Manuscript accepted on : 04-12-2021
Published online on: 09-12-2021
Plagiarism Check: Yes
Reviewed by: Dr. Rania I.M. Almoselhy
Second Review by: Dr. Sayed Hussain
Final Approval by: Dr. Fernando José Cebola Lidon
GC-MS Profiling and Antifungal Activity of Secondary Metabolite from Endophytic Fungus of Giloy
Akanksha Raj Sriwastava and Vivek Srivastava*
and Vivek Srivastava*
Department of biotechnology, Rama University Kanpur, India.
Corresponding Author E-mail: viveksrivastavabio@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2948
ABSTRACT:
The endophytic microbiota is considered to be one of the consistent and noble souce of potential and unique natural amalgams. These natural amalgams carry diverse pharmaceutical significance which the reason for their importance among research fields. The diversity of plants carries much more diversity of the endophytes as their mutual parts where both are benefited from each other. The current work deals with the isolation of the endophytic fungus from Tinospora cordifolia, for which the leaves were used after the surface sterilization, followed by the production of secondary metabolite by the endophytic isolates through submerged fermentation technique. The produced metabolite was extracted by liquid-liquid extraction technique, which was further used for evaluating its antifungal potential against Candida albicans and the obtained results show their considerable potential. The GC-MS profiling of secondary metabolite was conducted to determine the presence of some bioactive compounds in them, and as a result, some potential compounds detected are Levoglucosenone, Silanediol, Nonane, D-Allose, 5-Hydroxymethylfurfural. Since these compounds are biologically important in various aspects which gives the diversified application to the secondary metabolites. The study concludes the potential of secondary metabolites from endophytic fungus of Tinospora cordifolia and further investigation can be approached on determining the same from other plants, and also evaluating another bioactive potential of secondary metabolites.
KEYWORDS: Endophytic fungus; GC-MS analysis; Secondary metabolite; Tinospora cordifolia
Download this article as:| Copy the following to cite this article: Sriwastava A. R, Srivastava V. GC-MS Profiling and Antifungal Activity of Secondary Metabolite from Endophytic Fungus of Giloy. Biosci Biotech Res Asia 2021;18(4). |
| Copy the following to cite this URL: Sriwastava A. R, Srivastava V. GC-MS Profiling and Antifungal Activity of Secondary Metabolite from Endophytic Fungus of Giloy. Biosci Biotech Res Asia 2021;18(4). Available from: https://bit.ly/33fK5If |
Introduction
Microorganism accounts for the major source of new bioactive metabolites and up to 75% of the currently available metabolites are from their origin. The source of isolating these microbes usually refers to the soil but the pieces of evidence suggest that only a fraction of microbes can be isolated using the traditional techniques. Apart from this, less than 1% of the isolated bacteria and 5% of fungus are characterized, so there is a need to explore such alternative microbial habitats from which some novel microorganisms can be isolated, that are rich in their metabolite and functional diversity.1
The last few years have focused on collecting considerable knowledge regarding the biology and diversity of endophytic microbes2. Although they cover a large diversity the fungal endophytes are less explored3, they colonize the intercellular and intracellular spaces of tissues in plants and are known to impart no damage physically. Instead, they are the rich source of secondary metabolites4 and their mutualistic relation with their host plant benefits both in terms of survival and protection5.
A very less population of plant diversity has been evaluated for the presence of endophytic fungus and their potential secondary metabolite, and the thorough study in this area has led to the view that these endophytes could be an outstanding source of bioactive compounds with various potential biological activities. Endophytes help the host plant to tolerate biotic and abiotic stress, increase growth rate and extent of reproduction and hence improve the resistance of host medicinal plants by secretion of bioactive metabolites6. These endophytes are of biotechnological importance due to the potential nature of their secondary metabolite. The secondary metabolite from these endophytes is a diverse mixture of bioactive compounds hence are capable of carrying antimicrobial, anticancer, anti inflammatory, antimycotic, antioxidant and immunosuppressive properties.7,8,9
Tinospora cordifolia is a deciduous shrub climber that belongs to the family Menispermaceae and is commonly referred to as Giloy. The plant is very well documented in the Ayurveda and generally found on high altitudes. The plant is under prominent recognition due to its several biological importance including the properties like anti-spasmodic, anti-microbial, anti-inflammatory, anti-rheumatic, anti-diabetic, etc.10,11. The present study aims to isolate the endophytic fungus from this medicinally important plant followed by the production of the secondary metabolite from the isolated fungal endophytes. The antifungal potential of secondary metabolites is determined and the technique of GC-MS is incorporated for profiling the compounds present in secondary metabolites. Such profiling would help to determine the compounds which participate in the biological activity imparted by such metabolites. There is a need to consider some other well known and lesser-known medicinal plants to determine their endophytic biodiversity that could help to discover some potential endophytic microorganisms whose metabolite finds vivid application in present era industries.
Materials and Methods
Isolation of the Endophytic Fungus
For the isolation of endophytic fungus, fresh leaves of Tinospora cordifolia were collected and rinsed with distilled water twice. Then surface sterilization of the leaves was done using 0.1% sodium hypochlorite for 10 seconds followed by 45 seconds and 30 seconds of treatment with 70% ethanol and 30% ethanol respectively11. Then the leaves were thoroughly rinsed with sterile distilled water. Small uniform pieces of the leaves were kept on the potato dextrose agar media plate and incubated at 27ºC for 48hrs. The fungus growing near the leaf piece was transferred to the fresh media plate. Two isolates of the endophytes were selected and transferred to pure culture. The morphology of these isolates was studied via the lactophenol cotton blue staining.
Production and extraction of the secondary metabolite
For the production of secondary metabolite, the isolates were inoculated in 200ml of the sterile potato dextrose broth medium and incubated at 27ºC for 72hrs on the rotatory shaker at 130rpm. After the incubation period, the culture medium was filtered through the Whatman No. 1 filter paper and the filtrate was collected in clean capped bottles. For extraction purposes, the crude was transferred into the separating funnel and to the same 100ml, each ethyl acetate and methanol was added. The content was vigorously mixed for 10minutes and allowed to stand for the phase separation. The upper phase was saved from the separating funnel and from this complete evaporation of the solvent was achieved. The complete evaporation of the solvents leaves behind the extracted secondary metabolite in the form of a brown colored thick paste.
Evaluation of antifungal property
The antifungal activity of secondary metabolite obtained from both the fungal isolates was evaluated against Candida albicans by Agar well diffusion method. For this evaluation three different concentration of the secondary metabolite was prepared that is, 100mg/ml, 200mg/ml and 300mg/ml. The antifungal agent, fluconazole (1000ppm) was used as the positive control while methanol was taken as the negative control. After the incubation, the zone of inhibition was measured for the result evaluation.
Determination of the MIC and MFC value
Minimum inhibitory concentration for the secondary metabolite against C. albicans was determined by the broth microdilution method. The range of concentration used for the analysis was from 300mg/ml to 9.375mg/ml. The results were evaluated based on the least concentration of secondary metabolite at which no visible growth of the fungal isolate was seen. For determining the value of minimum fungicidal concentration, the aliquots from the wells showing no visible growth was inoculated over the PDA media plate and incubated. The minimum concentration of secondary metabolite at which no colony growth was seen on the solid media plate is taken as the MFC value for that secondary metabolite.
GC-MS analysis of the secondary metabolite
The GC-MS analysis was carried out for both the secondary metabolites to determine the types of compounds present in them. The GC-MS analysis was carried out in Agilent 7890B GC connected to 5977A MSD, Column-HP_5MS 5% Phenyl Methyl Silox-60ºC-325ºC, 30mx250µmx0.25µm, injector temperature 250ºC, detector temperature 280ºC, injection volume 1µl, splitless mode and oven program-as per the article given data compared with NIST MS2011 library.
Results and Discussion
Isolation of the Endophytic Fungus
The endophytic fungal isolates selected for the study showed the characteristic colony appearance on the potato dextrose agar media. The colony for Isolate 1 appeared as a white cotton-like colony with yellow spores in the center and the colony of Isolate 2 was dirty white cotton-like colonies with pale black spores at the center. The characteristic feature of these fungi was also studied by microscopy where isolate 1 showed the sporangium and hyphae close in appearance to Aspergillus spp. while isolate two showed the hyphae and scattered spores close in appearance to Trichoderma spp. These isolates were used for the production of secondary metabolite, obtained as a thick brown paste.
Evaluation of Antifungal Property, MIC and MFC value
The secondary metabolite from both isolates of endophytes showed a considerable antifungal potential against C. albicans (Figure 1). It shows the clear zone formed around the well loaded with secondary metabolite, the diameter of which is measured in mm as the zone of inhibition. The secondary metabolite from isolate 1 was showed 17mm Zone of inhibition (ZOI) and 14mm Zone of inhibition (ZOI) was obtained from isolate 2 at their concentration of 300mg/ml (Table 1). The results interpretation derived showed that the MIC value for secondary metabolite from isolate 1 and isolate 2 is150mg/ml and 300mg/ml respectively while similar MFC value (300mg/ml) was recorded for both isolates.
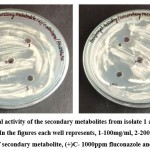 |
Figure 1: Antifungal activity of the secondary metabolites from isolate 1 and isolate 2 against Candida albicans; |
Table 1: Value of Zone of Inhibition obtained for the antifungal activity of secondary metabolite against Candida albicans
| Well code | Zone of Inhibition (mm) | |
| Secondary metabolite of isolate 1 | Secondary metabolite of isolate 2 | |
| 1-100mg/ml | Nil | Nil |
| 2- 200mg/ml | 12 | 10 |
| 3- 300mg/ml | 17 | 14 |
| (+)C- 1000ppm Fluconazole | 24 | 24 |
| (-)C- Methanol | – | – |
GC-MS analysis of the secondary metabolite
The GC-MS analysis was done for detecting compound profile of the secondary metabolites obtained from both isolates of endophytic fungus. The GC-MS derived chormatogram for the secondary metabolite samples as represented in (Figure 2 and 3), is between the time of detection of the compound versus its abundance. The profiling results shows that the metabolite is quite rich in the furfurals and their derivatives and a small proportion of the different kinds of acid including acetic acid, octadecanoic acid, oleic acid are also present. The compounds detected in both the secondary metaboltes via GC-MS analysis are represented (Table 2) and (Table 3) for the isolate 1 and 2 endophytic fungi respectively. Some of the major compounds detected in the metabolites inlcudes, Levoglucosenone, Furyl hydroxymethyl ketone, Silanediol, D-Allose, Octadecanoic acid, 5-Hydroxymethylfurfural, 4H-Pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl-, Oleic Acid etc.
 |
Figure 2: Chromatogram of GC-MS analysis of secondary metabolite obtained form the endophytic fungal isolate 1 |
 |
Figure 3: Chromatogram of GC-MS analysis of secondary metabolite obtained form the endophytic fungal isolate 2 |
Table 2: List of compounds detected in the secondary metabolite obtained from isolate 1 of endophytic fungus
| S. No. | Compound Name | Molecular formula | Retention Time (min) | #CAS |
| 1. | Acetic acid, methoxy-, anhydride | C6H10O5 | 2.610 | 19500-95-9 |
| 2. | Silanediol, dimethyl- | C2H8O2Si | 2.727 | 1066-42-8 |
| 3. | Furfural | C5H4O2 | 2.986 | 98-01-1 |
| 4. | Furfural | C5H4O2 | 3.274 | 98-01-1 |
| 5. | 2-Furancarboxaldehyde, 5-methyl- | C6H6O2 | 5.218 | 620-02-0 |
| 6. | 2,4-Dihydroxy-2,5-dimethyl-3(2H)-furan-3-one | C6H8O4 | 5.802 | 10230-62-3 |
| 7. | Arsenous acid, tris(trimethylsilyl) ester | C9H27AsO3Si3 | 7.045 | 55429-29-3 |
| 8. | Dimethyl(1-cyclopentylethoxy)silane | C9H20Osi | 7.648 | 105875-75-0 |
| 9. | Furyl hydroxymethyl ketone | C6H6O3 | 8.329 | 17678-19-2 |
| 10. | Levoglucosenone | C6H6O3 | 8.919 | 37112-31-5 |
| 11. | 5-Hydroxymethylfurfural | C6H6O3 | 9.395 | 67-47-0 |
| 12. | 5-Hydroxymethylfurfural | C6H6O3 | 9.597 | 67-47-0 |
| 13. | 4H-Pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl- | C6H8O4 | 10.200 | 28564-83-2 |
| 14. | Nonane, 4-methyl- | C10H22 | 11.608 | 17301-94-9 |
| 15. | D-(+)-Melezitose | C18H32O16 | 13.232 | 597-12-6 |
| 16. | 5-Hydroxymethylfurfural | C6H6O3 | 13.812 | 67-47-0 |
| 17. | 4H-Pyran-4-one, 5-hydroxy-2-(hydroxymethyl)- | C6H6O4 | 20.677 | 501-30-4 |
| 18. | D-Allose | C6H12O6 | 22.438 | 2595-97-3 |
| 19. | 1,6-Anhydro-α-d-galactofuranose | C6H10O5 | 25.254 | 33818-21-2 |
| 20. | Pentadecanoic acid, 14-methyl-, methyl ester | C17H34O2 | 27.938 | 5129-60-2 |
| 21. | n-Hexadecanoic acid | C16H32O2 | 29.044 | 57-10-3 |
| 22. | 2-Furancarboxaldehyde, 5-methyl- | C6H6O2 | 30.047 | 620-02-0 |
| 23. | 9,12-Octadecadienoic acid, methyl ester | C19H34O2 | 31.182 | 2462-85-3 |
| 24. | 8-Octadecenoic acid, methyl ester | C19H36O2 | 31.286 | 2345-29-1 |
| 25. | Oleic Acid | C18H34O2 | 32.336 | 112-80-1 |
| 26. | Octadecanoic acid | C18H36O2 | 32.736 | 57-11-4 |
| 27. | Cholesterol | C27H46O | 36.479 | 57-88-5 |
Table 3: List of compounds detected in the secondary metabolite obtained from isolate 2 of endophytic fungus
| S. No. | Compound Name | Molecular formula | Retention Time (min) | #CAS |
| 1. | Acetic acid, methoxy- | C3H6O3 | 2.642 | 625-45-6 |
| 2. | Silanediol, dimethyl- | C2H8O2Si | 2.718 | 1066-42-8 |
| 3. | Silanediol, dimethyl- | C2H8O2Si | 2.746 | 1066-42-8 |
| 4. | 2(5H)-Furanone | C4H4O2 | 2.930 | 497-23-4 |
| 5. | 1H-Imidazole, 2,4-dimethyl- | C5H8N2 | 2.967 | 930-62-1 |
| 6. | Furfural | C5H4O2 | 3.424 | 98-01-1 |
| 7. | 2-Furancarboxaldehyde, 5-methyl- | C6H6O2 | 5.472 | 620-02-0 |
| 8. | 2,4-Dihydroxy-2,5-dimethyl-3(2H)-furan-3-one | C6H8O4 | 5.971 | 10230-62-3 |
| 9. | Benzonitrile, m-phenethyl- | C15H13N | 7.243 | 34176-91-5 |
| 10. | 2-(2-(2-Butoxyethoxy)ethoxy)acetic acid | C10H20O5 | 7.817 | 75427-76-8 |
| 11. | 1-Propanone, 1-(2-furanyl)- | C7H8O2 | 8.575 | 3194-15-8 |
| 12. | Levoglucosenone | C6H6O3 | 9.140 | 37112-31-5 |
| 13. | 5-Hydroxymethylfurfural | C6H6O3 | 9.263 | 67-47-0 |
| 14. | 5-Hydroxymethylfurfural | C6H6O3 | 9.602 | 67-47-0 |
| 15. | 5-Hydroxymethylfurfural | C6H6O3 | 9.988 | 67-47-0 |
| 16. | 4H-Pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl- | C6H8O4 | 10.416 | 28564-83-2 |
| 17. | Octane, 2,5-dimethyl- | C10H22 | 11.820 | 15869-89-3 |
| 18. | 5-Hydroxymethylfurfural | C6H6O3 | 14.103 | 67-47-0 |
| 19. | 5-Hydroxymethylfurfural | C6H6O3 | 16.194 | 67-47-0 |
| 20. | D-Allose | C6H12O6 | 22.038 | 2595-97-3 |
| 21. | 1,6-Anhydro-α-d-galactofuranose | C6H10O5 | 25.211 | 33818-21-2 |
| 22. | Pentadecanoic acid, 14-methyl-, methyl ester | C17H34O2 | 28.112 | 5129-60-2 |
| 23. | n-Hexadecanoic acid | C16H32O2 | 29.199 | 57-10-3 |
| 24. | 2-Furancarboxaldehyde, 5-methyl- | C6H6O2 | 30.235 | 620-02-0 |
| 25. | Methyl 9-cis,11-trans-octadecadienoate | C19H34O2 | 31.351 | 2566-97-4 |
| 26. | trans-13-Octadecenoic acid, methyl ester | C19H36O2 | 31.450 | 1937-62-8 |
| 27. | Oleic Acid | C18H34O2 | 32.496 | 112-80-1 |
| 28. | Octadecanoic acid | C18H36O2 | 32.891 | 57-11-4 |
The endophytic fungi produce a wide variety of bioactive compounds. These compounds besides being involved in the host-endophyte relationship, also have potential applicability in pharmaceutical and agricultural industries. The compound, Levoglucosenone detected in the secondary metabolite of both endophytes isolates, is known to possess good antitumor activity against human hepatocarcinoma cell lines as reported by a study conducted by Giri12. The designing of protease inhibitor covers a major focus of pharmaceutical industries as the protease enzyme mediate a myriad of metabolic processes. These protease inhibitors help manage hypertension, AIDS, thrombosis13 and Cancer14and carry much other potential importance, the compound Silanediol and its derivative are reported to act as the protease inhibitor and their ability to inhibit serine protease is reported in a study conducted by Singh and Sieburth15.
Nonane is a bicyclo compound that is predominant in the essential oil, imparting its participation in the biological activity of oil, as predominantly detected in the essential oil from Vitex negundo16. A rare sugar, D-Allose has been detected in the current study, present in the secondary metabolite of endophyte isolate 2, is a biologically important monosaccharide, which can show an immunosuppressive effect17 and protective effect against liver damage18 in animals alongside it can also inhibit proliferation of cancer cell and inhibits the production of ROS in neutrophils as reported by some research studies19. The most prominent compound detected in the secondary metabolite of both endophytes isolates is 5-Hydroxymethylfurfural, the compound is a saccharide mainly synthesized by acid-catalyzed dehydration of hexose. This compound as reported by some study can be considered as a potential natural antioxidant agent carrying an application in cancer chemoprevention as it is capable of showing the antiproliferative property against human melanoma A375 cell line by inducing apoptosis and G0/G1 cell arrest20.
Conclusion
All the plants present in mother nature are the reservoir for many potential endophytes, residing as their mutual part where both get benefited from each other. The study concludes the considerable antifungal potential of secondary metabolites obtained from endophytes of Tinospora cordifolia. The study also reports that the bioactive nature of these metabolites is due to the presence of some potent compounds in them as revealed by the GC-MS profiling. Further work can be employed in the isolation of endophytes both bacteria and fungus from other parts of the same plant and other plants as well. The biological activities of secondary metabolites can be evaluated on various other parameters too.
Acknowledgement
I am very much indebted to founder of RAMA UNIVERSITY, Dr. B.S Kushwaha and higher authorities: Dr. Suraj B.S Kushwaha (Chancellor). and Dr. Janardhana Amaranath B.J (Vice-Chancellor) and Pranav Singh (Director) for their constant encouragement throughout the study of course M.Tech (Biotechnology).
I owe a debt of deepest gratitude to Dr. Ajay Kumar, Head of Department, Biotechnology and My Supervisor Dr. Vivek Srivastava, Rama university for their guidance, support, motivation and encouragement throughout the project work as well as during Academic Training.I also thanks Cytogene Research and Develpoment, Lucknow for guidance and support throughout the project. Sincerely I express my deep feelings to my friends who rendered a helping hand in the hour of need.
I am indebted to my PARENTS for their moral support and personal sacrifice to see me through this project work. I owe my soulful thank to Almighty for endowing his immense blessings that helped me towards the successful completion of my project work.
Conflict of Interest
The author declares no conflict of Interest.
Funding Sources
There is no funding source.
References
- Patil RH, Patil MP, Maheshwari VL. Bioactive secondary metabolites from endophytic fungi: a review of biotechnological production and their potential applications. Studies in natural products chemistry. 2016;49:189–205. https://doi.org/10.1016/B978-0-444-63601-0.00005-3
CrossRef - Firakova S, Sturdikova M, Muckova M. Bioactive secondary metabolites produced by microorganisms associated with plants. Biologia (Bratisl). 2007;62(3):251-257. https://doi.org/10.2478/s11756-007-0044-1
CrossRef - Perotto S, Angelini P, Bianciotto V, et al. Interactions of fungi with other organisms. Plant Biosyst. 2013;147(1):208-218. https://doi.org/10.1080/11263504.2012.753136
CrossRef - Molina G, Pimentel MR, Bertucci TC, Pastore GM. Application of fungal endophytes in biotechnological processes. Chem Eng Trans. 2012; 27.https://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.474.6613&rep=rep1&type=pdf.
- Kogel KH, Franken P, Hückelhoven R. Endophyte or parasite–what decides? Current opinion in plant biology. 2006;9(4):358–363. https://doi.org/10.1016/j.pbi.2006.05.001
CrossRef - Uzma F, Konappa NM, Chowdappa S. Diversity and extracellular enzyme activities of fungal endophytes isolated from medicinal plants of Western Ghats, Karnataka. Egypt j basic appl sci. 2016;3(4):335-342. https://doi.org/10.1016/j.ejbas.2016.08.007
CrossRef - Song X-Q, Zhang X, Han Q-J, et al. Xanthone derivatives from Aspergillus sydowii, an endophytic fungus from the liverwort Scapania ciliata S. Lac and their immunosuppressive activities. Phytochem Lett. 2013;6(3):318-321. https://doi.org/10.1016/ j.phytol. 2013.03.012
CrossRef - Sadananda TS, Govindappa M, Vinay Dutt G, Bhat B, Baishya P, Chandrappa CP. Isolation and characterization of antiviral and ribosome inactivating protein from the endophytic fungi Alternaria sp from Viscum album using MADLI-TOF-MS and their antibacterial activity. Drug Invention Today. 2014;6(2). https://www.researchgate.net/profile/ GovindappaMelappa2/publication /270103930
- Sharma D, Pramanik A, Agrawal PK. Evaluation of bioactive secondary metabolites from endophytic fungus Pestalotiopsis neglecta BAB-5510 isolated from leaves of Cupressus torulosa D.Don. 3 Biotech. 2016;6(2):210. https://doi.org/10.1007/s13205-016-0518-3
CrossRef - Sinha K, Mishra NP, Singh J, Khanuja SPS. Tinospora cordifolia (Guduchi), a reservoir plant for therapeutic applications: A Review. IJTK. 2004;3(3):257–270. http://nopr.niscair.res.in/handle/123456789/9359
- Kapoor N, Saxena S. Endophytic fungi of Tinospora cordifolia with anti-gout properties. 3 Biotech. 2018;8(6). doi:10.1007/s13205-018-1290-3. https://doi.org/10.1007/s13205-018-1290-3
CrossRef - Giri GF, Danielli M, Marinelli RA, Spanevello RA. Cytotoxic effect of levoglucosenone and related derivatives against human hepatocarcinoma cell lines. Bioorg Med Chem Lett. 2016;26(16):3955-3957. https://doi.org/10.1016/j.bmcl.2016.07.007
CrossRef - Mackman N. Triggers, targets and treatments for thrombosis. Nature. 2008;451(7181):914-918. https://doi.org/10.1038/nature06797
CrossRef - Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141(1):52-67. https://doi.org/10.1016/j.cell.2010.03.015
CrossRef - Singh S, Sieburth SM. Serine protease inhibition by a silanediol peptidomimetic. Org Lett. 2012;14(17):4422-4425. DOI: 10.1021/ol301933n
CrossRef - Pathak I, Niraula M, Thapa P. Biological and chemical studies of essential oil from Vitex negundo of Nepalese origin. J Nepal Chem Soc. 2018;39:18-24.https://www.nepjol.info/index.php/JNCS/article/view/27011/22386
CrossRef - Hossain MA, Wakabayashi H, Goda F, Kobayashi S, Maeba T, Maeta H. Effect of the immunosuppressants FK506 and D-allose on allogenic orthotopic liver transplantation in rats. Transplant Proc. 2000;32(7):2021-2023.http://pascal-francis.inist.fr/vibad /index.php?action=getRecordDetail&idt=948327
CrossRef - Hossain MA, Izuishi K, Maeta H. Protective effects of D-allose against ischemia reperfusion injury of the rat liver. J Hepatobiliary Pancreat Surg. 2003;10(3):218-225. https://doi.org/10.1007/s00534-002-0785-8
CrossRef - Sui L, Dong Y, Watanabe Y, et al. The inhibitory effect and possible mechanisms of D-allose on cancer cell proliferation. Int J Oncol. 2005;27(4):907-912.https://doi.org/10.3892/ijo.27.4.907
CrossRef - Zhao L, Chen J, Su J, et al. In vitro antioxidant and antiproliferative activities of 5-hydroxymethylfurfural. J Agric Food Chem. 2013;61(44):10604-10611.DOI: 10.1021/jf403098y
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





