwan, India
Corresponding Author E-mail: syam.nccs@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/3067
ABSTRACT: Immune homeostasis is crucial in a normal physiological, diseased, or pathogenic state and is mediated by numerous biological molecules. Certain pairs of costimulatory molecules, such as CD40-CD154 play major roles in many different situations. The functions of CD40-CD154 are pivotal for the maintenance of the immune system, cancer prevention, promotion of autoimmune disorders, and fighting against many pathogens. Since the discovery of CD40-CD154, numerous approaches have been taken to dissect this pathway favoring the interest of the host. Ranging from generating small peptides to mutated CD40L to agonistic or antagonistic antibodies have been tested in different models with varying levels of success. This review focuses on the various strategies that have been employed to manipulate CD40-CD154 dyad. Comprehensive knowledge of the understanding of different manipulation strategies of the CD40-CD154 pathway could be important for therapeutic purposes.
KEYWORDS: Agonist; Antagonist; Costimulatory molecules; CD40; CD154
Download this article as:| Copy the following to cite this article: Bandyopadhyay S. Advances in the CD40-mediated Manipulation Strategies. Biosci Biotech Res Asia 2023;20(1). |
| Copy the following to cite this URL: Bandyopadhyay S. Advances in the CD40-mediated Manipulation Strategies. Biosci Biotech Res Asia 2023;20(1). Available from: https://bit.ly/3lTnN8Q |
Introduction
CD40 belongs to the TNF receptor superfamily member, which qualifies as a transmembrane receptor. CD40 is observed to be expressed on the surface of many cell types such as B-cells, dendritic cells, macrophages, monocytes, etc. that engages its cognate ligand, called CD154 [also called CD40 ligand or CD40L] expressed mainly on the activated T-cells. During normal immune functions, depending on specific cell types the engagement of membrane-bound CD40 with its ligand evokes several important functions, including but not limited to differentiation of B lymphocytes, isotype switching of immunoglobulins, and development of the germinal center 1. Moreover, dendritic cells are licensed to stimulate T-cells that build up anti-tumor activity in a CD40-dependent manner, for the formation of a germinal center, B-cells expressed CD40 are essential 2,3. Contrary to this, the ligation of CD40 with CD40L serves as the driver of many chronic inflammatory diseases including atherosclerosis, lupus nephritis, systemic lupus erythematosus, colitis, autoimmunity, etc. 4–6. The information presented above attests to the fact that CD40 shows pleiotropism in its functions and can be considered as a double-edged sword in which, on one hand, the ligation of CD40 with CD154 is crucial for the execution of several important immunological functions that maintain immune homeostasis such as anti-leishmanial functions 7–9, on the other hand, successful CD40-CD154 engagement drives several pathological conditions in humans promoting diseases. This leads us to think about the feasibility of CD40 signaling being manipulated to evoke agonistic or antagonistic effector functions.
Since the discovery of CD40, numerous publications exist that describe how its agonists or antagonists can be designed 3,10–12. Agonists are molecules that evoke the same response as the original ligand does, whereas, antagonists are those that do bind with the receptor as the original ligand but there is no effect due to its binding. Antagonists rather function by preventing the binding of the original ligand and receptor. Agonists or antagonists modulate the functions of the CD40/CD40154 axis at the level of receptor-ligand interaction. The principles of how this binding of different molecules with the same receptor evokes differential functions were not clear. We propose this could be a function of the ligand binding to CD40. As a result, attempts to dissect the CD40/CD154 pathway using various ligands have been proven to be useful in many pathological cases including cancer, and leishmaniasis 3,13,14. How the property of ligands impacts the binding with CD40 leading to functional differentiation is intriguing. Here, we review the available literature describing the usages of different natural or modified agonistic or antagonistic ligands and the underlying principle that makes them so, which can be utilized to devise strategies to combat many diseases arising from a deficiency at the interface of the CD40 receptor-ligand dyad.
Strategies of Manipulation of CD40 on immune homeostasis and Disease Systems
CD40 influences a range of functions being part of the success of the immune system. Wide involvement of the CD40-CD154 dyad in various immune mechanisms and pathological conditions attracted scientists to manipulate this pathway. We categorize various strategies of manipulation as given and describe them in a comprehensive manner. We begin with a brief description of the interaction of CD40 with its natural ligand CD154.
Interaction of CD40/CD154
CD154 is the classical ligand for CD40 [Figure 1] and consists of 261 amino acids of which the first 22 amino acid residues make up the cytoplasmic domain and the next 24 amino acid residues make up the transmembrane domain embedded in the membrane. This is followed by an extracellular domain of CD40, which contains three Cysteine-rich domains. [CRD] stabilized by disulfide bonds 15. A few amino acid residues are important during contact with CD40. The crystal structure of CD40-CD154 suggests that two key residues at positions 143 and 145 [Tyrosine at 145 i.e., Y145, and Lysine at 143 i.e., K143] are crucial for binding with CD40. In addition, residues such as Serine at the 128th position [S128], and Glutamic acid at the 129th position [E129] might participate in binding with its receptor. A mutational study indicates that residues such as Serine at position l31, Asparagine at position l80, Phenylalanine at position 201, Glutamic acid at position 202, and Asparagine at position 240 are not crucial for binding, though their position in the binding site is evident.
CD40, on the other hand, consists of 277 amino acid residues, of which the first 21-193 residues compose the extracellular domain that has altogether three CRD stabilizing the structure, followed by 215 residues that make up the transmembrane domain and the cytoplasmic domain is comprising 216-277 residues. The extracellular domain of CD40 binds with the crevice composed of two CD154s where the hydrophilic and charge interactions are major forces. Whereas, CD154 supplies positively charged residues [Lys143, His249, Arg203, and Arg207], CD40 contributes negatively charged residues [Glu74, Asp84, and Glu117] 15. Besides, Tyrosine at position 82 and Asparagine at position 86 are also important for binding with their ligand 16. Altogether, we understand that certain amino acid residues play extremely important roles during the interaction phase impacting the final outcome of the CD40-mediated pathway.
Interaction of CD40 with Hsp70 and its analog
It was previously shown using a coimmunoprecipitation study that THP-1-expressed CD40 is able to physically associate with mycobacterial heat shock protein (Mtb HSP70) 17. The following year, another report came up that demonstrated that CD40 also serves as a cell-surface expressed receptor that responds to previously unreported molecules, called human Hsp70 in presence of ADP [Figure 1] via the ATPase domain which is present at the C-terminal domain of Hsp70 18,19 and the engagement leads to phosphorylation of p38.
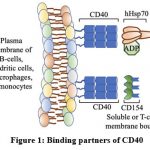 |
Figure 1: Binding partners of CD40. |
Mutational manipulation of CD154
Because amino acid residues are crucial for the effective interaction between CD40 and CD154, attempts have been made to perform point mutations on the ligand to alter the final outcome. Five residues were targeted for site-directed mutagenesis: S128, E129, K143, G144, and Y145 8. Substitution of S128 to V [S128V] shows a lower value of KD indicating reduced affinity of the interaction with CD40 as compared to the wild type. S128V is anti-inflammatory as evidenced by the induction of more IL-10 than IL-12 in assays. E129V serves as a pro-inflammatory ligand variant, though this, too, has reduced affinity for the receptor. Other point mutations such as K143A, G144V, and Y145F alter the affinity of the interaction, but they don’t significantly change the biological responses. Bioinformatic analysis suggested that these variants can bind at different sites in the binding region of CD40 influencing the conformation to bring about the differential functions. This study for the first time demonstrated residue-specific encryption of messages on the ligand that can dictate the duality of its functions.
Interaction analysis by Phage Display Derived Peptides
In the past, phage display technology was exploited to derive the sequences of dodecameric peptides, which were screened against the CD40-ECD for ensuring their binding with CD40. Finally, two peptides were derived with contrasting functions. While one peptide showed anti-inflammatory properties inducing more IL-10 than IL-12, another peptide induced more IL-12 than IL-10 7. Thus, these two peptides were contrasting in functions. Even though the anti-inflammatory peptide had reduced affinity compared to the wild-type CD154 [wtCD154], the affinity of the peptide with the pro-inflammatory property was comparable to that of the wtCD154. These data suggest that affinity does not have a direct correlation with agonistic or antagonistic activity. Interestingly, the pro-inflammatory peptide could possibly either cluster for interaction or it may interact with CD40 which is oligomeric. Again, the bioinformatic study suggested that these peptides bind to at least two different places on CD40 and that could probably explain its functional duality.
Interaction analysis by XIgM-related peptides
Patients suffering from XIgM have elevated levels of IgM and concomitant abnormally low levels of IgA, IgG, and IgE immunoglobulins and recurrent opportunistic infections. Many mutations have been mapped so far in XHIM patients to date 20. Many of these mutations impart structural instability to CD154, and a few have been demonstrated to generate ligands that fold and express properly but through minute conformational changes, they evoke differential CD40-mediated functions. 115-155 stretch of amino acid is considered to be the ‘hotspot’ for mutation on CD154 for XIgM patients. 41-mer peptides derived from this hotspot of CD154 on XIgM patients were analyzed using combinations of different biochemical and immunological techniques and demonstrated that they differentially altered CD40 signaling and various related biological responses including secretion of various pro- and anti-inflammatory cytokines, T-cell mediated anti-leishmanial responses.
Monoclonal Antibody
Monoclonal antibodies are very useful in the treatment of several diseases. Significant progress has been observed in recent years in the clinical use of monoclonal antibodies. Targeting CD40 using mAb is an attractive choice for many to treat CD40-mediated diseases. For example, a significantly higher expression of CD40 is seen in carcinoma and B-cell malignancies 21. A mAb called, SGN-14 is used to treat the disease.
Isotype switching
Certain antagonistic monoclonal anti-CD40 antibodies are used to treat cancer, such as 341G2 (bleselumab) 10. This antibody competes with CD154 and the binding of CD154 is inhibited when this antibody is present in a dose-related but isotype-independent way. This is not surprising, since the steric blockade is Fc-independent. Interestingly, switching isotypes have a distinct effect on the activity of the antibody in the CD40-mediated pathway. While switching to IgG1 and 4 from IgG2 retains its antagonistic activity. However, switching to the IgG2 isotype makes it super-agonistic, and the activity of the NF-kb pathway is comparable to trimeric CD154. The effect is mediated through the clustering of CD40 on the membrane promoting CD40 downstream signaling. Moreover, differential disulfide bonding contributes to the agonistic activity of the antibody, since the switching of a hinge region lacking specific cysteines causes the antibody to lose the agonistic activity. This is primarily because an inverse correlation exists between hinge flexibility and agonistic ability 22. This study also clearly emphasizes that the concept of switching isotype applies to at least one more CD40 antibody. Besides, the concept of isotype switching has also been used successfully in other systems such as 41BB, as well as members of the B7- CD28 superfamily 23. It is not yet clear how exactly this flexibility imparts agonistic activity for the CD40-mediated pathway, which is unknown.
Usage of Agonistic antibodies
Monoclonal antibodies such as ABBV-323 function as antagonistic antibodies through their capability to arrest the non-signaling dimeric form of CD40 which sterically prevents CD40L recognition. Certain substitution in amino acid sequences of Fab of ABBV-323 [from non-polar to basic polar] switches agonistic [called FAB516, FAB518] to antagonistic ligands [ABBV-323] through the formation of antiparallel dimeric CD40 that fails to bind its classical ligand, CD154 12. [Table 1].
Table 1: Summary of different manipulation strategies and their consequent impacts.
|
Manipulation Strategy |
Strategy Summary |
Impacts |
Model System/technique exploited |
|
Mutational manipulation of CD154 |
Point mutations on CD154 [S128V, E129V, K143A, G144V, and Y145F] |
S128V: reduced affinity with CD40 [anti-inflammatory]; E129V: reduced affinity with CD40 [pro–inflammatory] K143A, G144V, and Y145F: altered affinity without significant change in biological responses |
[A] Surface Plasmon Resonance for measuring In vitro interaction analysis; [B] THP1 cell lines for studying biological responses. |
|
Phage Display derived peptides |
Generating peptides capable to bind CD40 using Phage Display Library: Peptide 7: AETVESCLAKSH Peptide 19: HGWYFYTKPLHL |
Peptide 7: increased parasite load in macrophages; Peptide 19: reduced parasite load in macrophages
|
[A] Surface Plasmon Resonance and Atomic Force Microscope for measuring In vitro interaction analysis; [B] THP1-derived macrophages |
|
Agonistic antibodies |
Ravagalimab: mutating position (L)R32 to proline (FAB516) or leucine (FAB518) of anti-CD40 antagonist [ABBV-323] |
Reported assay demonstrated diminished luminescence in case of ABBV-323 |
HEK-293 cells |
|
XIgM-related peptides |
Peptide 7: DEDPQIAAHVVSEANSNAASVL Peptide 8: DEDPQIAAHVVSEANSNAASVL |
higher p38MAPK, Lyn, PKCbII, and PKCa phosphorylation |
Peritoneal macrophages of BALB/c mice |
|
Antagonistic antibodies |
Ch5D12 CHIR-12.12 |
Ch5D12: remission induction in Crohn’s disease; CHIR-12.12: diminishes CD40L-mediated growth and survival of CD40-expressing multiple myeloma cells |
Ch5D12: patients CHIR-12.12: patients |
|
Monoclonal antibodies |
SGN-14: CD40 receptor specific monoclonal antibody |
Together with IL-4, stimulates proliferation of B-cell |
peripheral blood B cells |
|
Isotype switching |
mAb 341G2 [bleselumab]: Isotype Switching to hIgG2 makes ‘Antagonist’ to ‘Super-agonist’ |
proliferation and homotypic cell-cell adhesion in B cells |
hCD40Tg splenic B cells and purified human B cells |
Usage of Antagonistic antibodies
Antibodies that are antagonists are used to treat various autoimmune and inflammatory disorders such as experimental colitis, and Crohn’s disease. Antagonizing the effect of ligation of CD40 and CD154 has a profound effect on various autoimmune and inflammatory disorders. Ch5D12 is an example of an antagonist that manipulates the CD40-CD154 dyad 24. CHIR-12.12 is another example of an antagonistic antibody that binds with the CD138+ multiple myeloma cells. CHIR-12.12 abolishes CD154-mediated viability of CD40+ multiple myeloma cells regardless of the presence or absence of bone marrow stromal cells (BMSC) through inhibition of PI3-K/AKT, NF-κB, and activation of kinases such as ERK induced by CD154 25.
Conclusion
Manipulating the CD40-CD154 pathway is not easy for the therapeutic purpose, since, this may lead to side effects. For instance, a higher-than-expected number of thromboembolic events is associated with the treatment of SLE patients using anti-CD154. Although manipulation of the CD40-CD154 pathway showed variable levels of outcome and success, this pathway can’t be ignored from the therapeutic perspective. Many diseases are the result of inappropriate activation of CD40 pathways, whereas, others may be attributed due to a deficiency in this pathway. Especially, certain pathogenic disorders impact the CD40 axis. Therefore, manipulating this pathway is crucial and a correct strategy must be devised depending on the situation to minimize side effects while simultaneously maximizing the desired effects. Modulation of the pathway can be brought about by generating CD40-interacting small peptides derived from Phage-Display screening. Although, in vivo how these peptides will behave is unknown yet. Further modifications may be required to make them physiologically more stable. Though it can’t be guaranteed, this problem could be presumably overcome by using natural ligands of CD40 modified by site-directed mutagenesis. Agonistic or antagonistic mAbs are useful as they are relatively stable in the physiological setup. A pictorial summary has been presented for ease of understanding of the current status of various strategies employed to dissect the CD40 pathway [Figure 2]. Our review establishes important aspects of the present scenario of the strategies for manipulating the CD40 pathway that is crucial considering the pleiotropic role of CD40 in immune homeostasis.
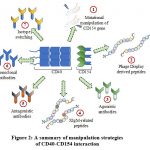 |
Figure 2: A summary of manipulation strategies |
Acknowledgment
I acknowledge the help of my institution for providing me with the necessary non-financial support and affiliation.
Conflict of Interest
There are no conflict of interest.
References
- Yazdany, J., & Davis, J. The role of CD40 ligand in systemic lupus erythematosus. Lupus, 2004, 13(5), 377–380.
CrossRef - Luo, W., Weisel, F., & Shlomchik, M. J. B Cell Receptor and CD40 Signaling Are Rewired for Synergistic Induction of the c-Myc Transcription Factor in Germinal Center B Cells. 2018, Immunity, 48(2), 313–326.e5.
CrossRef - Vonderheide, R. H. CD40 Agonist Antibodies in Cancer Immunotherapy. Annual review of medicine, 2020, (71) 47–58.
CrossRef - Seijkens, T. T. P., van Tiel, C. M., Kusters, P. J. H., Atzler, D., Soehnlein, O., Zarzycka, B., Aarts, S. A. B. M., Lameijer, M., Gijbels, M. J., Beckers, L., den Toom, M., Slütter, B., Kuiper, J., Duchene, J., Aslani, M., Megens, R. T. A., van ‘t Veer, C., Kooij, G., Schrijver, R., Hoeksema, M. A., … Lutgens, E. Targeting CD40-Induced TRAF6 Signaling in Macrophages Reduces Atherosclerosis. Journal of the American College of Cardiology, 2018, 71(5), 527–542.
CrossRef - Boumpas, D. T., Furie, R., Manzi, S., Illei, G. G., Wallace, D. J., Balow, J. E., Vaishnaw, A., & BG9588 Lupus Nephritis Trial Group. A short course of BG9588 (anti-CD40 ligand antibody) improves serologic activity and decreases hematuria in patients with proliferative lupus glomerulonephritis. Arthritis and rheumatism, 2003, 48(3), 719–727.
CrossRef - Sidiropoulos, P. I., & Boumpas, D. T. Lessons learned from anti-CD40L treatment in systemic lupus erythematosus patients. Lupus, 2004, 13(5), 391–397.
CrossRef - Khan, S., Alonso-Sarduy, L., Roduit, C., Bandyopadhyay, S., Singh, S., Saha, S., Tacchini-Cottier, F., Roy, S., Dietler, G., Kasas, S., Das, P., Krishnasastry, M. V., & Saha, B. Differential peptide binding to CD40 evokes counteractive responses. Human immunology, 2012, 73(5), 465–469.
CrossRef - Bandyopadhyay, S., Chandel, H. S., Singh, S., Roy, S., Krishnasastry, M. V., & Saha, B. Counteractive functions are encrypted in the residues of CD154. Human immunology, 2015, 76(9), 673–680.
CrossRef - Sarode, A. Y., Jha, M. K., Zutshi, S., Ghosh, S. K., Mahor, H., Sarma, U., & Saha, B. Residue-Specific Message Encoding in CD40-Ligand. iScience, 2020, 23(9), 101441. Advance online publication.
CrossRef - Yu, X., Chan, H. T. C., Fisher, H., Penfold, C. A., Kim, J., Inzhelevskaya, T., Mockridge, C. I., French, R. R., Duriez, P. J., Douglas, L. R., English, V., Verbeek, J. S., White, A. L., Tews, I., Glennie, M. J., & Cragg, M. S. Isotype Switching Converts Anti-CD40 Antagonism to Agonism to Elicit Potent Antitumor Activity. Cancer cell, 2020, 37(6), 850–866.e7.
CrossRef - Karnell, J. L., Rieder, S. A., Ettinger, R., & Kolbeck, R. Targeting the CD40-CD40L pathway in autoimmune diseases: Humoral immunity and beyond. Advanced drug delivery reviews, 2019, 141, 92–103.
CrossRef - Argiriadi, M. A., Benatuil, L., Dubrovska, I., Egan, D. A., Gao, L., Greischar, A., Hardman, J., Harlan, J., Iyer, R. B., Judge, R. A., Lake, M., Perron, D. C., Sadhukhan, R., Sielaff, B., Sousa, S., Wang, R., & McRae, B. L. CD40/anti-CD40 antibody complexes which illustrate agonist and antagonist structural switches. BMC molecular and cell biology, 2019, 20(1), 29.
CrossRef - Rub, A., Dey, R., Jadhav, M., Kamat, R., Chakkaramakkil, S., Majumdar, S., Mukhopadhyaya, R., & Saha, B. Cholesterol depletion associated with Leishmania major infection alters macrophage CD40 signalosome composition and effector function. Nature immunology, 2009, 10(3), 273–280.
CrossRef - Mathur, R. K., Awasthi, A., Wadhone, P., Ramanamurthy, B., & Saha, B. Reciprocal CD40 signals through p38MAPK and ERK-1/2 induce counteracting immune responses. Nature medicine, 2004, 10(5), 540–544.
CrossRef - An, H. J., Kim, Y. J., Song, D. H., Park, B. S., Kim, H. M., Lee, J. D., Paik, S. G., Lee, J. O., & Lee, H. Crystallographic and mutational analysis of the CD40-CD154 complex and its implications for receptor activation. The Journal of biological chemistry, 2011, 286(13), 11226–11235. https://doi.org/10.1074/jbc.M110.208215
CrossRef - Bajorath, J., Chalupny, N. J., Marken, J. S., Siadak, A. W., Skonier, J., Gordon, M., Hollenbaugh, D., Noelle, R. J., Ochs, H. D., & Aruffo, A. Identification of residues on CD40 and its ligand which are critical for the receptor-ligand interaction. Biochemistry, 1995, 34(6), 1833–1844.
CrossRef - Wang, Y., Kelly, C. G., Karttunen, J. T., Whittall, T., Lehner, P. J., Duncan, L., MacAry, P., Younson, J. S., Singh, M., Oehlmann, W., Cheng, G., Bergmeier, L., & Lehner, T. CD40 is a cellular receptor mediating mycobacterial heat shock protein 70 stimulation of CC-chemokines. Immunity, 2001, 15(6), 971–983.
CrossRef - Becker, T., Hartl, F. U., & Wieland, F. CD40, an extracellular receptor for binding and uptake of Hsp70-peptide complexes. The Journal of cell biology, 2002, 158(7), 1277–1285.
CrossRef - Facciponte, J. G., Wang, X. Y., MacDonald, I. J., Park, J. E., Arnouk, H., Grimm, M. J., Li, Y., Kim, H., Manjili, M. H., Easton, D. P., & Subjeck, J. R. Heat shock proteins HSP70 and GP96: structural insights. Cancer immunology, immunotherapy : CII, 2006, 55(3), 339–346.
CrossRef - Bajorath, J., Seyama, K., Nonoyama, S., Ochs, H. D., & Aruffo, A. Classification of mutations in the human CD40 ligand, gp39, that are associated with X-linked hyper IgM syndrome. Protein science : a publication of the Protein Society, 1996, 5(3), 531–534.
CrossRef - Francisco, J. A., Donaldson, K. L., Chace, D., Siegall, C. B., & Wahl, A. F. Agonistic properties and in vivo antitumor activity of the anti-CD40 antibody SGN-14. Cancer research, 2000, 60(12), 3225–3231.
- Liu, X., Zhao, Y., Shi, H., Zhang, Y., Yin, X., Liu, M., Zhang, H., He, Y., Lu, B., Jin, T., & Li, F. Human immunoglobulin G hinge regulates agonistic anti-CD40 immunostimulatory and antitumour activities through biophysical flexibility. Nature communications, 2019, 10(1), 4206.
CrossRef - White, A. L., Chan, H. T., French, R. R., Willoughby, J., Mockridge, C. I., Roghanian, A., Penfold, C. A., Booth, S. G., Dodhy, A., Polak, M. E., Potter, E. A., Ardern-Jones, M. R., Verbeek, J. S., Johnson, P. W., Al-Shamkhani, A., Cragg, M. S., Beers, S. A., & Glennie, M. J. Conformation of the human immunoglobulin G2 hinge imparts superagonistic properties to immunostimulatory anticancer antibodies. Cancer cell, 2015, 27(1), 138–148.
CrossRef - Kasran, A., Boon, L., Wortel, C. H., Hogezand, R. A., Schreiber, S., Goldin, E., Boer, M., Geboes, K., Rutgeerts, P., & Ceuppens, J. L. Safety and tolerability of antagonist anti-human CD40 Mab ch5D12 in patients with moderate to severe Crohn’s disease. Alimentary pharmacology & therapeutics, 2005, 22(2), 111–122.
CrossRef - Tai, Y. T., Li, X., Tong, X., Santos, D., Otsuki, T., Catley, L., Tournilhac, O., Podar, K., Hideshima, T., Schlossman, R., Richardson, P., Munshi, N. C., Luqman, M., & Anderson, K. C. Human anti-CD40 antagonist antibody triggers significant antitumor activity against human multiple myeloma. Cancer research, 2005, 65(13), 5898–5906.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





