Manuscript accepted on : 22-01-2022
Published online on: 27-01-2022
Plagiarism Check: Yes
Reviewed by: Dr. Matteo Bruno Lodi , Dr. Thaigarajan A/L Parumasivam
, Dr. Thaigarajan A/L Parumasivam
Second Review by: Dr. Mohammed Oday , Dr. Daya Shankar Gautam
, Dr. Daya Shankar Gautam
Final Approval by: Dr. Hifzur R. Siddique
Magnetic Drug Delivery System: New Hope for Cancer Patients
Shubham J. Khairnar1 , Diptanshu S. Kasar2*
, Diptanshu S. Kasar2* , Jivan G. Patil3
, Jivan G. Patil3 , Rahul S. Gayake2
, Rahul S. Gayake2 , Piyushgir S. Gosavi4
, Piyushgir S. Gosavi4 and Sonali R. Chavan1
and Sonali R. Chavan1
1Department of Pharmacology, MET’s Institute Pharmacy, Bhujbal Knowledge City, Adgaon, Nashik, Maharashtra, India, 422003.
2Department of Quality Assurance Techniques, MET’s Institute Pharmacy, Bhujbal Knowledge City, Adgaon, Nashik, Maharashtra, India, 422003.
3Department of Pharmaceutical Chemistry, MET’s Institute Pharmacy, Bhujbal Knowledge City, Adgaon, Nashik, Maharashtra, India, 422003.
4Department of Pharmaceutics, MET’s Institute Pharmacy, Bhujbal Knowledge City, Adgaon, Nashik, Maharashtra, India, 422003.
Corresponding Author E-mail: diptanshuskr.81298@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2978
ABSTRACT:
Now a day’s many types of research are carried out on drug delivery systems for early diagnosis and precise treatment with the primary aim of delivering the drug with maximum therapeutic action, fewer side effects, fast onset of action. Magnetic drug delivery can fulfil this entire requirement. Magnetic drug delivery system is defined as delivery of drug to the targeted tissue with help of magnet which may overcome many problems arises due to conventional drug delivery systems such as reticuloendothelial system clearance(RES clearance) and Target specificity. There have been very few studies on magnetic drug delivery systems, yet they can be quite helpful in treating life-threatening diseases like cancer and have very few side effects.
KEYWORDS: Cancer; Liposome; Magnetite; Magnetic Microsphere; Nanoparticles
Download this article as:| Copy the following to cite this article: Khairnar S. J, Kasar D. S, Patil J. G, Gayake R. S, Gosavi P. S, Chavan S. R. Magnetic Drug Delivery System: New Hope for Cancer Patients. Biosci Biotech Res Asia 2022;19(1). |
| Copy the following to cite this URL: Khairnar S. J, Kasar D. S, Patil J. G, Gayake R. S, Gosavi P. S, Chavan S. R. Magnetic Drug Delivery System: New Hope for Cancer Patients. Biosci Biotech Res Asia 2022;19(1). Available from: https://bit.ly/3KJDxCW |
Introduction
In conventional drug delivery system for cancer treatment anti-cancer drug given intravenously this drug may accumulate in cancerous cells which may contain a large number of leaking blood vessels, so due to accumulation of this drug it may also adversely affect on healthy tissue and show lots off side effect which is the biggest disadvantage of conventional drug delivery system. Some of the nanomedicines show high accumulation of drug on tumour site due to enhanced permeability and retention (EPR) effect it offers increases vascular permeability which is beneficial but EPR effect may vary from person to person and disease condition so it is insufficient to match challengeable and complicated tumour microenvironment. In a magnetic drug delivery system, we can manipulate a drug containing magnetic moment and block it to act on the only cancerous cell so the drug will not affect healthy tissue and these results in improvement of efficacy and reduce dosing of the drug (Fig 1). Magnetic drug delivery system can be used in the treatment of cancers, nervous system disorders, sudden sensorineural hearing loss, gene therapy, etc.
The magnetically modulated drug is also a good agent for Magnetic resonance imaging (MRI) and it gives the good advantage of diagnosis and treatment of disease by the single agent (Table 1) also used in molecular biology, cell isolation and purification, Hyperthermia, and radioimmunoassay1. Apart from advantages MDDS has several disadvantages such as high-cost technique, required specialized magnets for targeting, trained personnel required to perform drug therapy, cannot be used for cancerous cells that situated in organs deep inside the body but recent advancements are done in which magnet is implanted near the targeted area to overcome this problem2. Aim of work is to gain focus on magnetic drug delivery system, during the last few decades, magnetic drug delivery systems have been a popular approach for site-specific targeting of various pharmacological agents. It avoids the reticuloendothelial system and accurately sends the medications to the target with the help of a magnetic field.
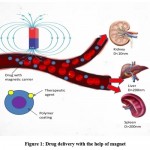 |
Figure 1: Drug delivery with the help of magnet |
History
Magnetic drug delivery system is comparatively newer technique Gilchrist in 1956 publish seminar that explains after injections of magnetism inducing particles in lymph nodes near surgically removed tumour inductive heating of lymph nodes takes place. In 1963 Meyers explain how they successfully guided and block small iron particles in dog leg veins with help of a horseshoe magnet1. In 1970 Widder, Senyi and colleagues proposed a concept of magnetic nanoparticles 2. In the year 1974 Hilal developed magnetic catheters and treat arteriovenous malformations with help of small magnets. Magnetic microsphere which in 1979 was first developed by Dr. Kenneth Widder and colleagues they developed Albumin microsphere. Magnetic drug delivery for the treatment of liver cancer was first used by Wu et al., Jones and Winter3. The first clinical trial was conducted for drugs delivered with help of a magnet in the year 1996.
Table 1: Conventional drug delivery and magnetic drug delivery difference
| Sr no. | Conventional drug delivery System | Magnetic drug delivery System |
| 1. | Slow onset of action | Fast onset of action |
| 2. | Not target specific | Target Specific |
| 3. | Accumulation of Drug may occur in healthy tissue hence show more adverse effects | No accumulation of drug in healthy tissue so have negligible side effect |
| 4. | Diagnosis and treatment by single agent not possible | Diagnosis and treatment can be possible by single agent |
| 5. | Cost is Low | Cost is high |
Various carriers of magnetic drug delivery system
Magnetic nanoparticles
Magnetic microsphere
Magnetic liposome
Magnetic microbubble
Magnetic microcapsule
Magnetic nanoparticle
Particles that have a size of less than 1 micrometer and can be manipulated using a magnetic field are magnetic nanoparticles. Magnetic nanoparticles have lots of advantages such as high stability, more carrier capacity can incorporate both hydrophilic and hydrophobic drugs and controlled release can also be possible which results in an increase in bioavailability and reduction in dosing frequency4, Magnetic nanoparticles also have a low sedimentation rate and higher tissue diffusion5. Magnetic nanoparticles did not possess any immunogenicity or virulence property6.
Magnetic material generally has a multi-domain structure but nano-sized magnetic particles have one domain structure and their magnetism property changes to paramagnetic nature7. Hence due to nano size, magnetic nanoparticles show super magnetism which allows easy targeting to cancerous cells.
Corona formation8 i.e. when nanoparticles come in contact with biological fluid they absorb proteins and lipids on their surfaces is a limitation of magnetic nanoparticles9.
Magnetic nanoparticles generally composed of
Magnetic core
Coating
Functional group on the surface by use of suitable surfactant surfaces of nanoparticle make functionalized by making them hydrophilic10. Coating is done to prevent aggregation and prevent interaction with system environment. Nanoparticles which contain metal oxides Fe2O3 and Fe3O4 mostly used(Table 2). Magnetic nanoparticles can be prepared by ionic and non ionic method 11
Table 2: Various types of nanoparticles and their compositions11
| Types | Contains |
| Metal nanoparticles | Gold(33),Silver(34), Iron, Cobalt, Nickel |
| Metal oxide nanoparticles | Iron oxides (γ-Fe2O3 and Fe3O4), ferrites (CoFe2O4 and Mn0.6Zn0.4Fe2O4) |
| Metal alloy nanoparticles | FeCo, FePt (32) |
Preparation of magnetic nanoparticles
Green synthesis of magnetic nanoparticles
Green nanotechnology is very efficient as it helps to reduce or eliminate toxic substances to restore the environment. Synthesis of nanoparticles from the plant is currently under development process. Green synthesis of a magnetic nanoparticle is a safe, non-toxic, and environment-friendly process. Inactivated plant tissue, plant exudates, plant extract, and other parts of living plant is used in green synthesis for the production of magnetic nanoparticles. The biological method can also be used for the preparation of magnetic nanoparticles in which micro-organisms, fungi, and enzymes are used but a preparation of nanoparticles by plant or plant extract is preferred over other biological processes as it eliminates the elaborate work involved in microbial culture maintenance.
Awwad A.M. and Salem N.M. recommended a method for the synthesis of magnetic nanoparticles in a single-step reaction through green synthesis. Extract of carob leaf, Ferric chloride tetrahydrate, ferric chloride hexahydrate, Sodium hydroxide used in the experiment. By this process, magnetic nanoparticles are obtained at a low temperature of about 80-85°C having 4 to 8 nm size and good mono dispersible properties.
Precipitation from solution
Precipitation of product from solution for nanoparticle preparation is one of the oldest methods. In this method metal precursors are dissolved in a solvent such as water and an insoluble solid is generated by the addition of precipitating agent. By homogeneous precipitation reaction, uniform particles are synthesized.
Co-precipitation
This is the most widely used method for the synthesis of magnetic nanoparticles. Aqueous salt solutions are used for the preparation of magnetic nanoparticles by base addition under an inert atmosphere at room temperature or high temperature. Spherical magnetic nanoparticles in solution is synthesized by two approaches: Partially oxidizing Fe(OH)₂ suspension with help of the different oxidizing agent and aging stoichiometric mixture of ferric and ferrous hydroxides in aqueous media which forms spherical homogeneous magnetic nanoparticles. Salt used, pH value, ferric and ferrous ion ratio, reaction temperature, ionic strength of media decides the size and shape of nanoparticles.
Microemulsion
It is isotropic, thermodynamically me as stable, transparent dispersion of two immiscible liquids such as oil and water stabilization is done by use of surfactant. At the water and oil interface surfactants form a monolayer, with hydrophobic tails in the oil phase and hydrophilic head dissolving in the aqueous phase. Advantages of using the micro emulsion technique are the use of simple equipment, which can be used to synthesize a large variety of materials and can control particle size and composition. Nanoparticles produced by this technique are small in size with higher saturation magnetization. The structure and type of surfactant decides the properties of nanoparticles.
Polyol method
The polyol method is used for the synthesis of uniform size nanoparticles which can be used in magnetic resonance imaging (MRI). In this method, fine metallic particles are synthesized by reducing dissolved metallic salt and precipitating metals from the solution including polyol.
Thermal decomposition of organic precursors
Samples with good size control, good crystallinity, and narrow size distribution have yielded after decomposition of iron (Fe) precursors in presence of hot organic surfactant. Thermal decomposition synthesized nanoparticles with the high level of mono dispersity.
Hydrothermal method
The solvothermal method is another name for the hydrothermal method. By this method, magnetic nanoparticles and ultrafine powders can be synthesized. This is one of the successful method to grow crystals of different material.
Chemical vapour deposition
Chemical vapour deposition is the method that can produce a wide range of materials and can take the benefits of a large database of chemistries that have been developed for this process. In this method, the particle size distribution of nanoparticles can be controlled by controlling the mixing of cold gas with hot gas carrying evaporated material.
Spray pyrolysis
In spray pyrolysis solid is prepared by spraying solution on reactors where the solvent evaporates from aerosol droplets with condensation of solute within droplet after that drying and thermolysis of the particle at high temperature is done. Maghemite nanoparticles start with Fe3+ salt and organic compound acts as reducing agent generated by most of the pyrolysis process.
Spray pyrolysis
In spray pyrolysis solid is prepared by spraying solution on reactors where the solvent evaporates from aerosol droplets with condensation of solute within droplet after that drying and thermolysis of the particle at high temperature is done. Maghemite nanoparticles start with Fe3+ salt and organic compound acts as reducing agent generated by most of the pyrolysis process.
Laser pyrolysis
It is a pyrolysis process in which laser energy is used for the synthesis of nanoparticles. This method allows rapid cooling and highly localized heating as compared to heating gases in a furnace. In this method flowing mixture of gases is heated with a carbon dioxide laser which initiates a chemical reaction.
Sonochemical reaction
Novel materials having unusual properties are synthesized by the sonochemical reaction. In sonochemical reaction, ultrasound energy is used. Nanopowder prepared by this method is generally amorphous, porous, and agglomerated12(Fig 2).
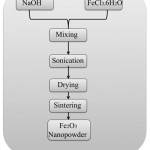 |
Figure 2: Sonochemical synthesis of iron oxide12 |
Applications of magnetic nanoparticles
Magnetic nanoparticles used as the carrier in conjugation with tetrahedral antibodies and chemotherapeutic agent13.
Antibody linked fluorescent MNP used for target imaging and treatment of GIT cancer14.
MNP can be used in hyperthermia and magnetofection.
MNP can replace fluorescent and optical labels in biosensors15.
For DNA absorption MNP coated with meso-2, 3-dimercaprosuccinic acid containing carboxylic acid group used16.
For Escherichia coli detection nanoparticles with iron oxide gold core-shell used17.
Human chorionic gonadotropin can be detected by SPR sensor chip which is the combination of MNP with antibodies.
Signal of fluorescence can be increased by nanoparticles18.
MNP can be used as the vector for gene transportation19.
MNP can be used in tumour thermotherapy as magnetic nanoparticles can produce the thermal effect in changing magnetic field6.
Microsphere
Magnetic microspheres are proteins or polymers having particle size of 1-100 micrometer and it is biodegradable20. Magnetic microspheres are small enough as can circulate through capillaries without forming embolic occlusion but can able to entrapped in micro vessels and dragged in adjacent tissues by using magnetic field21, 22.Without magnetic carrier major portion of the drug reaches to RES (Reticuloendothelial system) organs while drug with magnetic carrier all drug reaches to targeted tissue (Fig 3)23.
Magnetic microsphere can be used for the controlled release of drugs, antibodies, vaccines, hormones, etc. Lupron Depot®, Nutropin Depot® are products of polymeric microsphere22.By altering the size of the microsphere, changing drug content, altering magnetite content, changing hydration state, and changing drug release characteristics of carrier rate and amount of drug release can be controlled23.
Loaded magnetic microsphere injected in the blood vessel by using 18 or 16 number needle guided by the magnet and in a very short period, they gathered at the targeted site where they emit radiations to kill cancerous cells. There are two types of microsphere one is the therapeutic microsphere which is used for the treatment of disease and the other one is the diagnostic microsphere which is used for diagnosis e.g. Microsphere used for liver metastasis imaging, bowel loops, and other abdominal structures can distinguish by using magnetic microsphere24
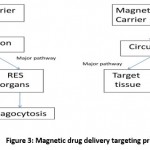 |
Figure 3: Magnetic drug delivery targeting principle23 |
Various methods of preparation are
Solvent evaporation
Solvent evaporation technique used to prepare polymer encapsulated microsphere. The auxiliary solution was prepared by the addition of drug, magnetite, and polymer in a volatile organic solvent. The resulting auxiliary solution is homogenized and stirred at a 22-30°c temperature which forms a magnetic microsphere that is separated by centrifugation and stored at 4°C25, 26.
Multiple emulsion method
In this method, w/o/w emulsion is formulated. Aqueous protein solution containing the active ingredient is dispersed in the lipophilic phase. The polymer solution is generally a continuous phase that encapsulates protein present in the dispersed aqueous phase, before adding to an aqueous solution of polyvinyl alcohol primary emulsion subjected to homogenization or sonication which forms multiple emulsion, and then solvent evaporation is done to form magnetic microsphere27.
Phase separation emulsion polymerization
The aqueous solution of polymer, drug, and magnetite is added in vegetable oil then emulsification is done by stirring heating is done at 100-150°c for stabilization of emulsion, then with the continuous stirring cross-linking agent is added which forms magnetic microsphere which is separated by washing25.
Dispersion co-polymerization
This method involves the reaction of various monomers at the interface between two immiscible liquid phases to form a film of polymer that envelopes the dispersed phase. In this method, two monomers are used one monomer dissolved in a continuous phase while the other monomer dispersed in a continuous phase. E.g. by dispersion co-polymerization of styrene and polyethylene oxide vinyl benzyl (PEO-VB)amphiphilic magnetic microsphere formed having a size range 5-100 micrometer25.
Hot melt microencapsulation
In hot melt microencapsulation initially, heating of polymer is done and then added into solid particles of the drug, this mixture suspended in an immiscible solvent, continuous stirring is done after that heating is carried out heating temperature should be 5°c more than the melting point of the polymer. Polymer particles solidified by cooling and microsphere is formed which then washed with petroleum ether26.
Microwave-assisted preparation of magnetic albumin microsphere
This method is faster than the traditional method and produces comparatively small particles. This method is mostly used for the preparation of magnetic protein microsphere28
Factors that influence properties of microsphere
Choice of solvent
The solvent should be chosen such that it can dissolve the selected polymer, more volatile and less toxic
Antifoaming agent
Foaming is one of the major problems which can disturb the formation of microspheres antifoaming agents such as dimethicone, spans are used to avoid foaming.
Surfactant
Surfactants generally stabilize emulsion by reducing surface tension and avoiding coalescence and agglomeration. Methylcellulose, tween, span, sodium dodecyl sulphate, etc. is used as a surfactant.
Application of magnetic microsphere
Magnetic microspheres used in immobilization of enzymes, isolation of cells, purification of proteins25, 29.
Mitoxantrone, paclitaxel, doxorubicin likes drugs can be incorporated in microsphere and used in cancer treatment30.
For a preclinical study to treat liver and brain tumour magnetic microsphere labelled with Rhenium-188 and Yttrium-90 used31.
Stem cell extraction and bone marrow purging possible by magnetic microsphere32.
For treatment of cancer by localized hyperthermia magnetic microsphere with paclitaxel and cisplastin used33.
Magnetic beads which are coated with streptavidin are used for the detection of bacteria34.
Magnetic Liposome
Liposomes are spherical vesicle that contains a minimum one lipid bilayer which contains cholesterol and natural non-toxic phospholipid. Liposome is advantageous as it has greater bioavailability, particle size can be adjustable, Hydrophilic, as well as hydrophobic drugs, can be incorporated, Surface modification can be possible which can help to pass biological barrier35. In the case of magnetic liposome magnetite is an additional component prepared by aentrapment of ferrofluid within the core of the liposome, which helps to guide the liposome to the targeted site with help of the magnet. Thermosensitive liposome release drug after heating by EMR1. There are two types of magnetic liposomes one which encapsulates metal-oxide ions in the aqueous layer while the other contains metal oxide enveloped in lipid layer36.
Application of magnetic liposome
Magnetic liposome use as cell or tissue site-specific accumulation drug delivery.
Magnetic liposome has specific functions in magnetic-related characteristics such as contrast agent, magnetic-targeted ability, and heating generation.
Magnetic microbubble
Magnetic microbubble is responsive to applied magnetic field changes and can be visible by ultrasonography used for magnetic drug delivery. Recently for the formulation of magnetic microbubble suspension of protamine functionalized microbubble mix with a suspension of heparinized NP41. Magnetic microbubble guided to tumours and imaging is done by ultrasonography. Focused ultrasound is used to collapse microbubble and then the drug is released37.
Magnetic Microcapsule
Magnetic microcapsule which is used in vivo and in vitro consisting of poly (allylamine hydrochloride) and poly (sodium 4-styrenesulfonate) and developed by LbL deposition10.This is the most promising drug delivery system with remote navigation by the magnetic field. Mesoporous mushroom (Agaricusbisporous) is used to prepare microcapsules called iMushbots. IMushbots show higher drug retaining properties at alkaline pH i.e. in blood while easy drug release in acidic medium (cancerous cells) 38.
Conclusion
Magnetic delivery is very useful in treating life-threatening diseases; Drug targeting is very easy with the magnetic drug delivery system which is the biggest advantage. Magnetic drug delivery is the latest technology that received attention in the 1990s.In the early 20th century Paul Ehrlich proposed the Magic bullet concept i.e. drugs reaches the right site in the body at right time at the right concentration and magnetic drug delivery systems fulfil all these objectives. It is a challenging area for researches carried out in the future so more researches, long-term toxicity study, and characterization should be done for continuous improvement in the field.
Conflict of Interest
There is no conflict of interest.
Funding Sources
There is no funding source.
References
- Koppisetti V, Sahiti B. Magnetically modulated drug delivery systems. Int J Drug Dev Res. 2011; 3(1):260-6.
- McBain SC, Yiu HH, Dobson J. Magnetic nanoparticles for gene and drug delivery. J. Nanomedicine. 2008;3(2):169.
CrossRef - Aggarwal A, Chhajer P, Maheshwari S. Magnetic drug delivery in therapeutics. J. Pharm. Sci. Res, 2012; 3(12):4670.
- Gelperina S, Kisich K, Iseman MD, Heifets L. The potential advantages of nanoparticle drug delivery systems in chemotherapy of tuberculosis. J. Respir. Crit 2005;172(12):1487-90.
CrossRef - Nitin N, LaConte LE, Zurkiya O, Hu X, Bao G. Functionalization and peptide-based delivery of magnetic nanoparticles as an intracellular MRI contrast agent. Biol. Inorg. Chem. 2004; 9(6):706-12.
CrossRef - Guo T, Lin M, Huang J, Zhou C, Tian W, Yu H, Jiang X, Ye J, Shi Y, Xiao Y, Bian X. The recent advances of magnetic nanoparticles in medicine. Nanomater.2018; 2018:1-8.
CrossRef - Blundell S. Magnetism in condensed matter. J. Phys.2003; 71(1):94-95.
CrossRef - Casals E, Puntes VF. Inorganic nanoparticle biomolecular corona: formation, evolution and biological impact. Nanomed. 2012; 7(12):1917-30.
CrossRef - Lima T, Bernfur K, Vilanova M, Cedervall T. Understanding the lipid and protein corona formation on different sized polymeric Sci.Rep.2020; 10(1):1-9.
CrossRef - Voronin DV, Sindeeva OA, Kurochkin MA, Mayorova O, Fedosov IV, Semyachkina-Glushkovskaya O, Gorin DA, Tuchin VV, Sukhorukov GB. In vitro and in vivo visualization and trapping of fluorescent magnetic microcapsules in a bloodstream.ACS Appl. Mater. Interfaces.2017; 9(8):6885-93.
CrossRef - Kalubowilage M, Janik K, Bossmann SH. Magnetic nanomaterials for magnetically-aided drug delivery and hyperthermia. Sci.2019;9(14):2927.
CrossRef - Majidi S, ZeinaliSehrig F, Farkhani SM, SoleymaniGoloujeh M, Akbarzadeh A. Current methods for synthesis of magnetic nanoparticles. Artificial. Artif Cells NanomedBiotechnol.2016; 44(2):722-34.
CrossRef - Wu M, Huang S. Magnetic nanoparticles in cancer diagnosis, drug delivery and treatmentMolClin Oncol.2017; 7(5):738-46.
CrossRef - Wang C, Bao C, Liang S, Zhang L, Fu H, Wang Y, Wang K, Li C, Deng M, Liao Q, Ni J. HAI-178 antibody-conjugated fluorescent magnetic nanoparticles for targeted imaging and simultaneous therapy of gastric cancer. Nanoscale Res.Lett.2014; 9(1):1-9.
CrossRef - Hussain C. M.: Magnetic nanomaterials for environmental analysis. In: Advanced Environmental Analysis: Applications of Nanomaterials.Royal.Society of Chemistry. 2016; pp 1-13.
CrossRef - Min JH, Woo MK, Yoon HY, Jang JW, Wu JH, Lim CS, Kim YK. Isolation of DNA using magnetic nanoparticles coated with dimercaptosuccinic acid.Biochem.2014; 447:114-8.
CrossRef - Li K, Lai Y, Zhang W, Jin L. Fe2O3@ Au core/shell nanoparticle-based electrochemical DNA biosensor for Escherichia coli detection.2011;84(3):607-13.
CrossRef - Wang Y, Dostalek J, Knoll W. Magnetic nanoparticle-enhanced biosensor based on grating-coupled surface plasmon resonance. Chem.2011; 83(16):6202-7.
CrossRef - Eslaminejad T, Noureddin Nematollahi- Mahani S, Ansari M. Glioblastoma targeted gene therapy based on pEGFP/p53-loaded superparamagnetic iron oxide nanoparticles. Gene Ther.2017; 17(1):59-69.
CrossRef - Prasanth V.V, Moy A.C, Mathew S. T,Mathapan, R.Microspheres-an overview. j. pharm.2011; 2(2):332-338.
- Pawan C, Hemchand P. Magnetic microsphere: as targeted drug delivery. Pharm. Res.2009; 2(5):964-6.
- Mathew Sam T, Devi Gayathri S, Prasanth VV, Vinod B. Suitability of factorial design in determining the processing factors affecting entrapment efficiency of albumin microspheres. Pharm. Res.2010; 3(5):1172-7.
- Kakar S, Batra D, Singh R, Nautiyal U. Magnetic microspheres as magical novel drug delivery system: A review. Acute Dis.2013; 2(1):1-2.
CrossRef - Shanthi, N. C, Gupta, R, Mahato, K. A. Traditional and emerging applications of microspheres: A review. J. Pharmtech Res.2010; 2(1):675-681.
- Vyas, Khar:Targeted and Controlled drug delivery,1st New Delhi, India: CBS Publishers & Distributors.2001; pp 1-594.
- Farah FH. Magnetic microspheres: a novel drug delivery system. J Anal Pharm Res. 2016; 3(5):00067.
CrossRef - Alagusundaram M, Chetty MS, Umashankari K, Badarinath AV, Lavanya C, Ramkanth S. Microspheres as a novel drug delivery system-a review. Int J Chem Tech Res.2009; 1(3):526-34.
- Chen CY, Long Q, Li XH, Xu J. Microwave-assisted preparation of magnetic albumin microspheres. Bioact. Compat. Polym.2008; 23(5):490-500.
CrossRef - Dynal Oslo: Molecular biology division, Cell separation and protein purification: Technical handbook. Dynalpubisher. Singapore.1996; pp.1-165.
- Burnside, B. A, Keith, A. D, Snipes W.Microporous hollow fibers as a peptide delivery system via the buccal cavity. In Int. Symp. Control. Release Bioact. Mater.1989; 16:93-94.
- Lübbe AS, Alexiou C, Bergemann C. Clinical applications of magnetic drug targeting. J Surg Res.2001; 95(2):200-206.95(2):200-6.
CrossRef - Jordan A, Scholz R, Maier-Hauff K, Johannsen M, Wust P, Nadobny J, Schirra H, Schmidt H, Deger S, Loening S, Lanksch W. Presentation of a new magnetic field therapy system for the treatment of human solid tumors with magnetic fluid hyperthermia. Magn.Magn.Mater.2001; 225 (1-2):118-26.
CrossRef - Schütt W, Grüttner C, Teller J, Westphal F, Häfeli U, Paulke B, Goetz P, Finck W. Biocompatible magnetic polymer carriers for in vivo radionuclide delivery. Organs.1999; 23(1):98-103.
CrossRef - TU SI, UKNALIS J, IRWIN P, YU LS. The use of streptavidin coated magnetic beads for detecting pathogenic bacteria by light addressable potentiometric sensor (LAPS). J RAPID METH AUT MIC.2000; 8(2):95-109.
CrossRef - TS A, Shalumon KT, Chen JP. Applications of magnetic liposomes in cancer therapies. Pharm. Des.2019; 25 (13):1490-504.
CrossRef - Ahmad M, Minhas MU, Sohail M, Faisal M, Rashid H. Comprehensive review on magnetic drug delivery systems: A novel approach for drug targeting. Journal of Pharmacy and Alternative Medicine. 2013;2(4):13-21.
- Price PM, Mahmoud WE, Al-Ghamdi AA, Bronstein LM. Magnetic drug delivery: where the field is going Chem.2018;6: 619.
CrossRef - Bhuyan T, Singh AK, Dutta D, Unal A, Ghosh SS, Bandyopadhyay D. Magnetic field guided chemotaxis of iMushbots for targeted anticancer therapeutics. ACS Biomater. Sci. Eng. ACS BIOMATER SCI ENG 2017; 3(8):1627-40.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





