Manuscript accepted on : 16-05-2022
Published online on: 24-05-2022
Plagiarism Check: Yes
Reviewed by: Dr. Jurdas
Second Review by: Dr. Amit Rastogi
Final Approval by: Dr. Hifzur R. Siddique
Sreepriya M , Bhimnaik S
, Bhimnaik S , Bhavyasai
, Bhavyasai , Rumana A
, Rumana A and Divya M
and Divya M
Department of Microbiology and Biotechnology, Bangalore University, Jnana Bharathi Campus, Bangalore – 560 056, Karnataka, India
Corresponding Author E-mail: mpriya7@yahoo.com
DOI : http://dx.doi.org/10.13005/bbra/2986
ABSTRACT: Phytoestrogens are phytoalexins, plant secondary metabolites produced in response to microbial attacks and other forms of external stress stimuli. These compounds are devoid of the steroidal structure of endogenous estrogens but could bind with estrogen receptors with great affinity as they have structural similarity with endogenous/synthetic estrogens. Phytoestrogens are reported to possess several health benefits including cardio protective, antidiabetic, antioxidant and anti osteoporotic properties. Phytoestrogens like genistein, daidzein, and equol have been reported to possess excellent anti proliferative and anticancer properties. Formononetin, is a phytoestrogen, isoflavone widely present in red clover (Trifolium pratense) and resveratrol is a phytoestrogen which is widely present in red grapes (Vitis vinifera). The cardio protective property of red wine has been attributed to the presence of resveratrol. Although formononetin and resveratrol have been investigated extensively for several pharmacological properties, reports on their anti proliferative effects especially against human pulmonary carcinoma cell lines are very less. Hence the current study aims to understand the influence of these two compounds on the growth of human pulmonary adenocarcinoma cell line A 549. MTT assay, cytomorphology analysis by phase contrast microscopy, DNA topology assay and DPPH assay were few assays which were performed to understand the antiproliferative and antioxidant effects of these compounds. Results of the study indicated the potent antiproliferative effects of these compounds against human pulmonary carcinoma cell lines implicating that these compounds could be potential therapeutic molecules with multifaceted roles as anticancer agents, potent antioxidants and anti inflammatory agents.
KEYWORDS: A 549 cell line; DNA topology assay; Formononetin; MTT assay; Resveratrol
Download this article as:| Copy the following to cite this article: Sreepriya M, Bhimnaik S, Bhavyasai B, Rumana A, Divya M. Antiproliferative Effects of Phytoestrogens Formononetin and Resveratrol Against Human Pulmonary Adenocarcinoma Cells A 549. Biosci Biotech Res Asia 2022;19(2). |
| Copy the following to cite this URL: Sreepriya M, Bhimnaik S, Bhavyasai B, Rumana A, Divya M. Antiproliferative Effects of Phytoestrogens Formononetin and Resveratrol Against Human Pulmonary Adenocarcinoma Cells A 549. Biosci Biotech Res Asia 2022;19(2). Available from: https://bit.ly/3wExvOU |
Introduction
The development of female reproductive system and its regulation is primarily controlled by the group of steroid hormone referred to as Estrogens 1. Estrone, estriol and estradiol are the three forms of estrogens with estradiol being the most prevalent and biologically active form of the hormone. Estrogens are produced predominantly by the ovaries with adrenal cortex, placenta and male testis also contributing to the production but to a lesser extent. It is observed that the circulating levels of estrogens are relatively lower than androgens in both sexes. Although referred to as female sex hormone, estrogens do have physiologically significant role in men such as sperm maturation and development of libido, but the levels of the hormone in males are comparatively lower than in females 2. Secondary sexual characters like breast enlargement and development, thickening of the endometrial wall of the uterus and regulation of the menstrual cycle are all influenced and controlled by estrogens.
Estrogens being important at physiological levels as natural hormones are also used exogenously as medications for numerous pathogenic conditions. Exogenous synthetic estrogens are extensively used in birth control pills and also for Estrogen replacement therapy in postmenopausal women with deficient levels of the hormone. Estrogens per se or as combination with synthetic progesterone are frequently used in in different formulations that have the ease of being administered in different routes 3.
Estrogen replacement therapy (ERT) is the gold standard therapy for osteoporosis and also for the management of perimenopausal and postmenopausal estrogen deficiency in elderly women. It also finds immense applications in younger women who underwent ovariectomy, have irradiated ovaries (radiation therapy for ovarian cancer) or who are diagnosed with primary ovarian failure. Long term estrogen replacement therapy is also associated with increased incidences of hormone responsive breast cancers expressing estrogen receptors. Hence in the recent years there has been renewed interest in the search for potential alternatives to synthetic estrogens especially from dietary sources which would possess the beneficial effects of estrogens but are devoid of the side effects produced by them.
Phytoestrogens or dietary estrogens are estrogens of plant origin obtained by consuming plant based diets containing such estrogenic compounds. Generally, these phytoestrogens are nonsteroidal compounds that lack the steroid backbone, but have structural similarity with estradiol (the biologically active form of estrogen). Owing to their similarities in structure with estradiol these compounds readily bind with receptors for estrogen and exert proestrogenic or antiestrogenic effects based on target tissue and hence display tissue selectivity 4. Phytoestrogens broadly fall under four categories-isoflavones, flavonoids (of which coumestans and prenylated flavonoids are the most common), stilbenes and lignans. Of the different types of phytoestrogens the best studied are the isoflavones which occur commonly in red clover and soy. Phytoestrogens taken in the diet are absorbed readily, circulated and excreted by humans 5 . In similarity with estrogens, phytoestrogens also possess protective effects on cardiovascular, nervous system, musculoskeletal system, reproductive system and consumption of phytoestrogens have been proven to be associated with reduced risk for certain types of cancer and in the management of postmenopausal symptoms 6. Phytoestogens are explored for the potential to be used adjunct therapy for cancer. Previous reports implicate the anti-inflammatory, anti-angiogenic, anti-invasive and anti-metastatic effects of phytoestrogens on in vivo and in vitro models. Phytoestrogens are also reported to be potent inhibitors of protein tyrosine kinases7, tumour necrosis factor alpha 14 and hydroxy steroid dehydrogenases 9, 10 and also function as effective antioxidants 8, In addition, they also inhibit the expression of mRNA and activity of the enzyme aromatase 11 and have proven to be potential agonists of peroxisome proliferation activated-receptor alpha and gamma (PPAR) 12, 13.
The absence of potential side effects observed with synthetic estrogens and the antagonistic effects on estrogen receptors based on tissue selectivity opens up a promising strategy in the treatment of breast cancers/endometrial cancers in postmenopausal women. It was reported that estrogen receptors are widely distributed and present in lung cancer tissues as well as in pulmonary adenocarcinoma cell lines 15. It was also observed that among the types of estrogen receptor, the estrogen receptor type beta (ERβ) is frequently observed in lung cancer tissues and A549 cells 16. Osteopontin is a glycoprotein that regulates several cell signaling pathways controlling cancer migration, invasion and metastasis 17. Overexpression of osteopontin is reported in patients with advanced lung cancer 18. Estrogen is known to influence overexpression of osteopontin and thereby promote lung cancer cell migration via the ERβ –mediated activation of several signaling pathways majorly MEK/ERK pathway 19. These adverse effects of estrogen limit their usage in HRT for several conditions including osteoporosis. Hence in the current study it was intended to study if a selective estrogen receptor modulator and phytoestrogen like formononetin and resveratrol could have an effect in controlling cell proliferation. Many phytoestrogens have been investigated for their anticancer and antitumour properties. But reports pertaining to the antitumour and anticancer effects of phytoestrogens formononetin, and resveratrol is very scanty. Hence this study was carried out to understand (i) The cytotoxic and antiproliferative activity of the phytoestrogens formononetin and resveratrol on human pulmonary adenocarcinoma cell line A 549 and (ii) To assess the effect of phytoestrogens on DNA topology in vitro. A 549 cell line is used as the in vitro model system in the current study as it is a pulmonary adenocarcinoma cell line and could be a close representative of Non small cell lung carcinoma (NSCLC).
Materials and Methods
Procurement and maintenance of Pulmonary adenocarcinoma cell line A 549
The Non Small Cell Lung Carcinoma (NSCLC) pulmonary carcinoma cell line A-549 (adenocarcinoma) was procured from National Centre for Cell Science (NCCS), Pune, India. The A-549 cells were grown and maintained in Hams F12K medium (AL106A- Himedia, India). The media was provided with 10% Fetal Bovine Serum (FBS, RM112, Himedia, India), 1X Antibiotic and Antimycotic solution (A007, Himdia, India) for active growth of cells. The cells required standard growth conditions (95% humidity, 5% CO2 and temperature 37◦C) and were maintained in a CO2 incubator (Forma Scientific, USA) till arriving at confluency.
Preparation of stock solutions of Phytoestrogens
A 1 mg/ ml concentration of the test compounds (stock solutions of Formononetin and Resveratrol) was prepared in Dimethyl sulphoxide (DMSO). It was ensured that the final concentration of DMSO in cell culture was less than 0.1% and stored at -20°C. The stock solution was appropriately diluted to get different concentrations of formononetin and resveratrol which were used for different assays.
Assessment of antiproliferative effects of the phytoestrogens on human pulmonary adenocarcinoma cells (Determination of Ic 50 by MTT assay)
MTT assay was performed as per the method of Mosmann, 1983 20. Briefly the cells (Human pulmonary adenocarcinoma cells A 549) were plated on to different wells of a 96 well microtiter plate and then added with various concentrations of Formononetin and Resveratrol and were grown for 48 hours. After 48 hours, the media was removed, washed with PBS twice and 30µl of MTT (5mg|ml) was added to different wells. This is followed by incubation of plates for two hours. After the incubation period, the insoluble formazan crystals were dissolved in 70µl of DMSO and the color intensity was read at 570 nm using a plate reader (Biorad, India). The concentration of the phytoestrogens which induced the death of 50% of the carcinoma cells was then calculated and the Ic 50 values were determined.
LDH cytotoxicity assay
The release of lactate dehydrogenase from treated and control cells was performed by using LDH–cytotoxicity colorimetric Assay kit (Biovision, catalog no.K313-500) as per the manufacturer’s recommendations. After the treatment of cells, the clear medium (10 μl/ well) from treated, control and high control groups was transferred into a clear 96-well plate. LDH reaction mixture (100 μl) was added to each well, and after that plates were incubated for 30 minutes at room temp. The reaction was stopped by adding 10 μl stop solution to each well. The red color intensity representing the LDH activity was measured at the absorbance of 450nm using a microplate reader.
Cytomorphology analysis- Phase contrast microscopy
Following the treatment of A 549 cells with the phytoestrogens, cytomorphology analysis was performed by observing the treated and untreated cells using an inverted microscope (Olympus, Japan). The cells were observed for changes like rounding of the cells, detachment from the substratum, floating of the cells, membrane blebbing and shrinkage. The change in morphology of the formononetin and resveratrol treated cells, if any, as compared to the untreated control cells were noted and photomicrographs were taken using a 12 megapixel digital camera (Sony, Japan) attached to the microscope.
DNA Topology assay to detect the proapoptotic effects of the phytoestrogens
The DNA topology assay involves the detection of topological change induced in DNA by agarose gel electrophoresis. The test was performed based on the methods described by Rauko et al 1994, Kogan et al 2000 and Cepak et al 2001 21,22, 23. Briefly, 20μl of the reaction mixture was adjusted to contain 1000ng plasmid DNA either in reaction buffer or with phytoestrogen combinations. DNA single stranded breaks were detected qualitatively by observing the conversion of super coiled DNA to circular DNA (relaxed). Topological changes of DNA molecules could be monitored by changes in the electrophoretic mobility of DNA. The changes were analyzed by performing agarose gel electrophoresis (1.5% agarose, 60min/60v) and visualizing the DNA under a UV-transilluminator. The changes observed in the different treatment groups were noted and the results analyzed.
DPPH assay for free radical scavenging activity
DPPH assay was performed following the method of Blois 1958 24. 2,2-diphenyl-1-picryl-hydrazyl-hydrate (DPPH) is a free radical, stable at room temperature which produces violet colour with ethanol. In the presence of a free radical scavenger (antioxidant), the violet colour is converted to yellow, the intensity of which gives a measure of the free radical scavenging potential of test compounds. Briefly, 0.5 mL of test sample, 3 mL of absolute ethanol and 0.3 mL of DPPH radical solution 0.5 mM in ethanol were mixed. The reaction between DPPH and antioxidants result in reduction of the compound owing to donation of hydrogen atom by the antioxidant compound. The colour change, if any (from deep violet to light yellow) was noted and measured at 517 nm after 100 min. Blank comprised 3.3 mL of ethanol and 0.5ml of sample and the control solution contained 3.5ml ethanol and 0.3ml of DPPH radical solution. The scavenging activity of the test compounds expressed in percentage was calculated by applying the formula
Statistical analysis
Statistical analysis was performed by carrying out Student’s t-test. Values were expressed as mean± SD. Experiments were conducted in triplicates on a minimum of three occasions and the replicates were considered for calculating mean. Comparisons were made between the control and test groups.
Results and Discussion
Formononetin, and resveratrol are estrogenic compounds produced as a defensive response by many plants to fight against pathogens. Having structural similarity with endogenous estrogens, these compounds can bind to estrogen receptors and influence the downstream signaling events controlling many physiological processes in humans. The current investigation aims at understanding the antiproliferative efficacy of two phytoestrogens formononetin and resveratrol against A 549 cells .
Influence of formononetin and resveratrol on the growth and viability of human pulmonary adenocarcinoma cell line A549 – MTT assay and LDH leakage assay for cytotoxicity.
As the compounds formononetin and resveratrol indicated strong antitumour properties in disc based bioassays in our preliminary experiments (data not shown) it was desirable to investigate the antiproliferative effects of these compounds on human carcinoma cell lines. This would help to confirm the results obtained with disc bioassays and also would provide an idea about the effectiveness of the compounds against human cancers. Figure 1a and 1b implicate the results of formononetin and resveratrol respectively on cell growth as assessed by MTT assay. In tandem, with the other assays, MTT assay on A 549 gave a clear picture about the antiproliferative properties of the compounds. The antiproliferative effects of formononetin was found to be superior to that observed with resverstrol. These changes observed in the treated cells were observed to be significant in comparison to the control cells. At a concentration of 750µg/ml of formononetin more than 50% inhibition of growth of A 549 cells were observed. This was in agreement with the earlier report pertaining to the antitumour properties of formononetin 25. The results of the MTT assay was in tandem with the results of LDH leakage test in which increase in cytotoxicity and growth inhibition was observed both with 500 and 750 µg/ml of formononetin and resveratrol on A 549 cells. LDH is a cytosolic enzyme which leaks in to the culture medium in the event of altered membrane permeability and integrity caused due to stress stimuli 26. Several reports have indicated increased leakage of LDH in the event of cytotoxicity and membrane damage induced by anticancer agents 27, 28, 29. In tandem with these reports, in the current study also increased leakage of LDH was observed in the cell culture supernatant of the formononetin and resveratrol treated group of cells as compared to untreated control cell. The results of LDH assay was in complete agreement with the results of MTT assay and confirmed the growth inhibitory properties of formononetin and resveratrol on pulmonary adenocarcinoma cell line A549.
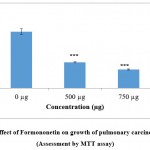 |
Figure 1(a): Effect of Formononetin on growth of pulmonary carcinoma cells A549 (Assessment by MTT assay). |
Figure indicates the influence of Formononetin on cell growth (MTT assay).Values were represented as mean ± SD (n = 6). The untreated control group was compared with the phytoestrogen treated groups. *** p <0.001.
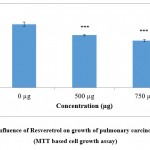 |
Figure 1(b): Influence of Resveretrol on growth of pulmonary carcinoma cells A549 (MTT based cell growth assay). |
Figure exhibit the efficacy of Resveratrol on cell growth (MTT assay).Values were expressed as mean ± SD (n = 6). The control group not treated with any drug is compared with the resveratrol treated group of cells *** p <0.001.
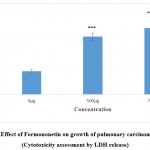 |
Figure 1(c): Effect of Formononetin on growth of pulmonary carcinoma cells A549 (Cytotoxicity assessment by LDH release). |
Figure indicates the influence of Formononetin on cytotoxicity and cell integrity (LDH leakage assay).Values were represented as mean ± SD (n = 6). The untreated control group was compared with the phytoestrogen treated groups. *** p <0.001.
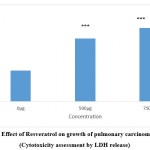 |
Figure 1(d): Effect of Resveratrol on growth of pulmonary carcinoma cells A549 (Cytotoxicity assessment by LDH release). |
Figure indicates the influence of Resveratrol on cytotoxicity and cell integrity (LDH leakage assay).Values were represented as mean ± SD (n = 6). The untreated control group was compared with the phytoestrogen treated groups. *** p <0.001.
Impact of formononetin and resveratrol treatment cytomorphology – Phase contrast microscopy analysis
Figure 2 shows the morphology of control A 549 cells and treated A 549 cells (with formononetin and resveratrol at a concentration of 500 and 750µg/ml respectively). In agreement with MTT assay typical cytotoxic changes like presence of floating cells, cell shrinkage, detachment of the cells from the substratum and rounding of the cells were noted in the formononetin and resveratrol treated cells. The cytotoxic effects observed was more pronounced in formononetin treated cells when compared with reseveratrol treated A 549 cells.
Antioxidant effects of formononetin and resveratrol
In the recent times, there is a renewed interest in research on antioxidants, especially those which have the potency to prevent the harmful effects of free radicals and inhibit fat deterioration in human body. Natural antioxidants are a preferred source for the induction of these effects as compared to synthetic antioxidants 30. The fact that formononetin and resveratrol exhibited strong antitumour properties stimulated the interest to understand the antioxidant activity of the compounds.
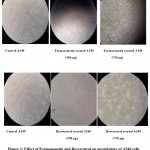 |
Figure 2: Effect of Formononetin and Resveratrol on morphology of A549 cells (Phase contrast microscopy studies-Magnification 40X). |
Figure illustrates that control cells show the typical epithelial morphology of the A 549 cells exhibiting confluent growth. Cells treated with formononetin and resveratrol at a concentration of 300 and 750µg/ml showed rounded appearance decreased cell numbers, signs of stress all indications of toxicity inflicted on pulmonary carcinoma cells.
Figure 3.a and 3.b indicate the results of DPPH assay carried out with different concentrations of the compounds. It was observed from the results of DPPH assay that a strong inhibition in the production of free radicals was observed with both the compounds (formononetin and resveratrol) as compared to control. Being effective quenchers of free radicals, these compounds might neutralize the effect of free radicals, enhance the antioxidative defenses and thereby can remove the altered cells (transformed tumor cells) from the body. The observed antioxidant effects could strongly contribute to the antitumour effects of the compounds as antioxidants are referred to as effective chemopreventive and anticarcinogenic agents. The antitumour and antiproliferative effects of the compounds could be attributed to the strong antioxidative capacities of the compounds.
Effect of formononetin and resveratrol on DNA topology
The ability of test compounds to induce or protect against DNA breaks could be assessed by DNA topology assay. Briefly, compounds that induces break in the DNA alters the topology of the DNA culminating in change from supercoiled form of DNA into linear form. Compounds that can induce single stranded breaks in the DNA could be effective in damaging the carcinoma cells. As the compounds formononetin and resveratrol exhibited strong antitumour effects, the ability of these compounds to induce nicks in the DNA was investigated. Figure 4 indicates the results of DNA topology assay. Treatment with formononetin and resveratrol induced strand breakage, resulted in the conversion of supercoiled DNA into linear DNA. This observation justifies the results obtained with other bioassays and support the antitumour properties of formononetin and resveratrol. These results were also consistent with reports obtained with other anticancer agents wherein a change in DNA topology was observed upon treatment 31. The results of the DNA topology assay has also given a clue that the observed growth inhibitory properties of the phytoestrogens could also be attributed to inhibition in the activity of topoisomerases. Few reports published are in support of this concept 32. However, further detailed investigations are needed to throw more light to understand the exact mechanisms of action involved.
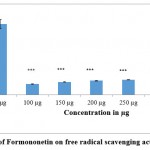 |
Figure 3(a): Effect of Formononetin on free radical scavenging activity (DPPH assay). |
Figure shows the effect of Formononetin on DPPH free radical scavenging activity. Values were represented as mean ± SD (n = 6). Control group was compared with phytoestrogen treated groups. *** p <0.001
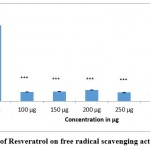 |
Figure 3(b): Effect of Resveratrol on free radical scavenging activity (DPPH assay). |
Figure depicts the scavenging activity of resveratrol on free radicals as assessed by DPPH assay. Values were shown as mean ± SD (n = 6). Comparisons were made between control Vs treated groups. *** p <0.001
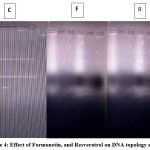 |
Figure 4: Effect of Formonetin, and Resveratrol on DNA topology assay. |
Figure shows the efficacy of Formononetin and Resveratrol on fragmentation of pBR 322 (DNA topology assay) where F represent Formononetin treated group and R represents Resveratrol treated group respectively. C represents the untreated control.
Conclusion
Formononetin, and resveratrol was found to exhibit strong growth inhibitory properties on human pulmonary adenocarcinoma cells A 549. The antiproliferative effects of formononetin was found to be superior to resveratrol. These two phytoestrogens can alter DNA topology from the supercoiled form to linear form and hence can function as effective anticancer agents. This effect observed could contribute to their antitumour and antiproliferative properties. Both the phytoestrogens taken up for investigation in the current study exhibited strong antioxidant property and resveratrol had better antioxidative properties than formononetin.
Acknowledgement
The authors thank the Chairman, Department of Microbiology and Biotechnology, Bangalore University, Jnana Bharathi Campus for the support, encouragement and for providing the infrastructure and other facilities required for this work.
Conflict of Interest
The authors declare that there is no conflict of interest involved in the manuscript.
Funding Source
The authors received no external funding support for this research work.
References
- Ryan K.J . Biochemistry of aromatase: significance to female reproductive physiology. Cancer Research, 1982; 42(8 Suppl): 3342s-3344s.
- Lombardi G, Zarrilli S, Colao A, Paesano L, Di Somma C, Rossi F, De Rosa M. Estrogens and health in males. Molecular and Cellular Endocrinology, 2001; 178(1–2): 51–5.
CrossRef - Kuhl H . Pharmacology of estrogens and progestogens: influence of different routes of administration” (PDF). Climacteric, 2005; 1 (8 Suppl): 3–63.
CrossRef - Yildiz F. Phytoestrogens in Functional Foods. Taylor & Francis Ltd.2005; pp. 3–5, 210–211.
CrossRef - Turner J.V, Agatonovic-Kustrin S, Glass B.D. Molecular aspects of phytoestrogen selective binding at estrogen receptors. Journal of Pharmaceutical Sciences, 2007; 96(8): 1879–85.
CrossRef - Rietjens I.M, Louisse J, Beekmann K (2017). The potential health effects of dietary phytoestrogens. British Journal of Pharmacology, 2017;174(11): 1263–1280.
CrossRef - Akiyama T, Ishida J, Nakagawa S, Ogawara H, Watanabe S, Itoh N, Shibuya M, Fukami Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem, 1987; 262(12):5592–5595.
CrossRef - Barnes S (2010) The biochemistry, chemistry and physiology of the isoflavones in soybeans and their food products. Lymphat Res Biol.8(1):89–98.
CrossRef - Medjakovic, S.; Jungbauer, A. (2008). Red clover isoflavonesbiochanin A and formononetin are potent ligands of the human aryl hydrocarbon receptor. The Journal of Steroid Biochemistry and Molecular Biology. 108 (1–2): 171–177.
CrossRef - Davis JN, Kucuk O, Sarkar FH (1999). Genistein inhibits NF-kappa B activation in prostate cancer cells. Nutr Cancer. 35(2):167–174.
CrossRef - Krazeisen A, Breitling R, Moller G, Adamski J (2001). Phytoestrogens inhibit human 17beta-hydroxysteroid dehydrogenase type 5. Mol Cell Endocrinol. 171(1-2):151–162.
CrossRef - Dang ZC, Audinot V, Papapoulos SE, Boutin JA, Lowik CW (2003). Peroxisome proliferator-activated receptor gamma (PPARgamma) as a molecular target for the soy phytoestrogen genistein. J Biol Chem. 278 (2):962–967.
CrossRef - Chacko BK, Chandler RT, Mundhekar A, Khoo N, Pruitt HM, Kucik DF, Parks DA, Kevil CG, Barnes S, Patel RP (2005). Revealing anti-inflammatory mechanisms of soy isoflavones by flow: modulation of leukocyte-endothelial cell interactions. Am J Physiol Heart Circ Physiol. 289(2):H908–H915.
CrossRef - Steiner C, Arnould S, Scalbert A, Manach C (2008). Isoflavones and the prevention of breast and prostate cancer: new perspectives opened by nutrigenomics. Br J Nutr. 99 E Suppl 1 ES78–108.
CrossRef - Hsu L, Chu N, Kao S. Estrogen, estrogen receptor and lung cancer. Int J Mol Sci, 2017; 18 (8): 1713.
CrossRef - Hiramitsu S, Ishikawa T, Lee W, Khan T, Crumbley C, Khwaja N, Zamanian F, Asghari A, Sen M, Zhang Y, Hawse JR, Minna JD and Umetani M. Estrogen receptor beta mediated modulation of lung cancer cell proliferation by 27 Hyrdroxy cholesterol. Front Endocrinol, 2018; 9: 470.
CrossRef - Zou X, Wang C, Liu K, Nie W, Ding Z. Prognostic significance of osteopontin expression in non-small cell lung cancer: A meta analysis. Molecular and Clinical Oncology, 2015; 26: 633-638.
CrossRef - Chang Y., Kim HJ, Chang J, Ahn CM, Kim SK, Kim SK. Elevated circulating level of osteopontin is associated with advanced disease state of non-small cell lung cancer. Lung Cancer. 2007; 57:373–380.
CrossRef - Hsu L., Liu KJ, Tsai MF, Wu CR, Feng AC, Chu NM, Kao SH. Estrogen adversely affects the prognosis of patients with lung adenocarcinoma. Cancer Sci. 2015; 106:51–59
CrossRef - Mosmann T (1983). Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. Journal of Immunological Methods; 65 (1-2): 55-63.
CrossRef - Rauko P, Novotny L, Dovinova I Hunakova L, Szekeres T, Jayaram HN (2001). Antitumour activity of benzamide riboside and its combination with cisplatin and staurosporine. Eur J Pharm Sci; 12(4): 387-394.
CrossRef - Cipak L, Miadokova E, Dingova H, Kogan G, Novotny L, Rauko P (2001). Comparative DNA protectivity and antimutagenicity studies using DNA topology and Ames assays. Toxicol In vitro; 15(6): 677-681.
CrossRef - Cipak L, Paulikova H, Novotny L, Jarosova M, Rauko P (2004). Potentiation of doxorubicin-induced apoptosis and differentiation by indomethacin in K-562 leukemia cells. Neoplasma;51(3): 188-92.
CrossRef - Blois, M.S (1958). Antioxidant determinations by the use of a stable free radical, Nature, 181: 1199-1200.
CrossRef - Tay KC, Tan LT, Chan CK, Hong SL, Chan KG, Yap WH, Pusparajah P, Lee LH and Goh BH (2019). Formononetin: A Review of its anticancer potentials and mechanisms. Front Pharmacol; 10: 820.
CrossRef - Cox CM, Mendes R, Silva F, Mendes TF, Lazo A, Halwachs K, Purkal JJ, Isidro IA, Felix A, Boghaert ER, Brito C. Application of LDH assay for therapeutic efficacy evaluation of ex vivo tumour models. Scientific Reports, 2021; 11: 18571.
CrossRef - Takahashi H, Yumoto K, Yasuhara K, Nadres ET, Kikuchi Y, Buttitta L, Taichman RS and Kuroda K. Anticancer polymers designed for killing dormant prostate cancer cells. Scientific Reports, 2019;9: 1096.
CrossRef - Zarandi PK, Mirakabadi AZ and Sootoodehnejadnematalahi. Cytotoxic and anticancer effects of ICD-85 (Venom Derived Peptides) on human breast adenocarcinoma and normal human dermal fibroblasts. Iran J Pharm Res, 2019; 18(1): 232-240.
- Wen J, Yang K, Huang J and Sun s. Recent advances in LDH-based nanosystems for cancer therapy. Materials and Design, 2021; 198: 109298.
CrossRef - Abdalla MA, Sulieman S, Muhling KH (2020). Regulation of selenium/sulfur interactions to enhance chemopreventive effects: Lessons to learn from Brassicaceae. Molecules; 25(24): 5846.
CrossRef - Buzun K, Bielawska A, Bielawski K, Gornowicz A. DNA polymerases as molecular targets for anticancer drugs. J Enzyme Inhib Med Chem, 2020; 35(1): 1781-1799.
CrossRef - Skok Z, Zidar N, Kikeli D, Ilaz J. Dual inhibitors of human DNA Topoisomerase II and other cancer related targets. J Med Chem., 2020; 63 (3): 884-904.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.






