Manuscript accepted on : 15-06-2022
Published online on: 17-06-2022
Plagiarism Check: Yes
Reviewed by: Dr. Robert Susło
Second Review by: Dr. Ali Elshafei
Final Approval by: Dr. Susana Rodriguez-Couto
Soham Lavande1, Shraddha Jaiswal1, Roshanee Deore1, Jayant Pawar2 and Vidya Tale1*
1Department of Microbiology, Rajiv Gandhi Institute of I.T and Biotechnology, Bharati Vidyapeeth, Pune, Maharashtra, India.
2Krishna Institute of Medical Sciences, Karad, Maharashtra, India.
Corresponding Author E-mail: vidya.tale@bharatividyapeeth.edu
DOI : http://dx.doi.org/10.13005/bbra/3002
ABSTRACT:
Nanoparticle synthesis using plant extracts is biologically safe, cost-effective, and environment-friendly, hence attracting many researchers owing to its advantages over chemical or physical methods. In the current study copper and silver nanoparticles have been synthesized by chemical and biological methods (using fruit extract). The leftover fruits collected from the fruit vendors and were used for the study, such as guava (Psidium guajava L), and tomato (Solanum lycopersicum) as a source of ascorbic acid, while lemon (Citrus limon (L.) Osbeck) and orange (Citrus X sinensis) as a source of citric acid. Quantification of ascorbic acid and citric acid present in fruit extract was performed by Iodometric and acid-base titrations, respectively, followed by Thin Layer Chromatography (TLC) to confirm their role in nanoparticle production. The synthesized nanoparticles were characterized by UV–visible (UV–VIS) spectroscopy and Fourier transform infrared (FT-IR) spectroscopy. The number of particles produced with fruit extract as a reducing agent was more compared to chemical methods. The size and structure of the synthesized nanoparticles produced using fruit extracts were similar to those produced chemically. Also, the antibacterial effect of Cu and Ag nanoparticles was seen against Escherichia coli and Streptococcus pyogenes strains.
KEYWORDS: Ascorbic Acid; Antimicrobial activity; Citric Acid; Copper Nanoparticles; leftover fruits extracts; Silver Nanoparticles
Download this article as:| Copy the following to cite this article: Lavande S, Jaiswal S, Deore R, Pawar J, Tale V. Metal Nanoparticle Synthesis Using Fruit Extracts as Reducing Agents and Comparative Studies with a Chemical Reducing Agent. Biosci Biotech Res Asia 2022;19(2). |
| Copy the following to cite this URL: Lavande S, Jaiswal S, Deore R, Pawar J, Tale V. Metal Nanoparticle Synthesis Using Fruit Extracts as Reducing Agents and Comparative Studies with a Chemical Reducing Agent. Biosci Biotech Res Asia 2022;19(2). Available from: https://bit.ly/3Ads0sS |
Introduction
Nanoparticles are defined as solid crystalline structures with at least one dimension between 1-100 nm. The key benefit of nanomaterials is that they can be easily altered by varying their size and shape. The unique chemical, physical, dielectric, optical properties of nanoparticles make them extremely suitable for designing new and improved sensing devices, especially electrochemical sensors and biosensors1. Amongst various metal nanoparticles, the copper and silver nanoparticles possess larger surface areas and many characteristics like biological, thermal, chemical, electrical, physical, mechanical, electronic, magnetic, and optical properties making them attractive tools for research work2.
The copper nanoparticles display unique characteristics including antifungal and antibacterial properties3. Copper oxide nanoparticles appear as a brownish-black powder. Copper oxide nanoparticles can be used as semiconductors, catalysts, superconducting materials, thermoelectric materials, sensing materials, glass, ceramics, and other fields and solar energy transformations4.
The silver nanoparticles exhibit a broad spectrum of bactericidal and fungicidal activity. Silver is a soft, white, lustrous transition metal possessing high electrical and thermal conductivity. Silver nanoparticles have received attention due to their physical, chemical, and biological properties found in applications in nanobiotechnological research. They are used as antimicrobial agents in wound dressings, as topical creams to prevent wound infections, and as anticancer agents5. It has been known for its medical and therapeutic benefits before the realization that microbes are agents for infections. It is used in many forms like coins, vessels, solutions, foils, sutures, and colloids as lotions, ointments, and so forth. It is the foremost therapeutic agent in medicine for infectious diseases and surgical infections. The benefits of silver are more than the risk factors6.
Nanoparticles are synthesized by different methods such as the physical, chemical and biological methods. The advantage of using biological methods for synthesizing nanoparticles is that they are cost-effective, economical, energy-efficient, safe, eco-friendly and produce less waste. Due to this, biological methods of nanoparticle synthesis are preferred although these produce large size nanoparticles. In the biological synthesis of nanoparticles microorganisms, enzymes, plants, and alga are used7.
The fruit of guava (Psidium guajava L) and Tomato (Solanum lycopersicum) is a source of ascorbic acid while lemon (Citrus limon (L.)Osbeck) and orange (Citrus X sinensis) is a source of citric acid. Ascorbic acid in tomato extract can reduce copper sulphate and also works as a protecting agent. Citric acid compounds can reduce silver nitrate solution releasing free metal ions or metal oxide ions in both cases. The fruit extracts are used as a source of ascorbic acid and citric acid to increase stability and have low toxicity and a higher production rate in nanoparticle synthesis8. In the present study we have tried to use waste from left over fruits as source of ascorbic acid and citric acid as reducing agent for the synthesis of copper and silver nanoparticles and compared its structural characteristic and antibacterial efficacy with one synthesized using chemical reducing agents.
Materials and Methods
Copper sulphate pentahydrate CuSO4 .5H2O, ascorbic acid C6H8O6 (0.2M), starch C6H10O5 (1.2%), silver nitrate AgNO3 (0.2M) and sodium hydroxide NaOH (1M) were of laboratory grade and purchased from Sigma Aldrich. Bacterial cultures of Escherichia coli and Streptococcus pyogenes are standard cultures characterized by microscopy and biochemical test. They were maintained in the Müller-Hinton medium (Himedia) as well as sub cultured regularly during the study.
Preparation of fruit extract
The fruit samples of guava, orange, tomato and lemon were obtained from the local market. The leftover fruits from vendors are used for the study to check their value addition. The mortar pestle and de-ionised water were used to prepare the fruit extract. The chemicals used for the estimation of acids were potassium iodide KI (0.6mol/L), starch as an indicator, dilute hydrochloric acid (1mol/L), and potassium iodate KIO3(0.002mol/L)
Synthesis of Copper and Silver nanoparticles using chemical reducing agents
Copper sulphate and Silver nitrate were used for the synthesis of copper and silver nanoparticles. For the chemical synthesis of these metal nanoparticles, ascorbic acid and citric acid were used as reducing agents. 50 mL of 10mM Copper (II) Chloride (CuCl2.2H2O) solution was heated continuously at 90oC in a water bath shaker. The 0.5M L-Ascorbic Acid was prepared in deionized water and added dropwise to the flask. The heating and mixing continued till the color changed to yellow, orange, brown and finally dark brown-black9.
50 ml of 1 mM AgNO3 were heated in an Erlenmeyer flask. Just before the boiling point, 5 ml of 1% trisodium citrate was added drop by drop to this solution. Vigorous mixing was carried out during the process to increase the interaction of the reagents, the reaction mixture was maintained at over 60 °C, until its color changed to grey10.
Synthesis of Copper and Silver nanoparticles using fruit extract
The tomato and guava extract is used as a replacement for Ascorbic acid whereas orange and lemon extract was used for Citric acid. Extracts were filtered and used for NP synthesis. Nanoparticles were synthesized using the same protocol used for synthesis using chemical reducing agent. The precipitate obtained was centrifuged, washed with ethanol and dried in a hot air oven for 24 hours. Then the sample was calcinated at 350 ̊C for 40 minutes for proper drying in a muffle furnace. The dried sample was crushed to fine powders for characterization and application purposes. After the preliminary test both the samples were prepared in bulk.
Thin Layer Chromatography
For the detection of organic acids present in the reaction mixture, TLC was performed. The mobile phase was prepared by a mixture of Chloroform, Acetone, Methanol and Ammonia in a ratio of 2:2:2:1. The stock solutions of standard Ascorbic Acid and Citric acid were prepared. The fruit extracts were used along with standards spotted on the thin silica gel before and after the synthesis of nanoparticles11.
Estimation of Ascorbic acid and Citric acid by titration
For ascorbic acid the titrant was a mixture of potassium iodide and iodine whereas for citric acid it was sodium hydroxide. The extract was collected after 8 hours of reaction time. Both the fresh and after the reaction extracts were titrated with their respective titrant and a burette reading was noted. The concentration of the ascorbic acid in fresh tomato and guava extract was calculated by the amount of fruit sample used and the amount of iodine required to neutralize the ascorbic acid in these extracts. The concentration of acids in the reaction mixture after the synthesis of nanoparticles was also calculated and compared with the acid concentration initially present in fruit extract12.
Characterization of Synthesized Nanoparticles
FTIR spectroscopy was done to determine various phytochemical constituents involved in the synthesis and stabilization of the Cu and Ag nanoparticles according to the KBr disc method and recorded in the range from 500 to 4000 cm– (Nicolet iS5-FTIR 143 Spectrometer). Scanning UV–Vis spectrophotometer (Genesys 10 UV) was used to determine the UV absorption spectra of the synthesized metal NPs.
Antibacterial assay of Cu and Ag nanoparticles
Antibacterial activity of the copper and silver nanoparticles was studied against Streptococcus pyogenes (Gram positive) and E.coli (Gram negative). Copper and silver nanoparticles (50 mg/ml and 100mg/ml) synthesized by both methods were used for the determination of their antimicrobial potential by agar well diffusion method using Muller and Hinton agar (MHA). A loop full of the overnight cultures of E. coli and Streptococcus pyogenes was added to 10ml of saline from this 0.1 ml of culture and was spread evenly on MHA plates. Wells (8mm in diameter) were prepared using sterile cork borer and 100µl of both the nanoparticles suspension were added to separate wells. The plates were incubated for 24hours at 37ᴼC and observed for the zone of inhibition along with control13. The experiment was repeated thrice.
Results and Discussion
Synthesis of copper and silver nanoparticles
Copper nanoparticles were brown whereas grey-colored silver nanoparticles were obtained using both methods. There was a slight variation in the intensity of the color of NP obtained by both methods. All the powders after drying and crushing are stored in dry plastic vials for further characterization and applications.
UV–Vis absorption spectroscopy analysis
Copper nanoparticles have shown UV absorption peaks in the range of 290-300nm using both the reducing agents. The absorbance of Cu nanoparticles is seen at this range due to the effect of L-ascorbic acid in the extract14. Silver nanoparticles show a UV absorption peak of around 430 nm15whereas Silver oxide nanoparticles show an absorption peak in the range of 230-260nm16 (Fig 1).
From this reading it is concluded that from the chemical method Silver Nanoparticles were produced evident from the Gaussian peak seen while from fruit extracts Silver Oxide nanoparticles were produced as the maximum absorption peak obtained was in the similar range which is in confirmation with previous similar research17. From UV spectroscopy it was seen that the structure of the synthesized nanoparticles produced using fruit extracts was similar to those produced using ascorbic and citric acid.
 |
Figure 1: UV spectroscopy graphs of copper nanoparticles synthesized using different reducing agents. |
Thin Layer Chromatography analysis
Thin Layer Chromatography was performed to confirm the presence of Ascorbic acid and Citric acid by comparing the spots of the samples of fruit extracts before and after synthesis with standards. The results show the presence of ascorbic and citric acid in fruit extract used for synthesis which was reduced to a non-detectable range after the reaction.
Ascorbic acid and citric acid analysis
Acid Base Titration
For titration of ascorbic acid the colour change was from colourless to dark blue with starch as an indicator. The samples rich in ascorbic acid require more volume of titrant to get the colour change. The samples were taken after 8 hour duration and the amount of titrant required to neutralize Ascorbic acid reduced gradually. The most rapid decrease in titrant volume was seen in the first 30 minutes after the synthesis reaction indicating the rapid utilization of Ascorbic acid for the reduction of Copper Sulphate.
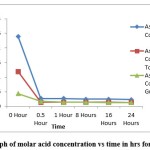 |
Figure 2: Graph of molar acid concentration vs time in hrs for ascorbic acid. |
Using pure Ascorbic acid, the concentration of the fresh solution was 0.024M and it got reduced to 0.0023M after the synthesis reaction. In the case of tomato the concentration of acid in the fresh extract was 0.012M which it got reduced to 0.0014M after the reaction. For guava, the concentration was 0.004M (Fresh) and it was 0.0013M after the reaction. For estimation of citric acid acid-base titration was done with colour change being colourless to light pink.
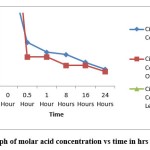 |
Figure 3: Graph of molar acid concentration vs time in hrs for citric acid. |
The amount of citric acid reduced from the sample was recorded, the concentration of acid was dropped to 0.007 M (After Reaction) from 0.05M (Fresh), in the case of Orange extract, the concentration was 0.095M (Fresh) and reduced to 0.006M (After reaction) and for Lemon extract, the concentration was 0.5M (Fresh) which was dropped to 0.16M (After reaction). All these readings indicate that the number of ascorbic acids and citric acids in the fresh extract was reduced after metal nanoparticle synthesis.
FTIR Spectra analysis
IR Spectrum of Copper nanoparticles shows absorbance at 3594.66 cm-1 indicating H-O-H stretching. Absorbance in the range of 706-878 cm-1 indicates the presence of Copper and oxygen as well as Cu-O stretching18. A sample of Silver nanoparticles showed absorbance at 3492.45 cm-1 due to the presence of alcohols and phenols and also –OH stretching. They also show absorbance at 2923.56 cm-1, 1756.83 cm-1, 1457.92 cm-1, 1110.8 cm-1, 832.133 cm-1, 794.493 cm-1 indicating the presence of Silver nanoparticles and functional groups like alkyl halides, amide groups, aldehydes, ketones and their bending, aromatic compounds of C=C stretching, esters of C=O stretching, Alkanes, alcohols and phenols with C-H stretching as well as –OH stretching16. The absorbance at different frequencies for both samples showed expected functional groups by FTIR analysis.
 |
Figure 4: Characterization by FTIR Analysis (A) Absorbance of Copper Nanoparticles Synthesized From Ascorbic Acid. (B) The absorbance of Silver Nanoparticles Synthesized from Citric Acid. |
Antibacterial Effect analysis
The antimicrobial assay of CuNPs and AgNPs nanoparticles synthesized by fruit extract and acid was compared. The cell membrane is essential for the survival of bacteria, the rupture of the cell membrane due to its interaction with copper nanoparticles causes bacterial cell lysis19. The antimicrobial activity of the synthesized copper and silver nanoparticles was tested against 2 bacterial strains: Escherichia coli and Streptococcus pyogenes. The good diffusion test for the presence of a clear zone around the well containing a suspension of CuNPs and AgNPs confirms the antibacterial activity of the aforementioned metal nanoparticles which can inhibit the growth of Escherichia coli and Streptococcus pyogenes. The results for the antibacterial activity of the CuNPs and AgNPs are summarized in tables 1 and 2 respectively. It was observed that chemically synthesized CuNPs and AgNPs exhibit similar antibacterial activities against both the test organisms whereas nanoparticles synthesized using fruit extract showed no inhibitory activity against S. pyogenes (fig 6).
Table 1: The antibacterial activity of the prepared copper nanoparticles at a concentration of 50µg/ml using different reducing agents against E. coli and S. pyogenes.
| Test organism
( Zone of inhibition in mm) |
||
| Reducing agent | E. coli | S. pyogenes |
| Ascorbic acid | 14 mm | 11 mm |
| Tomato Extract | 11 mm | No Inhibition |
| Guava Extract | 9 mm | No Inhibition |
Table 2: The antibacterial activity of the prepared silver nanoparticle in a concentration of 250µg/ml using different reducing agents against E. coli and S. pyogenes.
| Test organism
( Zone of inhibition in mm) |
||
| Reducing agent | E. coli | S. pyogenes |
| Citric Acid | 25mm | 12mm |
| Orange Extract | 22mm | No Inhibition |
| Lemon Extract | 20mm | No Inhibition |
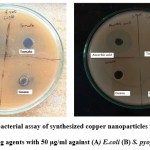 |
Figure 5: Anti-bacterial assay of synthesized copper nanoparticles using different reducing agents with 50 µg/ml against (A) E.coli (B) S. pyogenes |
 |
Figure 6: Anti-bacterial assay of synthesized silver nanoparticle using different reducing agents against with 250 µg/ml against (A) E. coli (B) S. pyogenes |
Generally, Ag and Cu NPs have been synthesized by reacting extract of fruit with metal ions17,20. The bioactive compounds such as proteins, vitamins, carboxylic acid, carbohydrates, saponins, polyphenols and amides found in the plant materials act as reducing and capping agents and use of hazardous reductants and surfactants can be reduced.21 The present study demonstrated successful synthesis of metal nanoparticles using extract of left over fruits as reducing agents. Antibacterial efficiency of the nanoparticles synthesized by using both the methods have shown similar antibacterial activity. The antimicrobial mechanisms of nanoparticles depend upon species of bacteria, size of the nanoparticles and concentration and purity of nanoparticles22. The E. coli was found to be more sensitive as compared to S. pyogenes to both the synthesized nanoparticles. This supports the previous work which shows Gram-positive bacteria are more resistant to nanoparticles 23.The nanoparticles synthesized by using extracts of left over fruits therefore gained the advantage of value addition and environment friendly process as compared to chemical synthesis. Fruit and vegetable wastes account for several thousand tonnes around the India. Therefore, utilization of these waste fruit and vegetable for various processes may strengthen the local economy and help mitigate environmental issues.
Conclusion
In this study copper and silver nanoparticles were successfully synthesized using Ascorbic acid and Citric acid as well as different fruit extracts rich in these acids. The quantity of particles formed after the reaction was more when produced with fruit extract as a reducing agent. By iodometric titration and thin layer chromatography it can be concluded that fruit extracts can be used in place of chemicals acting as reducing agents for the synthesis of metal nanoparticles with antibacterial properties. The results obtained in the study also suggest that further purification steps are required for increasing the concentration and purity of synthesised metal nanoparticles.
Acknowledgement
The authors highly acknowledge the Department of Microbiology for providing the research facilities.
Conflict of interest
The authors confirm that the content of this manuscript has no conflict of interest.
Funding Sources
There is no funding sources for this paper.
References
- Kumar H, Bhardwaj K, Dhanjal DS, Nepovimova E, Șen F, Regassa H, Singh R, Verma R, Kumar V, Kumar D, Bhatia SK, Kuča K. Fruit extract mediated green synthesis of metallic nanoparticles: A new avenue in pomology applications. International Journal of Molecular Sciences. 2020;21(22):1–18.
CrossRef - Nanda A, Saravanan M. Biosynthesis of silver nanoparticles from Staphylococcus aureus and its antimicrobial activity against MRSA and MRSE. Nanomedicine: Nanotechnology, Biology, and Medicine. 2009;5(4):452–456.
CrossRef - Wei Y, Chen S, Kowalczyk B, Huda S, Gray TP, Grzybowski BA. Jp1055683.Pdf. 2010:15612–15616.
CrossRef - Zoolfakar AS, Rani RA, Morfa AJ, O’Mullane AP, Kalantar-Zadeh K. Nanostructured copper oxide semiconductors: A perspective on materials, synthesis methods and applications. Journal of Materials Chemistry C. 2014;2(27):5247–5270.
CrossRef - Zhang XF, Liu ZG, Shen W, Gurunathan S. Silver nanoparticles: Synthesis, characterization, properties, applications, and therapeutic approaches. International Journal of Molecular Sciences. 2016;17(9).
CrossRef - Loo YY, Rukayadi Y, Nor-Khaizura MAR, Kuan CH, Chieng BW, Nishibuchi M, Radu S. In Vitro antimicrobial activity of green synthesized silver nanoparticles against selected Gram-negative foodborne pathogens. Frontiers in Microbiology. 2018;9(JUL):1–7.
CrossRef - Khan A, Rashid A, Younas R, Chong R. A chemical reduction approach to the synthesis of copper nanoparticles. International Nano Letters. 2016;6(1):21–26.
CrossRef - Mane Gavade SJ, Nikam GH, Dhabbe RS, Sabale SR, Tamhankar B V., Mulik GN. Green synthesis of silver nanoparticles by using carambola fruit extract and their antibacterial activity. Advances in Natural Sciences: Nanoscience and Nanotechnology. 2015;6(4):45015.
CrossRef - Umer A, Naveed S, Ramzan N, Rafique MS, Imran M. A green method for the synthesis of copper nanoparticles using l-ascorbic acid. Revista Materia. 2014;19(3):197–203.
CrossRef - Oprica L, Andries M, Sacarescu L, Popescu L, Pricop D, Creanga D, Balasoiu M. Citrate-silver nanoparticles and their impact on some environmental beneficial fungi. Saudi Journal of Biological Sciences. 2020;27(12):3365–3375.
CrossRef - Tlc-densitometry S. and D 3 in Pharmaceutical Products and Dietary. 2020;(Figure 1):1–13.
- Silva CR, Simoni JA, Collins CH, Volpe PLO. Ascorbic Acid as a Standard for Iodometric Titrations: An Analytical Experiment for General Chemistry. Journal of Chemical Education. 1999;76(10):1421–1422.
CrossRef - Balouiri M, Sadiki M, Ibnsouda SK. Methods for in vitro evaluating antimicrobial activity: A review. Journal of Pharmaceutical Analysis. 2016;6(2):71–79.
CrossRef - Caroling G, Priyadharshini MN, Vinodhini E, Ranjitham AM, Shanthi P. – Characterisation and Study of Antibacterial Effects. 2015;5(2).
- Yoksan R, Chirachanchai S. Silver nanoparticles dispersing in chitosan solution: Preparation by γ-ray irradiation and their antimicrobial activities. Materials Chemistry and Physics. 2009;115(1):296–302.
CrossRef - Pawar O, Deshpande N, Dagade S, Waghmode S, Nigam Joshi P. Green synthesis of silver nanoparticles from purple acid phosphatase apoenzyme isolated from a new source Limonia acidissima. Journal of Experimental Nanoscience. 2016;11(1):28–37.
CrossRef - Dhulappanavar G, Hungund B, Ayachit N, Bhakat A, Pal Singh P, Priya S, Pawar J, Vinchurkar P, Henry R. Characterization of silver nanoparticles biosynthesized using lemon juice. Proceedings of the International Conference on Nanoscience, Engineering and Technology, ICONSET 2011. 2011:258–262.
CrossRef - Markova-Deneva I. Infrared spectroscopy investigation of metallic nanoparticles based on copper, cobalt, and nickel synthesized through borohydride reduction method (Review). Journal of the University of Chemical Technology and Metallurgy. 2010;45(4):351–378.
- Li H, Chen Q, Zhao J, Urmila K. Enhancing the antimicrobial activity of natural extraction using the synthetic ultrasmall metal nanoparticles. Scientific Reports. 2015;5(November 2014):1–13.
CrossRef - Menamo DS, Ayele DW, Ali MT. Green synthesis, characterization and antibacterial activity of copper nanoparticles using L-ascorbic acid as a reducing agent. Ethiopian Journal of Science and Technology. 2017;10(3):209.
CrossRef - Ali S, Chen X, Ajmal Shah M, Ali M, Zareef M, Arslan M, Ahmad S, Jiao T, Li H, Chen Q. The avenue of fruit wastes to worth for synthesis of silver and gold nanoparticles and their antimicrobial application against foodborne pathogens: A review. Food Chemistry. 2021;359(March):129912.
CrossRef - Darvishi E, Kahrizi D, Arkan E. Comparison of different properties of zinc oxide nanoparticles synthesized by the green ( using Juglans regia L . leaf extract ) and chemical methods. Journal of Molecular Liquids. 2019;286:110831.
CrossRef - Slavin YN, Asnis J, Häfeli UO, Bach H. Metal nanoparticles: Understanding the mechanisms behind antibacterial activity. Journal of Nanobiotechnology. 2017;15(1):1–20.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





