Manuscript accepted on : 15-06-2022
Published online on: 27-06-2022
Plagiarism Check: Yes
Reviewed by: Dr. Sachchidanand Tewari
Second Review by: Dr. Thaigarajan Parumasivam
Final Approval by: Dr Eugene A. Silow
NDV as an Oncolytic Agent - Study in Cancer Cell Lines
Upasana Pathak1* , Nagesh Malik1
, Nagesh Malik1 and R. B. Pal2
and R. B. Pal2
1Vivekanand Education Society's College of Arts, Science and Commerce, Chembur, Mumbai-400071, Maharashtra, India.
2Sir H.N. Medical Research Society, Sir H.N. Reliance Foundation Hospital and Research Centre, Mumbai-400 004, Maharashtra, India.
Corresponding Author E-mail: upasana.pathak7@yahoo.com
DOI : http://dx.doi.org/10.13005/bbra/2996
ABSTRACT:
Newcastle disease virus (NDV) exhibits oncolysis in its natural form. This oncolytic virus (OV) has the potential to specifically infect, propagate, and lyse cancer cells while sparing the normal cells. This study was aimed to screen for oncolytic NDV strain isolated from poultry. A total of ten velogenic NDV strains were propagated in 10 day old embryonated SPF chicken eggs and allantoic fluid of these infected eggs was collected for further study. The virus enumeration was carried out by hemagglutination assay (HA) and end point dilution method. The cytopathic effect of ten NDV strains on cancer cell lines like MDA-MB-231, MCF-7, PC3, and A549 along with normal control cell line HEK293 was determined by MTT assay 72 hours post infection. These cell lines were infected with three doses (1, 0.1, and 0.01 MOI). DNA laddering effect of the screened NDV isolate was studied after infecting all cancer and normal cells at MOI 1. Morphological changes in MDA-MB-231 on infection with the screened NDV isolate were analyzed using H&E hematoxylin and eosin staining. The screened NDV isolate showed the maximum cytopathic effect i.e. 61.55% on MDA-MB-231 at MOI 1 but had no potent cytotoxic effect on HEK293. DNA laddering effect was observed which confirmed the mode of death to be apoptosis. All the observed morphological changes in MDA-MB-231 were typical of the cytopathogenic effects of NDV on cancer cell lines. In conclusion, the screened oncolytic NDV shows effective oncolysis against MDA-MB-231 cell line. However, further study is required to determine the exact mode of action involved.
KEYWORDS: Cytopathic effect; DNA laddering; Newcastle Disease Virus; Oncolytic; Velogenic
Download this article as:| Copy the following to cite this article: Pathak U, Malik N, Pal R. B. NDV as an Oncolytic Agent - Study in Cancer Cell Lines. Biosci Biotech Res Asia 2022;19(2). |
| Copy the following to cite this URL: Pathak U, Malik N, Pal R. B. NDV as an Oncolytic Agent - Study in Cancer Cell Lines. Biosci Biotech Res Asia 2022;19(2). Available from: https://bit.ly/3bxUTFy |
Introduction
Virotherapy with oncolytic viruses (OVs) has become the pivotal point of expeditiously growing cancer research. Conclusive data has been found that oncolytic virotherapy, by itself or in combination with chemotherapy, surgery or radiotherapy, considerably impacts deaths due to cancer and increases the remission rate 1, 2.
Oncolytic virotherapy involves the use of OVs which are natural or can also be genetically transformed viruses that are capable to selectively infect and kill cancer cells and effectively spare the healthy cells. This specificity depends on two key mechanisms for productive viral infection and causes host cell lysis. The first one being receptor-mediated uptake, where cancer cells overexpress virus entry specific receptors and second one involves virus adaptation to the host cancer signaling pathways for viral replication 3, 4. Some examples of OVs are Adenovirus, Reovirus, Herpes Simplex virus and NDV 5. Newcastle disease virus is one of the most economically important avian viruses which causes infection in domestic poultry, other bird species and thus affects the poultry industry worldwide 6. It is commonly known as the Ranikhet disease virus in India. It is ssRNA virus belonging to the Avulavirus genus of the paramyxoviridae family and is called Avian Paramyxovirus type 1(APMV-1) 7. While NDV is pathogenic to birds, in humans it is known to be avirulent and causes symptoms identical to flu or laryngitis and conjunctivitis 8. It is classified into three pathogenic types based on the disease severity that it causes in birds: (i) Velogenic (virulent), (ii) mesogenic (intermediate), and (iii) lentogenic (avirulent) 2. Cassel and Garret were the first scientist to report the anti-cancer effect of NDV in 1965 9. NDV induces an oncolytic effect by several mechanisms which have been confirmed in various cancer cell lines. First and foremost is by directly causing infection and then replicating in cancer cells. Normal cells quickly undergo apoptosis on virus infection, resulting in abortive infection, while cancer cells which have acquired apoptosis resistance survive longer, leading to production of more virus particles and initiating new replication cycles 10, 11. The non-lytic strains, present the viral proteins on the tumor cell membrane post infection and thus are capable to initiate and enhance the immune response 3, 12.
Secondly, immunostimulatory effect of the virus also contributes to the oncolytic potential. The virus stimulates the host for production of cytokines such as interferons (IFN) or tumor necrosis factors (TNF), which play an important role in activating macrophages, natural killer cells, and also ssensitizing Tcells 13.
Virotherapy has advanced from the use of wild type and attenuated strains (first generation) to genetically engineered viruses to improve their tumor selectivity (second generation) and finally to transgenic oncolytic viruses (third generation) hence the idea of “designer viruses” appears to be very promising 14, 15.
In India, there is a dearth of established oncolytic NDV strains which can be carried forward for clinical trials. We have intended 1) to screen oncolytic NDV from the poultry strains and 2) to determine and establish its oncolytic potential.
Material and Methods
Cell lines and cell culture
Chick embryonic fibroblast (CEF-DF1) cell line (UMNSAH/DF-1 ATCC) was used for virus culture which was procured from ATCC (USA). The cancer cell lines used in the study are breast cancer- MCF-7 and MDA-MB-231, lung cancer- A549, prostate cancer-PC3, and normal human embryonic kidney cell line- HEK-293. These were procured from NCCS, Pune. DMEM media supplemented with 10% FBS and antibiotics- 1% penicillin/streptomycin was used. The cells lines were maintained in T-25 flasks in complete DMEM 10% FBS at 37°C in a CO2 incubator.
NDV strains
10 velogenic NDV strains were screened for oncolytic effect out of which 5 NDV strains were from Venky’s Poultry Diagnostics and Research Centre, Pune, India and 2 isolates were given by TANUVAS, Chennai, India. Whereas 3 velogenic isolates were procured from IVRI, Bareilly, Uttar Pradesh, India. These strains were labelled serially along with the initial alphabet of the place of procurement.
NDV propagation
Original virus stocks were amplified by propagation in 10 day old, SPF embryonated eggs. 48 hours post inoculation in the SPF eggs the allantoic fluid containing virus (checked for embryo death by candling the egg) was harvested and the presence of the virus was detected by a slide hemagglutination test. The partial purification of the virus was carried out by filtering allantoic fluids using a 0.22μm syringe filter. This was used as seed virus. The seed viruses were then used to infect the CEF-DF1 monolayer (90% confluent). The monolayer was observed under an inverted microscope at a 24 hour interval for cytopathic effects (CPE) like monolayer shrinkage, rounding of cells, detachment of cells from culture flask, etc. After 72 hours, the passaged virus was harvested by freezing at -20°C and thawing twice. This virus culture was used to infect the CEF-DF1 monolayer again. Similarly, the virus was passaged thrice. The final harvest was filtered using a 0.22μm syringe filter and stored in 1.0 mL aliquots at -80°C.
Determination of virus HA titer
HA titer of NDV strains was determined by hemagglutination assay.
The 96-well plate was filled with 50 μL PBS up to a 10th well and a two-fold serial dilution of the virus was carried out. 50 μL of 1% chicken RBC suspension was added to all the wells and the plates were incubated at ambient temperature for 30 minutes. Positive results gave uniform lattice formation across the well. The end point of the titration was the highest dilution of the virus causing complete hemagglutination.
End Point Dilution Assay
The end-point dilution assay was used to measure virus titer by calculating TCID50 and MOI. CEF-DF1 cell line was seeded at a concentration of 2x 104/100 μL in a single well. 10-fold serial dilution of the NDV isolates was carried out from 10⁻3-10⁻10. Every dilution was used to infect CEF-DF1 in 6 replicates. 50μL of each dilution was added to the monolayer for 2 hours and then supplemented with 150μL of complete medium. The plates were incubated for 72 hours and checked for cytopathic effect. Post incubation, the medium was removed, and the cells were fixed with 3.4 % formaldehyde. These were then stained with crystal violet stain.
TCID50 was calculated from the data and expressed TCID50/ mL. The plaque forming units (PFU) of the virus were calculated by Reed and Muench method 16.
MTT Assay for cell viability
Cells from each of the five cell lines were seeded at a concentration of 2x 104cells/100 μL in a 96 well flat bottom microtiter plate and grown for 24 hours. All the 10 NDV isolates were used for infecting 5 cell lines at MOI of 1, 0.1, and 0.01. The infection was carried out in triplicates for 2 hours and then supplemented with 150 μL of complete medium. Post 72 hours incubation, 100 μL MTT solution was added and incubated for 4 hours in a CO2 incubator at 37oC. DMSO (100%) was added to dissolve formazan and the absorbance was read at 540nm/630 nm using an ELISA microplate reader.
Percentage of cell cytotoxicity calculation:
% Cytotoxicity = OD control – OD sample/OD control × 100.
DNA Laddering
All cancer and normal cell line HEK 293 monolayer (1 x 106) were infected with MOI 1 of NDV9B and incubation was carried out for 72 hours at 37°C. Sterile PBS was added instead of virus in controls. After 72 hours cell culture media, as well as adherent cells after trypsinization were centrifuged at 12,000rpm for 8 min. DNA extraction was carried out by modified Marmur’s method 17. Agarose gel electrophoresis was carried out at 60V. DNA ladder 25-700 bp was run along with the samples and gel was observed in the UV gel doc unit.
Morphological changes
Hematoxylin and Eosin (H&E) staining was used to demonstrate morphological changes and progression of infection in MDA-MB-231 cancer cell line by NDV9B at a time interval of 0, 24, 48, and 72 hours post infection. Cells were seeded at a concentration of 1×106 in 35 mm petri plates and incubated for 24 hours in CO2 incubator at 37oC. Monolayer formed was infected with NDV9B at MOI 1 and incubated in a CO2 incubator at 37°C. Sterile PBS was used instead of the virus for negative control. Fixation of the monolayer was carried out by the addition of 3.4% formaldehyde solution and plates were incubated for 1 hour at RT. After the fixation, the monolayer was washed in tap water and 1mL of hematoxylin (Gill no.3) was added to the plates for 5 minutes. The stain was then rinsed with water. The plates were dipped in differentiator 0.3% acid alcohol for a second and rinsed. Eosin was added to the plates for 2 minutes, and then washed off. The monolayers were observed under an inverted microscope at 400X magnification.
Statistical analysis
Statistical analysis was carried out using SPSS software, version 21.0 (SPSS, Chicago, IL, USA). Data presented for the MTT assay is mean ± Standard Deviation (SD) calculated from three independent experiments carried out in triplicates. The data was confirmed to be normally distributed by applying the Shapiro – Wilk normality test. Significance was determined by performing an independent sample T-test and defined as a p-value < 0.05.
Results
The presence of the NDV in the allantoic fluid was established by slide hemagglutination test with chicken RBC; Hemagglutination titer and end point dilution assay for titer of the CEF-DF1 cell line adapted 10 ND viruses were as shown in Table 1.
Table 1: Hemagglutination titer and end point dilution assay for titer of the CEF-DF1 cell line adapted 10 ND viruses.
|
Isolates |
Hemagglutination unit (HAU)/50 μL of the NDV-allantoic fluid stocks |
TCID50/mL of the CEF-DF1 adapted NDV |
PFU/ mL of the CEF-DF1 adapted NDV |
| NDV1P | 256 | 1.0×10⁷ | 6.9x 10⁶ |
| NDV2P | 64 | 3.4 x10⁶ | 2.3×10⁶ |
| NDV3P | 128 | 6.3x 105 | 4.3×10⁶ |
| NDV4 P | 128 | 6.3×10⁷ | 4.3×10⁷ |
| NDV5 P | 64 | 4.9×10⁶ | 3.3×10⁶ |
| NDV6C | 128 | 6.3×10⁶ | 4.3×10⁶ |
| NDV7C | 128 | 4.2×10⁶ | 2.8×10⁶ |
| NDV8B | 128 | 3.4×10⁶ | 2.3×10⁶ |
| NDV9B | 128 | 6.3×10⁶ | 4.3×10⁶ |
| NDV10B | 128 | 4.9×10⁶ | 3.3×10⁶ |
MTT Assay for cell viability
MTT assay was carried out as a screening test to determine the cytopathic effect of viral strains on the cell lines. The effect of all 10 NDV isolates at MOI 1, 0.1, and 0.01 on HEK293, MDA-MB231, MCF7, PC3, and A549, is represented in Figure 1a, 1b, and 1c.
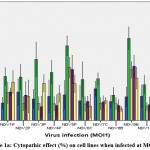 |
Figure 1a: Cytopathic effect (%) on cell lines when infected at MOI 1. |
 |
Figure 1b: Cytopathic effect (%) on cell lines when infected at MOI 0.1. |
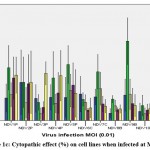 |
Figure 1c: Cytopathic effect (%) on cell lines when infected at MOI 0.01 |
There was least cytopathic effect observed in HEK 293 cell line by all the NDV strains. The maximum cytopathic effect was seen in the MDA-MB-231 by NDV9B 61.55±8.57 and NDV5P 55.89±7.36. In cancer cells the least cytopathic activity was seen in A549 lung cancer cell lines. NDV9B and NDV5P infection on all cancer cells at MOI1 was found to be comparable, p>0.05. NDV9B showed a relatively higher cytopathic effect and so was further tested for its efficacy.
DNA Laddering
DNA fragmentation is the biological hallmark of apoptosis. Unlike necrosis, apoptosis is a programmed process leading to DNA fragmentation due to caspase activated DNase. DNA laddering effect can be observed by running gel electrophoresis of the extracted DNA. The DNA of HEK293, MCF7, A549, MDA-MB-231, PC3 cells infected with NDV9B was extracted and analyzed by agarose gel electrophoresis (Figure.2). The controls for each cell line showed the presence of intact genomic DNA whereas DNA extracted from all cell lines infected with NDV9B showed very faint ladder formation characteristic of apoptosis. NDV9B infection on cell lines produced DNA fragments ranging from 100 bp to 600 bp and appeared as faint bands on the gel. However, no banding (fragmentation) was observed in HEK293 cell line DNA. MDA-MB-231 DNA showed DNA fragmentation as well as smearing which is also demonstrative of cytopathic effect.
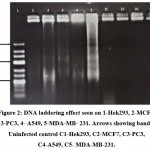 |
Figure 2: DNA laddering effect seen on 1-Hek293, 2-MCF7, 3-PC3, 4- A549, 5-MDA-MB- 231. Arrows showing bands. Uninfected control C1-Hek293, C2-MCF7, C3-PC3,C4-A549, C5- MDA-MB-231. |
Morphological changes
Morphological effects of NDV9B strain on MDA-MB-231 were observed by HE staining of the cell monolayer at 0, 24, 48, and 72 hours post infection. The NDV9B infected cells showed a change of cell morphology like rounding and clumping of cells, syncytia formation which was observed at 48 hrs. Cell lysis and detachment of cell monolayer were observed at 72 hours post infection in MDA-MB-231. The cells in untreated controls were found to be healthy, confluent with no sign of the cytopathic effect.
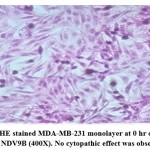 |
Figure 3a: HE stained MDA-MB-231 monolayer at 0 hr on infection with NDV9B (400X). No cytopathic effect was observed. |
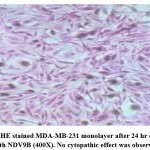 |
Figure 3b: HE stained MDA-MB-231 monolayer after 24 hr on infection with NDV9B (400X). No cytopathic effect was observed. |
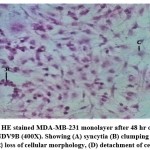 |
Figure 3c: HE stained MDA-MB-231 monolayer after 48 hr on infection with NDV9B (400X). Showing (A) syncytia (B) clumping of cells (C) loss of cellular morphology, (D) detachment of cells. |
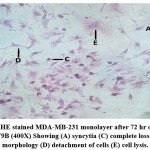 |
Figure 3d: HE stained MDA-MB-231 monolayer after 72 hr on infection with NDV9B (400X) Showing (A) syncytia (C) complete loss of cellular morphology (D) detachment of cells (E) cell lysis. |
Discussion
CEF-DF1 cell line was used successfully to culture and enumerate the NDV strains after passaging thrice in the cell line. The other most commonly used cell line for NDV culture is the Vero cell line 18, 19. However, CEF-DF1 proved to be a good host cell line and a decent pathogenic virus count was obtained due to which a calculated dosage of the virus could be further used for cancer cell line infection.
Previous study results of Umar Ahmed et al, have demonstrated that NDV AF2240 strain had no potent cytotoxic effects on normal cells such as HUVECs (endothelial cells), and Hs578Bst (epithelial breast cell line) but the virus was highly effective in killing MDA-MB-231 20. Yurchenko et al. demonstrated that normal PBMC primary cells maintained a high viability rate even after the 4th day of NDV infection 21. The normal cells have an effective antiviral interferon signaling which leads to effective clearance of the virus without giving it a chance to replicate 22. All the cancer cell lines showed variance in their susceptibility to the NDV strains. MCF-7 and A549 underwent the least cytolysis. The variability of the susceptibility percentage between MDA-MB-231 and MCF-7 according to Tianyan Liu et al., is owing to the levels of alpha-sialic acid acyltransferase which was upregulated in MDA-MB-231 cells in comparison to MCF7 cells, and the IFN levels were downregulated 23. Cells infected at MOI 1 showed maximum cytopathic effect by all the viral strains and the cytopathic effect decreased when cells were infected at lower MOI 0.1 and 0.01. Thus, NDV induces a cytolytic effect in dose dependent manner. This has been established by several earlier studies wherein, infection with higher HAU/ mL has proved to be more effective than lower titer 20, 24. It has been demonstrated that wild-type NDV strains show strong oncolytic effects through direct lysis in human cancer cell lines as well as effective on A549 carcinoma in SCID mice as the NDV infection led to arrest of tumor spread 22. Naturally, attenuated PV701 is one of the examples of non-recombinant, replication competent virus and also has broad spectrum anticancer specificity 25.
Several studies have long-established apoptosis as the primary mode in NDV induced cell death. DNA fragments of 180–200 bp on an agarose gel as a result of NDV AF2240 and V4-UPM infection on leukemia cells have been reported by Alabsi et al. 26 also NDV AF2240 on brain tumor cell (U-87MG) showed similar DNA fragments 27. P.V. Ravindra et al. demonstrated ladder formation in Vero cells 48 hours post infection with velogenic NDV strains establishing NDV induced cytopathic effect was due to induction of apoptosis 28.
The changes observed in cellular morphology in our study are in concurrence with the investigation carried out on NDVAF2240 and V4-UPM in HT-29 (human colorectal adenocarcinoma cells) which concluded that cells showed an increase in cell rounding, atrophy and became non adherent with an increase in time of viral exposure 25. Research has shown both velogenic and mesogenic NDV strains infected MCF7 cell line resulted in cell rounding, cytoplasmic fusion and syncytia formation leading to cytolysis 30. NDV HN (hemagglutinin-neuraminidase) and polybasic fusion site in F (fusion) protein enables the fusion of infected cells and uninfected cells in the vicinity, lead to production of syncytia which plays essential roles in NDV induced oncolytic effect. Thus, lytic strains cause lysis of host cancer cells through the production of infectious progeny virions which induce changes in plasma membrane and lead to syncytia formation 2, 30.
Conclusion
In India only, limited studies have been conducted to identify oncolytic NDV strains and its further exploration to be employed in cancer treatment is yet in the nascent stage. This study establishes that wild type NDV9B isolate has prominent effectiveness as an oncolytic agent against MDA-MB-231 with a very mild cytopathic effect on the normal HEK293 cell line. Apoptosis was found to be one of the primary mode of cell death however, further study is required to determine the precise signaling molecules and mechanism involved in NDV9B driven oncolysis.
Acknowledgment
Authors acknowledge the support of Venky’s Poultry Diagnostics and Research Centre, Pune, India for kindly providing 5 NDV isolates and Dr. Kumanan Kathaperumal, Former Director of Research, Tamil Nadu Veterinary and Animal Sciences University, Chennai, India for kindly sparing 2 NDV isolates.
Conflicts of interest
There is no conflict of Interest.
Funding Sources
Sir H.N. Medical Research Society, Sir H.N. Reliance Foundation Hospital & Research Centre, Mumbai-400 004, Maharashtra, India.
References
- Parsa N. Environmental factors inducing human cancers. Iran J Public Health, 2012;41(11):1–9.
- Fournier P, Schirrmacher V. Oncolytic newcastle disease virus as cutting edge between tumor and host. Biology (Basel), 2013;2(3):936–75.
CrossRef - Singh P.K, Doley J, Ravi Kumar G, Sahoo A.P, Tiwari A.K. Oncolytic viruses & their specific targeting to tumour cells. Indian J Med Res., 2012;136(4):571–84.
- Kaufman H. L, Kohlhapp F. J, Zloza A. Oncolytic viruses: A new class of immunotherapy drugs. Nat Rev Drug Discov.,2015;14(9):642–62.
CrossRef - Hemminki O, Manuel J, Hemminki A. Oncolytic viruses for cancer immunotherapy, 2020;1:1–15.
CrossRef - Costa-hurtado M, Afonso C. L, Miller P. J, Spackman E, Kapczynski D.R, Swayne D.E, et al. Virus interference between H7N2 low pathogenic avian influenza virus and lentogenic Newcastle disease virus in experimental co-infections in chickens and turkeys, 2014;1–11.
CrossRef - Ganar K, Das M, Sinha S, Kumar S. Newcastle disease virus: Current status and our understanding. Virus Res.,2014;184:71–81.
CrossRef - Chalovich J. M, Eisenberg E. Oncolytic Newcastle Disease Virus for cancer therapy: old challenges and new directions Dmitriy. Biophys Chem., 2005;257(5):2432–7.
- Cassel W. A, Garrett R. E. Newcastle disease virus as an antineoplastic agent. Cancer, 1965;18(7):863–8.
CrossRef - Zakay-Rones Z, Tayeb S, Panet A. Therapeutic potential of oncolytic Newcastle disease virus a critical review. Oncolytic Virotherapy, 2015:49.
CrossRef - Davis D. Application of Oncolytic Viruses for Cure of Colorectal Cancer. Cancer Res J.,2015;3(4):76.
CrossRef - Schirrmacher V, van Gool S, Stuecker W. Breaking therapy resistance: An update on oncolytic newcastle disease virus for improvements of cancer therapy. Biomedicines,2019;7(3):66.
CrossRef - Washburn B, Weigand M. A, Grosse-Wilde A, Janke M, Stahl H, Rieser E, et al. TNF-Related Apoptosis-Inducing Ligand Mediates Tumoricidal Activity of Human Monocytes Stimulated by Newcastle Disease Virus. J Immunol., 2003;170(4):1814–21.
CrossRef - Kelly E, Russell S. J. History of oncolytic viruses: Genesis to genetic engineering. Mol Ther., 2007;15(4):651–659.
CrossRef - Al-Humadi H, Zarros A, Al-Saigh R, Liapi C. Genetic basis and gene therapy trials for thyroid cancer. Cancer Genomics and Proteomics, 2010;7(1):31–49.
- Reed L. J, Muench H. A Simple Method of Estimating Fifty Percent Endpoints. American Journal of Epidemiology,1938;27:493–97.
CrossRef - Marmur J. A procedure for the isolation of deoxyribonucleic acid from micro-organisms Journal of Molecular Biology,1961;3(2):208-218.
CrossRef - Yurchenko K. S, Jing Y, Shestopalov A. M. Adaptation of the Newcastle Disease Virus to Cell Cultures for Enhancing Its Oncolytic Properties. Acta Naturae, 2019;11(40):66–73.
CrossRef - Ahamed T, Hossain K. M, Billah M. M, Islam K.M.D, Ahasan M. M, Islam M. E. Adaptation of Newcastle Disease Virus ( NDV ) on Vero Cell Line. International Journal of Poultry Science, 2004;3(2):153–156.
CrossRef - Ahmad U, Ahmed I, Keong Y. Y, Manan N. A, Othman F. Inhibitory and Apoptosis-Inducing Effects of Newcastle Disease Virus Strain AF2240 on Mammary Carcinoma Cell Line.Biomed Research International, 2015;2015.
CrossRef - Elankumaran S, Chavan V, Qiao D, Shobana R, Moorkanat G, Biswas M, et al. Type I Interferon-Sensitive Recombinant Newcastle Disease Virus for Oncolytic Virotherapy.J Virol., 2010;84(8):3835–44.
CrossRef - Yurchenko K. S, Zhou P, Kovner A. V., Zavjalov E. L, Shestopalova L. V., Shestopalov AM. Oncolytic effect of wild-type Newcastle disease virus isolates in cancer cell lines in vitro and in vivo on xenograft model. PLoS One, 2018;13(4):1–19.
CrossRef - Liu T, Zhang Y, Cao Y, Jiang S, Sun R, Yin J, et al. Optimization of oncolytic effect of Newcastle disease virus Clone30 by selecting sensitive tumor host and constructing more oncolytic viruses. Gene Ther, 2021; 697–717.
CrossRef - Sui H, Wang K, Xie R, Li X, Li K, Bai Y. NDV-D90 suppresses growth of gastric cancer and cancer-related vascularization. Oncotarget, 2017;8(21):34516–24.
CrossRef - Lam HY, Yeap SK, Rasoli M, Omar AR, Yusoff K, Suraini AA, et al. Safety and clinical usage of newcastle disease virus in cancer therapy. J Biomed Biotechnol, 2011:2011-718710.
CrossRef - Alabsi A. M., Aishah S., Bakar A., Ali R., Omar A. R.International journal of molecular sciences,2011;12(12):8645-60.
CrossRef - Assayaghi RM, Alabsi AM, Ali AM. Apoptosis Induction of Newcastle Disease Virus Strains ( AF 2240 & V4-UPM ) on HT-29 Human Colorectal Adenocarcinoma Cells. Journal of Cancer Research and Therapeutic Oncology,2017;4(101):1-8.
CrossRef - Ravindra PV, Tiwari AK, Ratta B, Chaturvedi U, Palia SK, Chauhan RS. Newcastle disease virus-induced cytopathic effect in infected cells is caused by apoptosis.Virus Res., 2009; 141 (1):13-20.
CrossRef - Ali R, Alabsi A. M, Ali A. M, Ideris A, Omar A. R, Yusoff K, et al . Cytolytic Effects and Apoptosis Induction of Newcastle Disease Virus Strain AF2240 on Anaplastic Astrocytoma Brain Tumor Cell Line. Neurochem Res., 2011; 36 (11):2051-62.
CrossRef - Jagtap R, Raja A. K, Parthiban M, Palanisamy M. Dose and Strain Dependent Induction of Cell Death of Human Breast Cancer Cells (Mcf-7) by Newcastle Disease Virus. Journal of Animal Health and Production, 2017;5(1):29-34.

This work is licensed under a Creative Commons Attribution 4.0 International License.





