Manuscript accepted on : 01-10-2021
Published online on: 12-10-2021
Plagiarism Check: Yes
Reviewed by: Dr. Nkoyo Nubila
Second Review by: Dr. Kor Yereli
Final Approval by: Dr. Muhammad Hamayun
Larvicidal Activity of Selected Plant Extracts Against the Screwworm Fly Chrysomya Albiceps
Mohammad M. Al-Jameeli
Department of Biology Sciences, Faculty of Sciences and arts, Northern Border University
Corresponding Author E-mail: dr.mo.198585@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2934
ABSTRACT:
Myiasis is a kind of parasitic disease originating from the invasion of tissues of domestic animals by dipteran larvae. Chrysomya albicebs is a type of screwworm fly spread in the tropical areas and known to cause myiasis among live human and animals leading to health problems and high economic losses to dairy producers. Management and control of this pest is needed to overcome these losses.
Nowadays, natural botanical products have been increasingly investigated as controlling agents against insects of medical and veterinary importance. This research was designed to evaluate the larvicidal effect of the total extracts of three plants, Ficus palmate, Juniperus procera and Nerium oleander against screwworm fly Chrysomya albiceps. The plants leaves were extracted with organic solvents mixture methanol : chloroform (1:1) and were tested against the second larval instar of C. albiceps using feeding and dipping methods. The extracts caused larval mortalities in the order of F. palmate> N. oleander > J. procer with IC50 values of 15.97, 33.73 and 37.24, respectively using feeding method and in the order N. oleander > F. palmate > J. procera with IC50 values of 43.12, 47.41 and 73.39, respectively using dipping method. It is concluded that the F. palmate followed by N. oleander and J. procera are candidates to use in controlling the larvae of myiasis-caused fly C. albiceps.
KEYWORDS: Botanical Extracts; Chrysomya Albiceps; Larvicidal Bioassay; Myiasis
Download this article as:| Copy the following to cite this article: Al-Jameeli M. M. Larvicidal Activity of Selected Plant Extracts Against the Screwworm Fly Chrysomya Albiceps. Biosci Biotech Res Asia 2021;18(3). |
| Copy the following to cite this URL: Al-Jameeli M. M. Larvicidal Activity of Selected Plant Extracts Against the Screwworm Fly Chrysomya Albiceps. Biosci Biotech Res Asia 2021;18(3). Available from: https://bit.ly/3BE4nYc |
Introduction
A number of Diptera species have medical importance, as they cause myiasis and act as vectors for some bacterial, viral and protozoan pathogens. Families Muscidae, Sarcophagidae, and Calliphoridae contain the major flies which are vectors to many diseases such as bacillary dysentery, trachoma virus, tuberculosis, cholera, enteric infection spoliomyelitis, typhoid fever, etc 1,2. The family Calliphoridae is commonly known as blowflies. There are medical, veterinary and forensic importance for these insects particularly in tropical regions 3. The worldwide dominant fly species Chrysomya bezziana and Chrysomya albiceps cause cutaneous myiasis. These flies are mechanically transmit myiasis and pathogens for animal4,5 as well as this fly reported that cause myiasis infection among humans live in developing countries6.
The fly pest C. albiceps spread throughout Southeast Asia, India, Africa and Arabian Peninsula 7,8. It has been reportedly seen in Jeddah9, North, Center, East, South of Kingdom of Saudi Arabia (KSA)10. Wohlfahrtia nuba (Wied), C. albiceps and C. bezziana are the major species responsible for inducing cutaneous myiasis in KSA11,12.
Myiasis-caused flies use body tissues and fluids of vertebrate as a source for food.
Myiasis is common parasitic disease that infect animals and causes economic losses in livestock production worldwide 13. Dairy farmers face severe economic losses due to myiasis disease that cause mortalities in domestic animals and lead to reduction in productivity. Before the control plan of myiasis-caused screwworm fly, the economic loss in the USA was estimated to be 140 million US dollars per year in livestock industry 14. Also the annual average loss in the sheep industry in Australia is estimated to be $280 millions 15. Drawbacks associated with wide spread use of conventional insecticides for controlling housefly populations resulted in the development of insect resistance to different insecticides, environmental pollution and negative side effects to non-target organisms including humans. Therefore, there is a need to search for safe alternatives to combat these flies such as insect growth regulators (IGR) and plant-based ingredient 16,17. This study aimed to test the larvicidal effect of three botanical extracts against screwworm fly Chrysomya albiceps using feeding and dipping methods under laboratory conditions.
Materials and Methods
Rearing of flies:
The blowfly, Chrysomya albiceps adults were collected from Jeddah slaughterhouse and transferred to insect raring laboratory, King Abdulaziz University, Kingdom of Saudi Arabia (KSA) and maintained for several generations under standard conditions, temperature of (27±2ºC), relative humidity (75±5%) and photoperiods (14h light: 10h dark). Collected insects were identified to species by a taxonomist in biological department, King Abdulaziz University using a key 18. Flies were reared according to method 19 with some modifications. Adults were reared in mesh cages (45×45×45cm) with three sides made of the wire and feed on milk powder and sucrose solution. A plastic dish containing fresh cow liver was provided inside the cages for oviposition and as a diet for the larvae. The late 3rd instar larvae were picked up and transferred into plastic pans (25 x 30 x 15 cm) containing 300cm of softwood sawdust and left to pupate and adult emergence.
Plant’s materials
Three plant species Ficus palmate, Juniperus procera and Nerium oleander were taken from AL-Baha area in the south-west of Saudi Arabia during June 2020. The plants were identified by taxonomy specialist in King Abdulaziz University. Plants leaves were rinsed with distilled water, put in the shade at room temperature until complete dryness, then grounded to a fine powder.
Preparation of plant extracts
Nearly 100 gm of the fine powder of selected plants were individually extracted by chloroform-methanol mixture (1:1) under reflux. The supernatant was filtered and evaporated under reduced pressure by rotary evaporator at temperature not exceeding 45 oC. the extraction was repeated up to three times. The dry extracts were kept in the refrigerator (4oC) till the bioassay test 20.
Testing technique
The larval bioassay was carried out as described by Singh and Kaur, 2015 21 with some modifications:
Dipping method
The second instar larvae of Chrysomya albiceps were exposed to botanical extracts under investigation at five different concentrations. The extracts were prepared by dissolving 1 g in 99.5 ml distilled water and 0.5 ml of the emulsifier triton x100 to obtain complete solubility. Series of concentrations were prepared in plastic jar (6cm x 9cm) using distilled water. the larvae were wrapped in a small cloth bag and gently immersed into extract solutions for 30 second for each concentration, whereas those of the controls were immersed in distilled water. the larvae were transferred to the rearing box containing non-treated food. The experiments were replicated five times for each concentration and the larval mortalities were recorded 24 h post treatment.
Feeding method
Larvae were transferred to jars containing treated fresh cow liver after discarding the distilled water completely. In the control experiments, distilled water only was added to the food. Larval mortality was recorded 24h after treatment. The experiments were replicated five times for each concentration. Twenty larvae were added in each duplicate. Abnormal larvae, pupae and adults were removed and placed in labeled glass vials containing 70% ethanol then photographed under a binocular microscope.
Statistical analyses
The percentage of larval mortality were corrected according to abbot’s formula 22. LC50 values (concentration which kill 50 % of the larvae) and LC90 values (concentration which kill 90 % of the larvae) were calculated by probit model by using Ldp-line program and also compute slope of the toxicity line and Chi square according to Finney 23.
Results and Discussion
The control of insect pests of medical and veterinary importance, including flies, depends mainly on the use of chemical pesticides, but the residues of these pesticides are harmful to the environment, humans and domestic animals.
For this reason, many techniques have emerged that can be used in pest control, such as the use of natural enemies like insects, viruses, bacteria and fungi, as well as the use of natural components of plant origin 24.
Many researchers have reported that some of the metabolites extracted from plants can be used to control insects and thus provide safe alternatives to synthetic chemical pesticides 25,26,27.
Alkaloids, terpenes and other plant metabolites have proven their ability to combat insect pests, and the use of these compounds have a promising future due to their desirable environmental and economic properties 28.
The effects of selected botanical extracts on larval survival and development by feeding and dipping methods are demonstrated in (table 1). The toxicity in terms of IC50 and IC90 values with the three extracts applied through the feeding and dipping methods are shown in figure (1, 2 and 3). Figure (4 & 5) compare the toxicity lines between the three extracts applied with the feeding and dipping methods. Figure 6 shows the deformations in the larvae down to the full insect stage. The mortality percentage of larvae increased as the extract concentration increased. The mortality rate in control experiments did not exceed 4%. The efficacy of the plant extracts was expressed by IC50 values. The results demonstrated that F. plamate (IC50=15.9675) was 2.3 and 2.1 times more effective than J. procera (IC50=37.2409) and N. oleander (IC50= 33.7264) Extracts (Table 1).
Table 1: The effect of selected plant extracts on the development of the second instar larvae of Chrysomya albiceps using the feeding and dipping method.
| Relative toxicity | Dipping bioassay method | Feeding bioassay method | Statical parameters |
| J. procera | |||
|
1.971
|
73.3902
65.4985 – 82.4329 |
37.2409
31.7133- 42.6185 |
IC50(ppm)
95% (F. L.) |
| 239.5213
194.5083- 317.8668 |
136.5096
113.6238- 174.4432 |
IC90 (ppm)
95% (F. L.) |
|
| 2.4948 | 2.2718 | Slope | |
| 7.81 | 7.81 | Tabulated (Chi)2 | |
| 4.366 | 5.6209 | Calculated (Chi)2 | |
| N. oleander | |||
| 1.279 | 43.1236
37.4522- 49.5311 |
33.7264
29.5912- 37.8548 |
IC50(ppm)
95% (F. L.) |
| 194.702
144.4576- 307.0039 |
113.6993
93.9919- 147.9467 |
IC90 (ppm)
95% (F. L.) |
|
| 1.9577 | 2.4282 | Slope | |
| 7.81 | 7.81 | Tabulated (Chi)2 | |
| 7.0673 | 5.99 | Calculated (Chi)2 | |
|
F. plamate |
|||
| 2.969 | 47.406
29.8556 – 77.8389 |
15.9675
4.7337 – 21.6508 |
IC50(ppm)
95% (F. L.) |
| 208.9072
181.2835 – 1032.0047 |
99.5855
78.2297- 438.618 |
IC90 (ppm)
95% (F. L.) |
|
| 1.9897 | 1.6122 | Slope | |
| 7.81 | 7.81 | Tabulated (Chi)2 | |
| 9.306 | 8.7079 | Calculated (Chi)2 | |
 |
Figure 1: Toxicity line show the relationship between J. procera extract concentrations and larval mortality percentage using feeding and dipping methods |
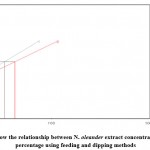 |
Figure 2: Toxicity line show the relationship between N. oleander extract concentrations and larval mortality percentage using feeding and dipping methods |
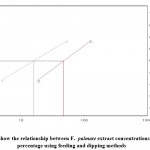 |
Figure 3: Toxicity line show the relationship between F. palmate extract concentrations and larval mortality percentage using feeding and dipping methods |
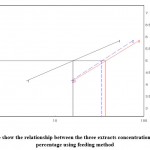 |
Figure 4: Toxicity line show the relationship between the three extracts concentrations and larval mortality percentage using feeding method |
 |
Figure 5: Toxicity line show the relationship between the three extracts concentrations and larval mortality percentage using dipping method |
Generally, the three extracts proved larvicidal effect in both the techniques, where in the feeding method, Ficus palmate extract revealed the highest larvicidal activity followed by N. oleander and J. procera extracts with IC50 values 15.97, 33.73and 37.24 ppm, respectively whereas in the dipping application method, N. oleander extract recorded the highest mortalities followed by Ficus palmate extract then J. procera extract with IC50 values 43.12, 47.41 and 73.39 ppm, respectively.
Some active ingredients of the plant have the ability to penetrate the body of the larva by direct ingestion if the feeding method is applied or enter through the skin during the application of the dipping technique. Studies have demonstrated that Four wild plants components of Artemisia herba-alba, Artemisia monosperma, Euphorbia aegyptiaca and Francoeuria crispa can penetrate the abdomen of the larva, which leads to damage to the epithelial lining, causing it to die or change its feeding behavior. In this field, histological analysis of the intestines of larvae treated with some extracts of a wild medicinal plants showed damage of the epithelial lining in the dead larvae 29.
The properties of botanical natural products make it suitable for use to combat insect pest. They affect their life cycle and alter their developmental process leading to inhibition of adults emergence. Botanical compounds such as azadirachtin, salanin and nimbin in Azadirachta indica plant exhibited insecticidal, antifeedant and insect growth inhibitor activities 30. Compounds such as azadirachtin belong to the class of tetranortriterpenoids compounds and is similar in its chemical composition to the insect growth regulator called ecdysone. This hormone affects and inhibits the developmental process of the insect 31.
Researchers have reported insecticidal effect for the tested extracts in this study against different insects, for examples J. procera extract had insecticidal effect against anopheles mosquito Anopheles arabiensis larvae 32.
Also the essential oil content of J. procera exhibited larvicidal effect against Anopheles Arabiensis with LC50= 14.4 mg/L and LC90=24.65 mg/L 33.
The extracts of the three types of Nerium oleander flower, (pink, red and white) had larvicidal effect against Anopheles stephensi, Culex quinquefasciatus and Aedes aegypti with LC50 values of 94.60, 101.21 and 121.79 mg/L, respectively [34]. Additionally, the Ficus benghalensis showed lethal effect on early second, third and fourth instar larvae of Culex quinquefasciatus, Aedes Aegypti and Anopheles stephensi by LC50 41.4, 58.2 and 74.3 ppm, 56.5, 70.3 and 80.6 ppm and 60.4, 76.4 and 89.6 ppm respectively 35.
Morphological changes of treated larvae
Deformities were observed in the dead individuals as a result of the treatment with the three plant extracts (Fig. 6). The deformities included pigmentation and the dead larvae appeared partially exuviated as well as deformities of dead adults included small sized, twisting wings, shrunk appearance (segment body contraction) in addition to poorly developed and deformed wing and legs.
 |
Figure 6: Morphological abnormalities resulting from treatment with plants extracts. |
Conclusion
The plant extracts tested in this study have proven their effectiveness in controlling the screwworm fly Chrysomya albiceps, and this makes them suitable as better alternative to the chemical insecticides used in the current control programs, which have significant collateral damage in terms of their accumulation in the food chain and the negative effects on humans, animals and the environment. In this study, the individual components of the extracts were not investigated, but the evaluation of the total extracts of plants was done. Therefore, we recommend further studies to investigate the active components present in the extracts.
Conflict of Interest
The authors declare no conflict of interest.
Funding Sources
There is no funding or financial support for this research work.
References
- Greenberg, B. (1973). Flies and Diseases. Biology and Disease Transmission, vol. 2. Princeton University, Princeton.
- Zumpt, F., (1965). Myiasis in Man and Animals in the Old World: A Textbook for Physicians, Veterinarians and Zoologists. Butterworths, London.
- Oliveira-Costa, J. (2011). Entomologia Forense quando os insetos são vestígios. Campinas: Millennium.
- Marinho, C. R.; Barbosa, L. S.; Azevedo, A. C. G.; Queiroz, M. M.C.; Valgode, M. A. and Aguiar-Coelho, V. M. (2006). Diversity of calliphoridae (Diptera) in Brazil’s tingua biological reseve. Brazilian Journal of Biology, 66(1A), 95-100.
CrossRef - Al-Ghamdi ,K.M.; Alikhan ,M.; Mahyoub, J.A.; Alanazi, N.A.; Al-Najada, A.R.; Nassar, M.I. and Alfarhan ,B. Z. (2015). Characterization of forensically important Necrophagous Flies (Diptera) of Jeddah, Saudi Arabia. Advances in Environmental Biology, 9 (8): 58–71.
- Singh A, Singh Z. (2015) Incidence of myiasis among humans – a review. Parasitol Res. 2015; 114: 3183-3199.
CrossRef - Norris, K.R. and Murray, M. D. (1964). Notes on the screw-worm fly, Chrysomya bezziana (Diptera: Calliphoridae) as a pest of cattle in New Guinea. Tech. Pap. Div Ent. CSIRO Aust., No.
- Spradbery, J. P. and Kirk, J. (1992). Incidence of Old World screw-worm fly in the United Arab Emirates, Veterinary Records, 127: 33-37.
CrossRef - Al-Shareef , L. A.H. and Almazyad , M.M.F.(2016 ). Comparative study of fly species diversity and their succession on rabbit carcasses in three different habitats in Jeddah city, Kingdom of Saudi Arabia. AJBAS, 10(18): 336-345.
CrossRef - Setyaningrum, H. and Al Dhafer, H M. (2014). The Calliphoridae the blow flies (Diptera: Oestroidea) of Kingdom of Saudi Arabia. Egyptian Academic Journal of Biological Sciences, 7(1): 49-139.
CrossRef - Badawi, A. (1994). Arthropods of medical and veterinary importance in the kingdom of Saudi Arabia. King Saud Univ. Press, Riyadh.
- Alahmed, A. M. (2001). Incidence of myiasis in sheep caused by Chrysomya bezzianain Saudi Arabia. Journal of King Saudi Univerdity, 14 (2): 109-112.
- Colebrook E and Wall R. (2004) Ectoparasites of livestock in Europe and the Mediterranean region. Vet Parasitol. 2004; 120: 251-274.
CrossRef - Peter RJ, Van den Bossche RJP, Penzhorn BL, Sharp B. (2005) Tick, fly, and mosquito control–lessons from the past, solutions for the future. Vet Parasitol. 2005; 132: 205-215.
CrossRef - Sackett D, Holmes P, Abbott K, Jephcott S, Barbaer M. (2006) Assessing the economic cost of endemic disease on the profitability of Australian beef cattle and sheep producers. A report to meat and livestock Australia. North Sydney. Austalia. 2006.
- Bisselleua, H.B.O; Gbewonyo, S.W.K and Obeng – Ofori, D. (2008). Toxicity, growth regulatory and repellent activities of medicinal plant extracts on Musca domestica L. (Diptera: Muscidae). African Jourrnal of Biotechnology 7 (24): 4637- 4642.
- Begum, N., Sharma, B. and d Pandey, R.S. (2013). Calotropis procera and Annona squamosa: Potential alternatives to chemical pesticides. British J. Appl. Sci. Technol. 3(2): 254-267.
CrossRef - Shaumar, N.F.; Mohammed, S.K. and Mohammed S.A., (1989). Keys for identification of species of family Calliphoridae (Diptera) in Egypt. Journal of Egyptian Society Parasitology, 19: 669-681.
- Singh, A. and Kaur, J. (2017). Activity of foliage extracts of Ricinus communis L.(Euphorbiaceae) against myiasis causing larvae of Chrysomya bezzianaVilleneuvae (Diptera: Calliphoridae). Journal of Veterinary Medicine and Research, 4(1): 1070.
- El-Sheikh, T. M., Al-Fifi, Z. I. (2016) Alabboud, M. A. Larvicidal and repellent effect of some Tribulus terrestris L.,(Zygophyllaceae) extracts against the dengue fever mosquito, Aedes aegypti (Diptera: Culicidae). Journal of Saudi Chemical Society, 2016; 20:13-19.
CrossRef - Singh, A. and Kaur, J. (2015). The bioefficacy of crude extracts of Azadirachta indica (Meliaceae) on the survival and development of myiasis-causing larvae of Chrysomya bezziana (Diptera: Calliphoridae).Tropical Animal Health and Production,47(7).
CrossRef - Abbot, W. S. (1925): A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 18: 265 – 26
CrossRef - Finney, D. J. (1971) Statistical Method in Biological Assay, 3 rd edn. Griffn, London.
- Lovatto, P.B., Goetze, M., and Thomé, G.C.H. (2004) Efeito de extratos de plantas silvestres da família Solanaceae sobre o controle de Brevicoryne brassicae em couve (Brassica oleracea var. acephala). Cienc. Rural 34, 971-978.
CrossRef - Koul, O., Walia, S. and Dhaliwal, G.S. (2008). Essential oils as green pesticides: Potential and Constraints. Biopest. Int. 4, 63-84.
- Dayan, F.E., Cantrell, C.L. and Duke, S.O. (2009). Natural products in crop protection. Bioorg Med Chem 17:4022–4034.
CrossRef - Bilal, H. and Hassan, S.A. (2012) Plants secondary metabolites for mosquito control. Asian Pac. J. Trop. Dis. 168-168.
CrossRef - Rattan, R. S. (2010). Mechanism of action of insecticidal secondary metabolites of plant origin. Crop. Protection 29, 913-920.
CrossRef - Abdel-Shafy, S., El-Khateeb, R.M., Soliman, M.M. and Abdel-Aziz, M.M. (2009). The efficacy of some wild medicinal plant extracts on the survival and development of third instar larvae of Chrysomya albiceps (Wied) (Diptera: Calliphoridae). Tropical Animal Health and Production, 41, 1741–1753.
CrossRef - Evans, W. C. (2009). Trease and Evans’ Pharmacognosy. (Saunders Elsevier, London), pp. 433–434.
- Mukandiwa, L., Eloff, J.N. and Naidoo, V. (2012). Evaluation of plant species used traditionally to treat myiasis for activity on the survival and development of Lucilia cuprina and Chrysomya marginalis (Diptera: Calliphoridae). Veterinary Parasitology, 190 (3–4), 566– 572.
CrossRef - Ersino W., Teressa H. and Alemayo T. (2020) Larvicidal activity of Juniperus procera extract against anopheles mosquito in in vitro, North Western Ethiopia, Vol. 14(9), pp. 445-450
CrossRef - Karunamoorthi K., Girmay, A. and Fekadu S. (2014) Larvicidal efficacy of Ethiopian ethnomedicinal plant Juniperus procera essential oil against Afrotropical malaria vector Anopheles arabiensis (Diptera: Culicidae), Asian Pac J Trop Biomed; 4(Suppl 1): S99-S106
CrossRef - Rajasingh R. Muniyasamy P., Fauzia A., Daniel R., Samuel T., Subramanian A. and Manickkam J. (2017) Bioefficacy of Nerium oleander Linnaeus (Apocynaceae) floral extracts on the larva of three vector mosquitoes of medical importance, International Journal of Mosquito Research 2017; 4(6): 65-77
- Govindarajan, M. (2010) Larvicidal efficacy of Ficus benghalensis plant leaf extracts against Culex quinquefasciatus Say, Aedes aegypti L. and Anopheles stephensi L. (Diptera: Culicidae), Eur Rev Med Pharmacol Sci, 14(2):107-11.

This work is licensed under a Creative Commons Attribution 4.0 International License.





