Manuscript accepted on : 02-08-2021
Published online on: 06-09-2021
Plagiarism Check: Yes
Reviewed by: Dr. Debajyoti Kundu
Second Review by: Dr. Okon-akan Omolabake
Final Approval by: Dr. Dr. Eugene A. Silow
Paradigm of Climate Change and its Influence on Zooplankton
Mohammad Yasir Arafat1, Yahya Bakhtiyar1*, Zahoor Ahmad Mir1 and Hamid Iqbal Tak2
1Department of Zoology, School of Biological Sciences, University of Kashmir, Hazratbal, Srinagar, 190006.
2Department of Applied Biotechnology, College of Applied Sciences, Sur, Oman
Corresponding Author E-mail: yahya.bakhtiyar@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2929
ABSTRACT:
Zooplankton are the precious elements of aquatic ecosphere playing a significant role in some ecological phenomena viz., biomonitoring, ecological indication, link between primary producers and higher trophic levels, aquaculture,and maintenance of balance in aquatic food webs.The climate,being a dynamic abiotic entity, changed many times during the history of earth particularly before and after the industrial revolution.The unending materialistic benefits of human beings have been increasing the concentration of greenhouse gases such as carbon dioxide, methane, nitrous oxide, and fluorinated gases since the last few decades that is enough to raise the global temperature. It is a fact that both biotic and abiotic factors affect the dynamics of aquatic biota due to which the aquatic ecosystems and the organisms inhabiting them such as zooplankton are becoming the worst targets of the climate change phenomenon. Some of the significant consequences of climate change posing threats for the zooplankton community include increased temperature, acidification, nutrient enrichment,and increasing ultraviolet (UV) environment of the aquatic ecosystem that significantly affect theirsurvival, behaviour, nutritional procurement, reproduction,and their overall population dynamics.Due to the profound effects of climate change on the zooplankton community, the entire aquatic food web gets crushed away leading to more severe concerns about the higher trophic levels and overall dynamics of the aquatic biota. Thus,unending loss in the dynamics of the aquatic ecosystem could prevailand will go on expanding if the causal factors of climate change continue to operate beyond their limits unless a strong scientific policy and framework in contrary to climate change are reinforced with the key focus on aquatic biota especially zooplankton.
KEYWORDS: Aquaculture; Biomonitoring; Climate Change; Food Web; Greenhouse Gases; Zooplankton
Download this article as:| Copy the following to cite this article: Arafat M. Y, Bakhtiyar Y, Mir Z. A, Tak H. I. Paradigm of Climate Change and its Influence on Zooplankton. Biosci Biotech Res Asia 2021;18(2). |
| Copy the following to cite this URL: Arafat M. Y, Bakhtiyar Y, Mir Z. A, Tak H. I. Paradigm of Climate Change and its Influence on Zooplankton. Biosci Biotech Res Asia 2021;18(2). Available from: https://bit.ly/3jMS84W |
Introduction
Weather across the entire planet over time has always varied and is still varying because of the interactions between the components in the climate system (atmosphere, oceans, ice sheets, etc.). Climate change is a long-term swing in the weather statistics such as temperature, precipitation, or wind1and is considered the major significant environmental issue for the current generation2.The amount of energy in the entire climatic system is changing due to change in energy received from the sun and the amount of greenhouse gases in the atmosphere, which in turn affect every module in the system leading to Climate change. The results of climate change are havoc ranging from melting of glaciers resulting in extreme floods upto the change in species distribution3,4. Climate is changing due to natural attributes (variations in the sun’s output and earth’s orbit around the sun, volcanic eruptions,and internal fluctuations in the climate system such as El Niño and La Niña)as well as through anthropogenic influences (e.g., industrialization and emission of the huge amount of greenhouse gases).The evidence from the “fingerprint” studies of carbon dioxide that compares the average CO2 emissions from volcanoes and human activities revealed that humans are emitting an estimated 36 billion tons of CO2 each year, 80-85% of which are from fossil fuels butvolcano emissions are only about 200 million tons per year5,therefore a clear prediction is that natural causes alone are inadequate to explain the recent observed changes in the climate.Climate warming is clear and researchers around the globe are 90% certain that mostly it is caused by the rise in greenhouse gas concentration due to anthropogenic activities viz., fossil fuel burning and deforestation6,7,8. Therefore, additional CO2 get released into the atmosphere much more rapidly than in the natural carbon cycle. With the result, there is disturbance in temperature distribution, clouds, air-currents, rainfall, evapo-transpiration, melting of polar ice-caps and risingof sea level that could adversely affect natural ecosystems like fresh water resources, agriculture and food supply, biodiversity and human health9. The global climate is constantly changing and the last decade of the 20th century up to the beginning of the 21st century observed the highest temperature record and hottest climate globally1.The studies have shown that the current climate disturbance is contributing towardsthe surface temperature of earth10, rise in sea temperature11,12, change in the flux of particulate organic matter (POC) to the sea13,14, the decline in pH15, and deoxygenation16,17. Over and out, all the above changes are bellwethers for what climate scientists forecast, will make dramatic impacts on the biosphere in decades to come18.
Zooplankton comprise of minute aquatic organisms ranging in size from a few microns to a few millimetres or more, are either non-motile or weak swimmers drifting in oceans, seas, or freshwater bodies, and are significantly associated with changes in phytoplankton community19.Zooplankton play a vital role in the aquatic food web by feeding on the phytoplankton and other members of zooplankton20 and hence they act as a major agent in the energy transfer between phytoplankton and fish21. The diversity and abundance of the zooplankton community strongly affect the biotic components of the aquatic ecosystem22.The freshwater zooplankton group includes Rotifera, Cladocera, Copepoda, and Ostracoda. Rotifers comprise microscopic, soft-bodied invertebrates, which serve as a major source of food for fishes21and also act as bioindicators23. Cladocerans are known to be the most significant herbivore in the lake plankton community24 and are dominated by filter-feeding species. Copepods act as a vital source of food for many larger invertebrates and vertebrates including zooplanktivorous fishes and prawns24 and thus encompass a major portion of the consumer biomass in aquatic habitats. Ostracods are of great interest as they are found in heavily polluted areas25 therefore, can be used as indicator species of climate and ecosystem changes26.
Since the communities in any ecosystem are structured by a combination of both intrinsic (competition, parasitism, predation, and mutualism) and extrinsic (interactions involving effects of the environment on them) interactions27,28. Both types of interactions affect the dynamic pattern of individual taxa because climate change could modify communities in unpredicted ways viz. when the effect of climate on one individual is transmitted to other species via biotic interactions like food webs and other aquatic biological phenomena like eutrophication29. Climatic change in combination with other natural factors possibly makes the ecosystems less flexible and therefore suddenly restructure the communities and cause drastic modifications to ecosystem structure and function30. Different abiotic factors (viz., availability of light, temperature, salinity, nutrients, and pH) and some biotic factors (viz., parasites, predators) are controlling elements of plankton community structure31,32. Allied to this, some meteorological attributesviz., direction and intensity of wind andthe Atlantic Multidecadal Oscillation (AMO), the North Atlantic Oscillation (NAO), and El Niño possess significant effect to alter hydrography and ocean stratification that contributesto long-term variation in the diversity and abundance of plankton33. Zooplanktonquickly react to variation in the physico-chemical and biological attributes of their environment (bioindicators of climate change), because of their poikilothermic nature, sensitiveness, short life cycle, and free-floating behaviour for their entire life, thus making them immediate victims of the climate change34,35. Therefore, the overall growthand abundance of plankton vary with respect to season, climate, and water properties, which will reveal the diversity of these important organisms within their ecosystem36,37,38,10. Hence the climate is significantly accepted as a key factor for the determination of long-term fluctuations in plankton communities, both in marine as well as limnetic ecosystems.Studies around the world predicted the three most important facets of climate change affecting the zooplankton viz., ‘temperature’39, ‘nutrient enrichment’40 and ‘acidification’41 (Figure-1 and Table-1). Thus, keeping in view of the significant importance of zooplankton and one of the worst victims of changing climate patterns, the aim of the present compiled reviewwas to highlight the significant effects of changing climatic patterns on zooplankton based on the field as well as laboratory experiments, that could pave way for the conservation and management of suchvaluable creatures of the aquatic ecosphere.
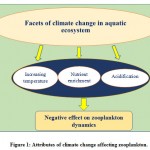 |
Figure 1: Attributes of climate change affecting zooplankton. |
Table 1: Various attributes of climate change affecting zooplankton.
| S.no | Climate change attributes | Effect on Zooplankton | Affected component of zooplankton | Reference |
| 1 | Increasing temperature in aquatic ecosystem | Behaviour | Alteration of grazing rate | Hu et al. (2018)65 |
| Disturbance in migration pattern | Jonkerset al. (2019)66 | |||
| Survival | Mortality in fourth instar or pupal stage | Hanazato and Yasuno (1989)61 | ||
| Growth | Rise in metabolic rate | (Rosenfeld et al. 2015)70 | ||
| Hypothermic stress | Khan and Khan (2008)79 | |||
| Reduction in body size | Havens et al. (2015)80 | |||
| Development | Size at maturity | Buns and Ratte (1991)74 | ||
| Reproduction | Decline in reproductive success | (Rhyne et al. (2009)84; Doan et al. (2019)85 | ||
| Decline in egg production | Ruiz et al. (2021)86 | |||
| 2 | Nutrient enrichment in aquatic ecosystem | Fitness | Egg abortion | Bednarska&Slusarczyk. (2013)124 |
| Growth and reproduction | Low ingestibility for large algal particles | Porter and McDonough. (1984)126 | ||
| Diversity | Decline in survival | Bockwoldtet al. (2017)133 | ||
| 3
|
Acidification in aquatic ecosystem | Trophic interactions | Mortality of herbivorous zooplankton density | Murphy et al. (2020)50 |
| Life span | Impairment of reproductive maturity | Havens et al. (1993)138 | ||
| Decreased egg hatching | Invidia et al. (2004)97 | |||
| Calcification process | Negative effect on shell formation in planktonic calcifiers | Fabry et al. (2008)142 | ||
| 4 | Change in ultraviolet exposure in aquatic ecosystem | Behaviour | Alteration in vertical migration of zooplankton | Balseiroet al. (2008)168 |
| Growth and fitness | Negative impact on molting process | Wolinskiet al. (2020)167 |
Mechanism of Climate Change
Due to the importance of climate change globally, several high-level meetings have been held since 1967 after Nobel laurate Swedish scientist Svante Arrhenius in 1896, first calculated the warming power of excess carbon dioxide (CO2)8 and projectedout that if human activities increase CO2 levels in the atmosphere, a warming trend would result.From 1800 to 2012, the atmospheric CO2 increased by about 40%, projected as a result of direct measurements of CO2 in the atmosphere and air trapped in ice42.According to Intergovernmental Panel on Climate Change (IPCC), anthropogenic sources of greenhouse gases are responsible for the rise in global surface temperature from 1951 to 201043. The elementary understanding of climate change is based on the heat-trapping property of greenhouse gases. The composition of greenhouse gases (carbon dioxide, methane, nitrous oxide, and water vapour) comprises a little portion of the earth’s atmosphere and form a blanket type of structure in the atmosphere that keeps the heat in the lower atmosphere, thus are valuable for keeping enough optimal warmth of the planet forthe sustenance of life. On one side, there occurs a progressive increase in the CO2 concentration and influx of greenhouse gases viz., methane, nitrous oxide that trigger global climate change and thus contributing towards change in the ecological regime of water resources, ice melting as well as altered precipitation44. On the other side in nature, a continual exchange of CO2occurs between the atmosphere, animals, and plantsvia photosynthesis, respiration, and decomposition. Allied to this, CO2 exchange also occurs between the atmosphere and oceans. Soon after theenergy from solar radiations hit the earth’s surface, a portion of it is reflected but most of it gets absorbed by oceans and land but is later on radiated back in the form of heat. The absence of huge concentration of greenhouse gases makes it possible for heat to escape to space due to which average temperature of the surface of the planet remains below freezing but some portion of this heat is redirected downwards that keep on heat near the surface of the earth. The upsurge in the normal concentration of greenhouse gasesin the atmosphere intensifies the natural greenhouse effect of the earth (forming a thickblanket) potentially raise the surface temperatures. Temperature rise, however,is not only the sole phenomenon associated with climate change but deviation in precipitation process, wind, and recurrent floods, droughts, etc. all these signify major attributes of climate change, thus disturbing the abiotic and biotic template45.Thus, global climate change possesses numerous effects on aquatic ecosystems and its bad results are predicted to expand largely in the near future46.
Effect of Climate Change Induced Increasing Temperature on Zooplankton
As different species show distinctive responses with respect to change in environmental temperature, therefore, due to climate change, unexpected costs result47,48,49. Large zooplankton (crustaceans) are known to be more sensitive to elevated temperatures than smaller taxa45.It has been shown that the top of plankton food webs are prone to climate warming and that top-down effects possess a stronger effect in shaping cascading interactions among the plankton community50. Generally,higher trophic levels show more susceptibility towards elevated temperature, because the consumer metabolic demands possess more sensitivity to warming than primary producers51. This leads to higher grazing rates and ultimately reduced consumer fitness when intake of energy by consumers cannot stay in touch with their metabolic demands52.In general,with increasing or decreasing temperatures, different rate processes in poikilotherms get altered, and thus, due to annual temperature fluctuation of a lake, the zooplankton production, their physiological life span, and generation time are greatly affected. For example,water temperature exhibited considerable upsurge in cladoceran concentrations (over 3-fold increase since 1946) and somehow declines in copepods53,54. Generally the effect of temperature on zooplankton may be direct (viz., effects on growth, development, reproduction, behaviour, and population dynamics) as well as indirect (mediated by changes in the algal community and algal food quality), all of which can affect the outcome of zooplankton interspecific interaction or competition55.
Effect on Behaviour and Survival
Zooplankton are known toquickly adapt their behaviour to changes in their environmentand as per various studies, behaviourbeing the first responseagainstdeviation in the environment, marks effect on individual fitness as well as species interactions, thus influencing the whole ecology of the ecosystem56. Generally filtering, ingestion, and respiration rates in zooplankton increase until the temperature where the maximum filtration rate is achieved57. Since temperatures near or slightly above 20oC are often reported as optimum for Cladoceran filteration58. Above the thermal optima, filtering rates decline as the temperature approaches lethality. Elevated temperature alters water viscosity and dissolved oxygen, therefore, indirectly affect zooplankton feeding which is differentially affected by water viscosity, and the water viscosity favours different species at different temperatures59. The less dissolved oxygen at higher temperatures can also diminish feeding rates, existence, and growth of the zoolplankton60. Some zooplankton species survive at temperatures as high as 30oC to 32oC55, but survival and longevity generally increase at low temperatures. Above 25oC some species of zooplankton can’t survive e.g., Chaoborus flavicans, when reared from hatching to pupation at 30oC, shows 100% mortality in the fourth instar or pupal stage61. Other poor survivors among zooplankton against elevated water temperature include Mysis relicta and Senecella calanoides62.It was shown that as the temperature increases, there is a reduction in the size of marine phytoplankton63, but phytoplankton size being an important factor in determining trophic connections64, it is likely that warming lessensthe strength of interaction between zooplankton and phytoplankton by choosing for small cell sizes that are grazed by zooplankton less efficiently.Anothersimilar kind of study revealed that a significant reduction in the grazing rates of tropical copepods was observed when there was a 6 oCrise in temperature from a coastal power plant65 while in other the alteration in migration pattern of marine zooplankton migration, thus creating an unsuitable area to live that affects the aquatic life in the oceans66.
Effect on Growth, Development, and Reproduction
Since metabolic rate influences the growth of organisms and temperature is a primary variable affecting biological activities by influencing metabolic rates67. It is also pertinent to mention that warmer temperature acts as an induction to reduce the size of planktonic species68. Thus, there occurs an inverse relationship between warming and species growth69 and also rise in metabolic rate, reduce the species growth rate70.Temperature and the rate of development in many aquatic invertebrates also possess inverse linear relation71. Since temperature and food availability are among the most vitalfeaturesthat control the abundance of freshwater zooplankton. Temperature generally controls the growth and hatching rates whereas the food availability affects the fertility of females and the survival of their offspring72. Growth rates of individuals and size at maturity are strongly affected by elevated temperature in zooplankton like rotifer73, Chaoborus74, Copepods75,and Cladocera76.In general, growth rates increase with temperature until a thermal optimum is exceeded, and then growth declines. Antagonistic to this, there is a decline in body size at maturity usually with rearing temperature even when food and other resources are not limiting77. Daphnia magna is found to possess toleration towards high temperature (25 oC)78 but the hypothermic stress (decline in body mass, decrease in body size, and loss of body water) was observed after acclimation at 27- 29 oC for one month that indicated similar kind of responses in field population due to climate change79. Also, the reduction in body size of Cladocera was observed in lakes at a latitude from 6◦ to 74◦ with the rise in temperature 80. Some researchers found during their experimental studies that, survival and reproduction in harpacticoid copepods declined when exposed to thermal stress81,82. Some Splashpool harpacticoids can withstand a wide range in temperature fluctuations during the day83, but a rise of temperature continued over time resulted in lethal and sublethal effects82. There is an obvious adverse effect of a 4 o Crise of temperature on the reproductive success in tropical copepods and an increase of 2−4 oC temperature leads to a decline in survival and reproduction 84,85. Similarly moderate to intensive heat waves result in thermal stress that resulted in a decline in survival and egg production of a copepod (Centropagesvelificatus)86.
Effect of Climate Change Induced Nutrient Enrichment on Zooplankton
The precipitation swings, rise in air temperature, and increasedconcentration of greenhouse gases (particularly CO2) are the major consequences of climaticvariations to freshwater ecosystems87. In addition to high runoff, the elevated water temperatures and more prolonged summer stratificationincreases the soil erosion and significantly cause extensive climate-related eutrophication88because increased runoff and soil erosion causes enrichment of nutrient load from nitrogen to lakes and rivers. There occurs an effect on lakes due to climatechangeinduced storms by cooling, mixing, and perhaps by destratification of the water column89. Allied to this, the water quality around the world is in constant danger due to changing climate and anthropogenic input of nutrients90. Due to the climatechangeinduced rise of net precipitation in winter and increase in extreme rainfall events,phosphorous loading of lakes takes place91. The change in the concentration and ratio of limiting nutrients i.e. Nitrogenand phosphorus in aquatic systems affectthe system productivity and composition of phytoplankton community92,93. The rise in Phosphorous inputs and elevated temperatures in freshwater ecosystems could be the causal agents of noxious cyanobacteria blooms94. In addition to many physicochemical parameters (low CO2 supply and light limitation), the changing nutrientand temperature conditions are the potential drivers for the development of blooms of cyanobacteria95,96. Thus, increased nutrients and higher water temperature leads to overgrowth of blue-green algae, resulting in harmful toxic blooms in lakes and estuaries that potentially decreasewater transparency, causing deoxygenation (hypoxia) and increased occurrence of fish kills.When the concentration of oxygen falls below species-specific thresholds, reproduction and survival of zooplankton are directly affected97,98,99. In one of the experiment, it has been found that due to hypoxia, the dominance of gelatinous zooplankton increase relative to crustacean zooplankton, cyclopoid copepod dominance increase relative to calanoidsand total abundances and biomass of zooplankton decrease100. Some other studies also revealed the upsurge in gelatinous zooplankton inhabiting eutrophic and otherwise stressed coastal waters101,102.Hypoxia possesses a negative effect on total biomass and abundance of zooplankton103,104, but some studies are contrary105,106,107, e.g. polychaete larvae show inverse relation with oxygen concentration107,108. The hypoxia can also lead to shifts in pelagic community structure temporally, thus favouring some taxa with greater tolerance towards hypoxic conditions109. In addition to this, the nutrient enrichment phenomenondue to climate disturbances reduces the ability of zooplankton to control algal blooms as it becomes very difficult and harder for them to feed and digest the blue-green algae which are very dominant in eutrophic lakes110. Since Daphnia is most sensitive to warming water and susceptible ofbeing eaten by fish and on the other side, the lake with warmer waters has higher densities of fish that eat zooplankton110. So, climate change and eutrophication reinforce the blooms to become more and more.Amongst other observable changes, increases of dissolved organic matter (DOM) resulted from the climate change111,112, has resulted in “browning” of waters in the recent past113,114,115. The rising of DOM leads to light attenuation, thus reducing photosynthetically active radiation and negatively impact primary producers of aquatic ecosystem116 that in turn could affect zooplankton species.
Effect of Climate Change Induced Eutrophic Blooms on Zooplankton
Climate change leads to an altered stratification pattern of the aquatic ecosystems that significantly impact the nutrient regime117 and availability118. It has been found that stronger stratification may lead to community shifts, thus taxa with greater ability to regulate buoyancy (such as cyanobacteria) are favoured119.Cyanobacteria are considered as insufficientnutrition for zooplankton due to less nutritional values120, inappropriatesize and shape121, andtheir significant toxicity122,123. Thus, some zooplankton species show decreasing fitness because of higher respiration rate or egg abortion due to the high abundance of large colonial or filamentous cyanobacteria124. Also, the significant effect of cyanobacteria on zooplankton is the mechanical interference of food gathering by their filaments125,126. The large algal particles could potentially reduce the filtering efficacy of zooplankton grazers and the same was analyzed experimentally wherein poor growth and reproduction were observed in zooplankton which were fed on the diets of cyanobacteria in the laboratory that could be attributed mainly due to high energetic budgetfor low ingestibility126 and insufficient nutritional makeup127. Other laboratory studies predicted the toxicity of cyanobacteria such as Anabaena flosaquae128,129and Microcystis130,131 could also be responsible fordamaging effects to Daphnia and such kind of toxic effects are expected to occur in nature also. Some field-based studies suggested that large zooplankton grazers such as Daphnia pulexselectively ingest competitive phytoplankton and thus helpin the selective growth of colonial cyanobacteria, that gets support from experimental evidence of Haney (1987)132wherein, in eutrophic lakes dominated by cyanobacteria, zooplankton graze on small particles which are 100% per day but as compared to smaller phytoplankton, the colonial cyanobacteria werepreferably not grazed rapidly. Furthermore, zooplankton grazers face deleterious effects from cyanobacteria, and some filamentous cyanobacteria e.g.,Anabaena and Oscillatoria can hinderthe filtering behaviour of Cladocerans thus retarding their growth and reproduction and it was also confirmed in the laboratory studies that the zooplankton face clear detrimental effects from nutritional deficiencies and toxins of cyanobacteria132. As per the experiments across the shallow sites in lake Champlain USA133, the negative relationship occurred between metrics of cyanobacterial density and zooplankton diversity whereas, a positive relationship occurred between phytoplankton and zooplankton diversity because, with an increase in cyanobacteria density at shallow sites, phytoplankton richness got reduced thatindirectly decrease the diversity of zooplankton by diminishing resource heterogeneity (i.e., phytoplankton richness) therefore, providing evidence in favour of the model that shows damaging effects of cyanobacteria on zooplankton diversity.
Effect of Climate Change Induced Acidification and Response of Zooplankton
Due to climate change, a rise in CO2 concentrations could be responsible for the acidification of freshwaters in crystalline and bed-rock areas that is similar to what is also predicted for the oceans134. It has been estimated that about 25 billion tons of CO2is released into the atmosphere each year135. Some of the studies focus on ocean acidification as well as carbon capture and storage activities that result from continued anthropogenic emission of CO2 and the resulting acidification due to increased CO2 absorption from the atmosphere, thus decreasing the pH of the water and subsequent alteration of ocean chemistry136,137. This increased acidity in the water bodies may also pose a deleterious effect on zooplankton communities. During the field survey and laboratory studies of various lakes in Ontario, the six common crustacean zooplankton taxa were analyzed for their acid sensitivity and were subsequently ranked wherein the ranking (from most to least sensitive) of zooplankton include, Daphnia galeatamendotae, Daphnia retrocurva, and Skistodiaptomus oregonensis > Diaphanosomabirgei >Mesocyclopsedax >Bosminalongirostris, andthe finding also predicted the widespread damage in the zooplankton community due to acidification in Ontario and North Eastern U.S. lakes 138. The conditions of decreased pH caused by different means viz., ocean acidification due to climate change and carbon capture and storage leaksare found to significantly impact foraminifera, pteropods, and copepod s139. Acidic environmentsare predicted to eliminate sensitive zooplankton species by impairing their reproductive maturity and increasing their death rates138. Significant mortality was also observed in the Acartiatonsa at pH < 6.7140, whereassome of the experimental studies showed the effect of decreased pH (due to acidification) on some zooplankton speciesviz., Acartiatonsa a copepod that showed decreasing egg hatching and life span when exposed to pH below 6.597, Calanus glacialis showed a decrease in hatching success when exposed to pH of 6.9 for a period of 9 days141and negative effects on shell formation and calcification rate in planktonic calcifiers142.Ocean acidification is considered a major threat for numerous calcifying planktonicorganisms (e.g.,pelagic gastropods; Limacina spp.)143. Acidifications cause direct mortality to herbivorous zooplankton density, thus acidification together with warmingalter the trophic interactions in planktonic community food webs from bottom and top50. Thus, overall findings also significantly predict detrimental effects of acidification of water bodies on zooplankton species in their wild habitats if the causal factors for climate change continue to remain operating.
Climate Change Induced Elevated Temperature and Change in Ultraviolet (UV) Response in Zooplankton
Due to climate warming, ultraviolet exposure has increased at mid to high latitudes144. Some important factors that play important role in UV exposure of planktonic organisms are, the timing and extent of ice coveras due to mixing of water after ice-out, the UV transparency is greater in water as compared to ice145. Climate change alters the lake thermal regime146and during recent decades, it has been observed that due to climate change the timing and extent of ice cover have changed as evident fromthe Northern Hemisphere lakes wherein the ice-out occurs 6.5 days earlier per hundred years147and in case of Arctic sea ice cover, the areal extent has also been decreasing significantly148. Due to these alterations in the extent and timing of ice cover around the globe, the thermal stratification process of lakes will change due to which there might be an altered UV exposure in lakes. It has been observed that the altered stratification can also lead to accelerated photobleaching and therefore the surface water of lakes showsincreasedUV transparency149. Some workersopined that due to stratification, the less motile plankton speciesare easily trapped in high UV surface waters. Since photo enzymatic repair (PER), a light-dependent DNA repair phenomenon provides a sort of tolerance against UV exposure in somezooplankton species but is absent or weak in some species150. Some workers somehow opine that UV: T(ultraviolet:temperature) ratios play an important role in UV tolerance in zooplankton(Williamson et al., 2002)151 and according to them, the DNA repair process will be decelerated and net DNA damage is increased during high UV:T ratios and vice versa. Therefore, the UV exposure shows its effectiveness as a function of ambient temperature which causes major threat to this important biotic community. Studies done by Williamson et al. (2002)151have shown that Daphnia catawba, Leptodiaptomusminutus, and Asplanchnagirodiwhen exposed to UV-B at four different temperatures;10, 15, 20, and 25oC, the D. catawba and L. minutus species showed increased UV tolerance at elevated temperatures that depend heavily on photoenzymatic repair (PER), but decreased UV tolerance in A.girodi, a species that has less PER. Also, theDaphnia showed a decreasein body size with increasing UV dose. Therefore, it may be concluded here that the altered thermal regimes and creation of underwater UV environment due to climate change and also the dependence of PER on temperature will result in trouble in the normal process of UV response in zooplankton.Extensive studies regarding the diverse behavioural responses of zooplankton to ultraviolet radiation (UVR) have been carried out by various workers152,153. There occur some significant adaptations of zooplankton with respect to UVR viz., avoidance of predators154, flight from UVR155,156,157, and grazing migration158,159. At times, the dissolved organic matter (DOM) in the aquatic ecosystem seemingly relieves the detrimental effects of UVR by absorbing UVR molecules160. Daphnids have compound eyes that possess the ability to recognize harmful UVR161 and the firstresponse they show towards the UVR is the movement into deeper waters162,155,163,164, thus potentially eliminating the metabolic harm that could have resulted from UVR165. Another target of increased UVR in water bodies is the moltingprocess of some zooplankton. Moltinginvolves chitobiase (chitinolytic enzyme) and the process of apoptosis (caspase-3 activity)166,167. It has been found that UVR negatively impacts moltingphenomenon in Daphnia commutataleading to its growth reduction, therefore changing its fitness and overall population dynamics167. Some reactive oxygen species are alsoproduced due to UVR that can disturb vertical migration ofzooplankton viz., Daphnia commutata168, change its pigmentation169, the integrity of DNA170, and activity of alkaline phosphatase171.
Conclusion
Though knowledge about the effect of climate change on zooplankton seems scanty but climate change pose significant effect on the zooplankton as evident from thelaboratory-cum field observations and the detrimental effects are likely to expand in near future. Since zooplankton act as a major link in the aquatic food webs and thus it may be predicted that if the causal factors of climate change continue to operate beyond their limits, then there is maximum possibility of a major shift in the aquatic ecosystem dynamics as far as its biota and stability is concerned.Various research problems taken in hand about the zooplankton dynamics must include their relationship with biotic and abiotic parameters that could provide a wide understanding of their response towards changing climate. In addition, there must be the reinforcement of the policies, which are meant to mitigate the factors responsible for climate change, with strong government support and political will, only then we can combat this war of climate change.
Acknowledgement
The authors are highly thankful to the Head, Department of Zoology, the University of Kashmir for the necessary facilities in the department. The present review paper was prepared under the framework of DST-SERB Government of Indiafunded project, with grant number EMR/2017/003669/AS (Ver-1), and the authors highly acknowledge the same.
Conflict of Interest
The authors have no conflict of interests to declare that are relevant to the content of this article.
References
- Atehmengo N., Idika I. K., Shehu A., Ibrahim R. Climate change/global warming and its impacts on parasitology/entomology. Open Parasitol. 2014;5:1-11.
CrossRef - Miranda L. E., Coppola G., Boxrucker J. Reservoir fish habitats: A perspective on coping with climate change. Fish. Sci. Aquac. 2020;28(4):478-498.
CrossRef - Sutherst R.W. Global change and human vulnerability to vector-borne diseases. Microbiol. Rev. 2004;17(1), 136-173.
CrossRef - Melillo J. M., Richmond T.C., Yohe G.W. Climate change impacts in the United States: The Third National Climate Assessment. U.S. Global Change Research Program. 2014; Available online: https://nca2014.glob- alchange.gov/downloads.
CrossRef - Climate Change Evidence and Causes. 2010; Http://royalsociety.org/WorkArea/DownloadAsset.assp?id=4294972963 [Assessed in March 2012]
- NOAA National Weather Service. Climate change. 2007; http://www.ncdc.noaa.gov/paleo/abrupt/story2.html
- Council NR. America’s Climate Choices: Panel on advancing the science of climate change; 2010;Report No: ISBN 0‐309‐14588‐
- Environmental Defense Fund (EDF). 2012, http://www.edf.org/ climate/human-activity-cause-warming# [Assesed in August 2012].
- Climate Change, 1995. Impacts, Adaptation and Mitigation of Climate change. 1996; Cambridge. Cambridge University Press England.
- Sarker, S., Yadav, A. K., Akter, M., Hossain, M. S., Chowdhury, S. R., Kabir, M. A., Sharifuzzaman, S. M. Rising temperature and marine plankton community dynamics: Is warming bad?. Complex. 2020;43:100857.
CrossRef - Purkey S. G., Johnson, G. C. Warming of global abyssal and deep Southern Ocean waters between the 1990s and 2000s: Contributions to global heat and sea level rise budgets. Clim. 2010;23(23):6336-6351.
CrossRef - Boyd, P. W., Law, C. S., Doney, S. C. A climate change atlas for the ocean. 2015;24:4.
- Ruhl H. A., Smith, K. L. Shifts in deep-sea community structure linked to climate and food supply. Science. 2004;305(5683):513-515.
CrossRef - Smith K. L., Ruhl H. A., Kahru M., Huffard C. L., Sherman A. D. Deep ocean communities impacted by changing climate over 24years in the abyssal northeast Pacific Ocean. Natl. Acad. Sci. 2013;110(49):19838-19841.
CrossRef - Byrne R. H., Mecking S., Feely R. A., Liu X. Direct observations of basin‐wide acidification of the North Pacific Ocean. Res. Lett. 2010;37(2).
CrossRef - Keeling R. F., Körtzinger A., Gruber N. Ocean deoxygenation in a warming world. Rev. Mar. Sci. 2010;2:199-229.
CrossRef - Stramma L., Prince E. D., Schmidtko S., Luo J., Hoolihan J. P., Visbeck M., Wallace D. W. R., Brandt P.,Körtzinger A. Expansion of oxygen minimum zones may reduce available habitat for tropical pelagic fishes. Clim. Change. 2012;2(1):33-37.
CrossRef - Maclean I. M., Wilson, R. J. Recent ecological responses to climate change support predictions of high extinction risk. Natl. Acad. Sci. 2011;108(30):12337-12342.
CrossRef - Perbiche-Neves G., Portinho J. L., Ferreira R. A. R., Antonia R., Gomes N. M. Increases in microcrustaceans (Cladocera and Copepoda) associated with phytoplankton peaks in tropical reservoirs. Ecol. 2016;57(3):523-532.
- Ward B. A., Dutkiewicz S., Jahn O., Follows, M. J. A size‐structured food‐web model for the global ocean. Oceanogr. 2012;57(6):1877-1891.
CrossRef - Telesh I. V. Plankton of the Baltic estuarine ecosystems with emphasis on Neva Estuary: a review of present knowledge and research perspectives. Pollut. Bull. 2004;49(3):206-219.
CrossRef - Jose R., Sanalkumar M. G. Seasonal variations in the zooplankton diversity of River Achencovil. IJSRP. 2012;2(11):1-5.
CrossRef - Shayestehfar A., Soleimani M., Mousavi S. N., Shirazi F. Ecological study of rotifers from Kor river, Fars, Iran. Environ. Biol. 2008;29(5):715-720.
- Sommer U., Sommer F. Cladocerans versus copepods: the cause of contrasting top-down controls on freshwater and marine phytoplankton. Oecologia. 2006;147(2):183-194.
CrossRef - Edmondson W. T. Freshwater Biology. Edn 2, John Wiley and Sons. Inc, London-Chapman and Hall Limited, New York, USA. 1959; pp1248.
CrossRef - Martens K., Schon I., Meisch C., Horne D. J. Global diversity of Ostracods (Ostracoda: Crustacea) in freshwater. In: Freshwater animal diversity assessment (Balian E., et al., eds). Hydrobiologia. 2008;595:185-193.
CrossRef - Menge B. A., Sutherland J. P. Species diversity gradients: synthesis of the roles of predation, competition, and temporal heterogeneity. Am. Nat. 1976;110(973):351-369.
CrossRef - Harley C. D. Abiotic stress and herbivory interact to set range limits across a two‐dimensional stress gradient. Ecology. 2003;84(6):1477-1488.
CrossRef - Carter J. L., Schindler D. E., Francis T. B. Effects of climate change on zooplankton community interactions in an Alaskan lake. Climate Change Responses. 2017;4(1), 1-12.
CrossRef - Scheffer M., Carpenter S. R. Catastrophic regime shifts in ecosystems: linking theory to observation. Trends Ecol. Evol. 2003;18(12):648-656.
CrossRef - Horne A. J., Goldman C. R. Limnology. 2nd edn. McGrawHill, New York, 1994; pp 1-576.
- Wiltshire, K. H., Boersma, M., Carstens, K., Kraberg, A. C., Peters, S., Scharfe, M. Control of phytoplankton in a shelf sea: determination of the main drivers based on the Helgoland Roads Time Series. Sea Res.2015;105:42-52.
CrossRef - Sarker, S., Lemke, P., Wiltshire, K. H. Does ecosystem variability explain phytoplankton diversity? Solving an ecological puzzle with long-term data sets. Sea Res.2018;135:11-17.
CrossRef - Richardson A. J. In hot water: zooplankton and climate change. ICES J. Mar. Sci. 2008;65(3):279-295.
CrossRef - Sahu, B. K., Pati, P., Panigrahy, R. C. Impact of climate change on marine plankton with special reference to Indian Seas. Indian J. Mar. Sci.2018;47(02):259-268.
- Rao S. N., Chabeg R., Srinivasan K. V. Ganga water quality in Bihar, India. Environ. Health. 1990;52:393-400.
- Boyd C. E., Tucker C. S. Ecology of aquaculture ponds. In: Pond aquaculture water quality management. 1998; pp 8-86.
CrossRef - Xiong W., Li J., Chen Y., Shan B., Wang W., Zhan A. Determinants of community structure of zooplankton in heavily polluted river ecosystems. Rep. 2016;6(1):1-11.
CrossRef - Roemmich D., McGowan J. Climatic warming and the decline of zooplankton in the California. Sci. 1995;267(5202):1324-1326.
CrossRef - Richardson A. J., Hobday A. J., Poloczanska E. S. Australia’s marine life. Report Card of Marine Climate Change for Australia, NCCARF Publication. 2009;05:9.
- Halder P. M., Jana P. K., Chakraborty S. K., Banerjee, S. Relative tolerance of some tropical freshwater microcrustaceans to acidification. J. Zool.2013;37(2):228-237.
- National Research Council. Climate change: evidence, impacts, and choices: PDF booklet. National Academies Press. 2012.
- Bindoff N. L. et al. Detection and attribution of climate change: from global to regional. In: Climate Change: The Physical Science Basis (Stocker, TF, et al., ed). 2013; pp 867–952.
- Danovaro R. Climate change impacts on the biota and on vulnerable habitats of the deep Mediterranean Sea. Lincei Sci. Fis. Nat. 2018;29(3):525-541.
CrossRef - Vadadi-Fülöp C., Sipkay C., Mészáros G., Hufnagel L. Climate change and freshwater zooplankton: what does it boil down to? Ecol. 2012;46(4):501-519.
CrossRef - Climate Change 2013: The Physical Science Basis. In: Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change (Stocker TF, et al. eds). Cambridge University, 2013.
- Parmesan C., Yohe G. A globally coherent fingerprint of climate change impacts across natural systems. Nature. 2003:421(6918):37-42.
CrossRef - Winder M., Schindler D. E. Climate change uncouples trophic interactions in an aquatic ecosystem. Ecology. 2004;85(8):2100-2106.
CrossRef - De SenerpontDomis L. N., et al. Plankton dynamics under different climatic conditions in space and time. Biol. 2013;58(3):463-482.
CrossRef - Murphy, G. E., Romanuk, T. N., Worm, B. Cascading effects of climate change on plankton community structure. Evol.2020;10(4):2170-2181.
CrossRef - López-Urrutia, Á., San Martin, E., Harris, R. P., Irigoien, X. Scaling the metabolic balance of the oceans. Natl. Acad. Sci. 2006;103(23):8739-8744.
CrossRef - Rall, B. C., Vucic‐Pestic, O. L. I. V. E. R. A., Ehnes, R. B., Emmerson, M., Brose, U. Temperature, predator–prey interaction strength and population stability. Change Biol. 2010;16(8):2145-2157.
CrossRef - Hampton S. E., Izmest’eva L. R., Moore M. V., Katz S. L., Dennis B., Silow E. A. Sixty years of environmental change in the world’s largest freshwater lake–Lake Baikal, Siberia. Glob. Change. Biol. 2008;14(8):1947-1958.
CrossRef - Mac Nally R., et al. Analysis of pelagic species decline in the upper San Francisco Estuary using multivariate autoregressive modelling (MAR). Appl. 2010;20(5):1417-1430.
CrossRef - Moore M. V., Folt C. F., Stemberger R. S. Consequences of elevated temperatures for zooplankton assemblages in temperate lakes. ArchivfürHydrobiologie. 1996;135(3):289-319.
CrossRef - Sih, A., Stamps, J., Yang, L. H., McElreath, R., Ramenofsky, M. Behavior as a key component of integrative biology in a human-alteredworld. Comp. Biol. 2010;50(6):934-944.
CrossRef - Mourelatos S., Lacroix G. In situ filtering rates of Cladocera: effect of body length, temperature and food concentration. Limnol. Oceanogr. 1990;35(5):1101-1111.
CrossRef - Lampert, W. Feeding and nutrition in Mem. Ist. Ital. Idrobiol. 1987;45:143-192.
- Vanderploeg, H. A. Feeding mechanisms and particle selection in suspension feeding zooplankton. In: The biology of particles in aquatic systems (Wotton, RS ed). CRC press, Boca Raton. 1990; pp 184-212.
- LaBerge S., Hann, B. J. Acute temperature and oxygen stress among genotypes of Daphnia pulex and Simocephalusvetulus (Cladocera, Daphniidae) in relation to environmental conditions. J. Zool. 1990;68(11):2257-2263.
CrossRef - Hanazato & Yasuno M. Effect of temperature in laboratory studies on growth of Chaoborusflavicans (DipteraChaoboridae).Arch. Hydrobiol. 1989;114:497–504.
- Dadswell M. J. Distribution, ecology, and postglacial dispersal of certain crustaceans and fishes in eastern North America(Doctoral dissertation, Carleton University). 1973
- Sommer, U., Peter, K. H., Genitsaris, S., Moustaka‐Gouni, M. Do marine phytoplankton follow Bergmann’s rule sensulato?. Rev. 2016;92(2):1011-1026.
CrossRef - Boyce, D. G., Frank, K. T., Leggett, W. C. From mice to elephants: overturning the ‘one size fits all’paradigm in marine plankton food chains. Lett. 2015;18(6):504-515.
CrossRef - Hu, S., Liu, S., Wang, L., Li, T., Huang, H. Feeding response of the tropical copepod Acartiaerythraea to short-term thermal stress: more animal-derived food was consumed. 2018;6:e6129.
CrossRef - Jonkers, L., Hillebrand, H., Kucera, M. Global change drives modern plankton communities away from the pre-industrial state. 2019;570(7761):372-375.
CrossRef - Brown, J. H., Gillooly, J. F., Allen, A. P., Savage, V. M., West, G. B. Toward a metabolic theory of ecology. 2004;85(7):1771-1789.
CrossRef - Atkinson, D., Ciotti, B. J., Montagnes, D. J. Protists decrease in size linearly with temperature: ca. 2.5% C− 1. R. Soc. Lond. Series B: Biological Sciences. 2003;270(1533):2605-2611.
CrossRef - Avery-Gomm, S., O’Hara, P. D., Kleine, L., Bowes, V., Wilson, L. K., Barry, K. L. Northern fulmars as biological monitors of trends of plastic pollution in the eastern North Pacific. Pollut. Bull.2012;64(9):1776-1781.
CrossRef - Rosenfeld, J., Van Leeuwen, T., Richards, J., Allen, D. Relationship between growth and standard metabolic rate: measurement artefacts and implications for habitat use and life‐history adaptation in salmonids. Animal Ecol.2015;84(1):4-20.
CrossRef - Edmondson W. T., Vinberg G. G. Manual on methods for the assessment of secondary productivity in fresh waters.B.P. Handbook No. 17, Blackwell, Oxford. 1971.
- George D. G., Hewitt D. P., Lund J. W. G., Smyl W. J. P. The relative effects of enrichment and climate change on the long‐term dynamics of Daphnia in Esthwaite Water, Cumbria. Biol. 1990;23(1), 55-70.
CrossRef - Duncan A. Assessment of factors influencing the composition, body size and turnover rate of zooplankton in Parakrama Samudra, an irrigation reservoir in Sri Lanka. Hydrobiologia. 1984;113(1):201-215.
CrossRef - Büns M., Ratte H. T. The combined effects of temperature and food consumption on body weight, egg production and developmental time in Chaoboruscrystallinus De Geer (Diptera: Chaoboridae). Oecologia. 1991;88(4):470-476.
CrossRef - Jamieson C., Burns C. The effects of temperature and food on copepodite development, growth and reproduction in three species of Boeckella (Copepoda; Calanoida). Hydrobiologia. 1988;164(3):235-257.
CrossRef - Hanazato T., Yasuno M. Effect of temperature in the laboratory studies on growth, egg development and first parturition of five species of Cladocera. Japanese J. Limnnology. (RikusuigakuZasshi). 1985;46(3):185-191.
CrossRef - Atkinson D. Temperature and organism size: a biological law for ectotherms? Ecol. Res. 1994;25:1-58.
CrossRef - Lagerspetz K. Y. Thermal avoidance and preference in Daphnia magna. Therm. Biol. 2000;25(6):405-410.
CrossRef - Khan M. A. Q., Khan M. A. Effect of temperature on waterfleaDaphnia magna (Crustacea:Cladocera). 2008;1-1. Available from Nature Precedings http://hdl.handle.net/ 10101/npre.2008.1909.1.
- Havens, K. E., Pinto-Coelho, R. M., Beklioğlu, M., Christoffersen, K. S., Jeppesen, E., Lauridsen, T. L., …. Vijverberg, J. Temperature effects on body size of freshwater crustacean zooplankton from Greenland to the tropics. 2015;743(1):27-35.
CrossRef - Koch, J., Bui, T. T., Lundström Belleza, E., Brinkmann, M., Hollert, H., Breitholtz, M. Temperature and food quantity effects on the harpacticoid copepod Nitocraspinipes: Combining in vivo bioassays with population modeling. PloS One, 2017;12(3): e0174384.
CrossRef - Siegle, M. R., Taylor, E. B., O’Connor, M. I. Prior heat accumulation reduces survival during subsequent experimental heat waves. Exp. Mar. Biol. Ecol. 2018;501:109-117.
CrossRef - Burton, R. S., Feldman, M. W., Curtsinger, J. W. Population genetics of Tigriopuscalifornicus(Copepoda: Harpacticoida): I. Population structure along the central California coast. Ecol. Prog. Ser. 1979;1:29-39.
CrossRef - Rhyne, A. L., Ohs, C. L., Stenn, E. Effects of temperature on reproduction and survival of the calanoid copepod Pseudodiaptomuspelagicus. 2009;292(1-2):53-59.
CrossRef - Doan, N. X., Vu, M. T., Pham, H. Q., Wisz, M. S., Nielsen, T. G., Dinh, K. V. Extreme temperature impairs growth and productivity in a common tropical marine copepod. Rep.2019;9(1):1-9.
CrossRef - Ruiz, L. H., Ekumah, B., Asiedu, D. A., Albani, G., Acheampong, E., Jónasdóttir, S. H., … Nielsen, T. G. Climate change and oil pollution: A dangerous cocktail for tropical zooplankton. Toxicol. 2021;231:105718.
CrossRef - Dokulil M. T., Teubner K. Eutrophication and climate change: present situation and future scenarios. In: Eutrophication: causes, consequences and control (Ansari AA, et al. eds). Springer, Dordrecht. 2010; pp 1-16.
CrossRef - Dokulil M. T., et al. The impact of climate change on lakes in Central Europe. In:The impact of climate change on European lakes(George DG, ed). Springer, Dordrecht. 2010; pp 387-409.
CrossRef - Berger S. A., Diehl S., Kunz T. J., Albrecht D., Oucible A. M., Ritzer S. Light supply, plankton biomass, and seston stoichiometry in a gradient of lake mixing depths. Oceanogr. 2006;51(4):1898-1905.
CrossRef - Michalak A. M. Study role of climate change in extreme threats to water quality. Nature News. 2016;535(7612):349-350.
CrossRef - Mooij W. M., et al. The impact of climate change on lakes in the Netherlands: a review. Ecol. 2005;39(4):381-400.
CrossRef - Rhee G. Y., Gotham I. J. Optimum N: P ratios and coexistence of planktonic algae. Phycol. 1980;16(4):486-489.
CrossRef - Hecky R. E., Kilham P. Nutrient limitation of phytoplankton in freshwater and marine environments: a review of recent evidence on the effects of enrichment. Oceanogr. 1988;33(4part2):796-822.
CrossRef - Brookes J. D., Carey C. C. Resilience to blooms. Science. 2011;334(6052), 46-47.
CrossRef - Havens K. E., James R. T., East T. L., Smith V. H. N: P ratios, light limitation, and cyanobacterial dominance in a subtropical lake impacted by non-point source nutrient pollution. Environ. Pollut. 2003;122(3), 379-390.
CrossRef - Poste A. E., Hecky R. E., Guildford S. J. Phosphorus enrichment and carbon depletion contribute to high Microcystis biomass and microcystin concentrations in Ugandan lakes. Oceanogr. 2013;58(3):1075-1088.
CrossRef - Invidia M., Sei S., Gorbi G. Survival of the copepod Acartiatonsa following egg exposure to near anoxia and to sulfide at different pH values. Ecol. Prog. Ser. 2004;276:187-196.
CrossRef - Ruz, P. M., Hidalgo, P., Yáñez, S., Escribano, R., Keister, J. E. Egg production and hatching success of Calanus chilensis and Acartiatonsa in the northern Chile upwelling zone (23 S), Humboldt Current System. Mar. Syst.2015;148:200-212.
CrossRef - Grodzins, M. A., Ruz, P. M., Keister, J. E. Effects of oxygen depletion on field distributions and laboratory survival of the marine copepod Calanus pacificus. Plankton Res. 2016;38(6):1412-1419.
CrossRef - Keister, J. E., Winans, A. K., Herrmann, B. Zooplankton community response to seasonal hypoxia: a test of three hypotheses. 2020;12(1):21.
CrossRef - Mills, C. E. Jellyfish blooms: are populations increasing globally in response to changing ocean conditions?. 2001;451(1):55-68.
CrossRef - Purcell, J. E. Jellyfish and ctenophore blooms coincide with human proliferations and environmental perturbations. Rev. Mar. Sci.2012;4:209-235.
CrossRef - Keister, J. E., Houde, E. D., Breitburg, D. L. Effects of bottom-layer hypoxia on abundances and depth distributions of organisms in Patuxent River, Chesapeake Bay. Ecol. Prog. Ser. 2000;205:43-59.
CrossRef - Park, G. S., Marshall, H. G. Estuarine relationships between zooplankton community structure and trophic gradients. Plankton Res.2000;22(1):121-136.
CrossRef - Zhang, H., Ludsin, S. A., Mason, D. M., Adamack, A. T., Brandt, S. B., Zhang, X., … Boicourt, W. C. Hypoxia-driven changes in the behavior and spatial distribution of pelagic fish and mesozooplankton in the northern Gulf of Mexico. Exp. Mar. Bio. Ecol. 2009;381:S80-S91.
CrossRef - Roman, M. R., Pierson, J. J., Kimmel, D. G., Boicourt, W. C., Zhang, X. Impacts of hypoxia on zooplankton spatial distributions in the northern Gulf of Mexico. Coast.2012;35(5):1261-1269.
CrossRef - Keister, J. E., Tuttle, L. B. Effects of bottom‐layer hypoxia on spatial distributions and community structure of mesozooplankton in a sub‐estuary of Puget Sound, Washington, USA. . Limnol. Oceanogr.2013;58(2):667-680.
CrossRef - Kehayias, G., Aposporis, M. Zooplankton variation in relation to hydrology in an enclosed hypoxic bay (Amvrakikos Gulf, Greece). Mar. Sci.2014;15(3):554-568.
CrossRef - Ekau, W., Auel, H., Pörtner, H. O., Gilbert, D. Impacts of hypoxia on the structure and processes in pelagic communities (zooplankton, macro-invertebrates and fish). Biogeosciences. 2010;7(5):1669-1699.
CrossRef - Moss B., et al. Allied attack: climate change and eutrophication. Inland waters. 2011;1(2):101-105.
CrossRef - Larsen, S., Andersen, T. O. M., Hessen, D. O. Climate change predicted to cause severe increase of organic carbon in lakes. Chang. Biol. 2011;17(2):1186-1192.
CrossRef - de Wit, H. A., Valinia, S., Weyhenmeyer, G. A., Futter, M. N., Kortelainen, P., Austnes, K., … Vuorenmaa, J. Current browning of surface waters will be further promoted by wetter climate. Sci. Technol. Lett.2016;3(12): 430-435.
CrossRef - Erlandsson, M., Buffam, I., Fölster, J., Laudon, H., Temnerud, J., Weyhenmeyer, G. A., Bishop, K. Thirty‐five years of synchrony in the organic matter concentrations of Swedish rivers explained by variation in flow and sulphate. Chang. Biol.2008;14(5):1191-1198.
CrossRef - Solomon, C. T., Jones, S. E., Weidel, B. C., Buffam, I., Fork, M. L., Karlsson, J., … Saros, J. E. Ecosystem consequences of changing inputs of terrestrial dissolved organic matter to lakes: current knowledge and future challenges. 2015;18(3): 376-389.
CrossRef - Rantala, M. V., Nevalainen, L., Rautio, M., Galkin, A., Luoto, T. P. Sources and controls of organic carbon in lakes across the subarctic treeline. 2016;129:235–253.
CrossRef - Thrane, J. E., Hessen, D. O., Andersen, T. The absorption of light in lakes: negative impact of dissolved organic carbon on primary productivity. Ecosystems. 2014;17(6): 1040-1052.
CrossRef - Livingstone, D. M. Impact of secular climate change on the thermal structure of a large temperate central European lake. Change. 2003;57(1):205-225.
CrossRef - Reynolds, C. S. The ecology of phytoplankton. Cambridge University Press. 2006.
CrossRef - Carey, C. C., Ibelings, B. W., Hoffmann, E. P., Hamilton, D. P. Brookes, J. D. Eco-physiological adaptations that favour freshwater cyanobacteria in a changing climate. Res. 2012;46(5):1394-1407.
CrossRef - Von Elert, E., Wolffrom, T. Supplementation of cyanobacterial food with polyunsaturated fatty acids does not improve growth of Daphnia. Oceanogr. 2001;46(6):1552-1558.
CrossRef - DeMott, W. R., Gulati, R. D., Van Donk, E. Daphnia food limitation in three hypereutrophic Dutch lakes: Evidence for exclusion of large‐bodied species by interfering filaments of cyanobacteria. Oceanogr. 2001;46(8):2054-2060.
CrossRef - Ger, K. A., Arneson, P., Goldman, C. R., Teh, S. J. Species specific differences in the ingestion of Microcystis cells by the calanoid copepods Eurytemoraaffinis and Pseudodiaptomusforbesi. Plankton. Res. 2010;32(10):1479-1484.
CrossRef - Hong, J., Talapatra, S., Katz, J., Tester, P. A., Waggett, R. J., Place, A. R. Algal toxins alter copepod feeding behavior. One.2012;7(5):e36845.
CrossRef - Bednarska, A., Slusarczyk, M. Effect of non-toxic, filamentous cyanobacteria on egg abortion in Daphnia under various thermal conditions. Hydrobiologia. 2013;715(1):151-157.
CrossRef - Burns C. W. Direct observations of mechanisms regulating feeding behavior of Daphnia, in lakewater. Rev. ges. Hydrobiol. Hydrogr. 1968;53(1):83-100.
CrossRef - Porter K. G., McDonough R. The energetic cost of response to blue‐green algal filaments by cladocerans . Oceanogr. 1984;29(2):365-369.
CrossRef - Arnold D. E. Ingestion, assimilation, survival, and reproduction by Daphnia pulex fed seven species of blue-green algae. Oceanogr. 1971;16(6):906-920.
CrossRef - Porter K. G., Orcutt J. D. Jr. Nutritional adequacy, manageability, and toxicity as factors that determine the food quality of green and blue–green algae for Daphnia. In: Evolution and ecology of zooplankton communities (Kerfoot WC, ed). University Press of New England, Hanover, NH. 1977; pp 268–281.
- Ostrofsky M. L., Jacobs F. G., Rowan, J. Evidence for the production of extracellular herbivore deterrents by Anabaenaflos‐Freshw. Biol. 1983;13(6):501-506.
CrossRef - Lampert W. Inhibitory and toxic effects of blue‐green algae on Daphnia. Rev. ges. Hydrobiol. Hydrogr. 1981;66(3):285-298.
CrossRef - Nizan S., Dimentman C., Shilo M. Acute toxic effects of the cyanobacterium Microcystis aeruginosa on Daphnia magna. Oceanogr. 1986;31(3):497-502.
CrossRef - Haney J. F. Field studies on zooplankton‐cyanobacteria interactions. Z. J. Mar. Freshwater Res. 1987;21(3):467-475.
CrossRef - Bockwoldt K. A., Nodine E. R., Mihuc T. B., Shambaugh A. D., Stockwell J. D. Reduced phytoplankton and zooplankton diversity associated with increased cyanobacteria in lake Champlain, USA. Contemp. Water Res. Educ. 2017;160(1):100-118.
CrossRef - Caldeira K., Wickett M. E. Anthropogenic carbon and ocean pH. Nature. 2003;425(6956), 365.
CrossRef - Stephens J. C., van der Zwaan, B. The case of carbon capture and storage. Sci. Technol. 2005;22:68–76.
- Doney S. C., Fabry V. J., Feely R. A., Kleypas J. A. Ocean acidification: the other CO2Annu. Rev. Mar. Sci. 2009;1:169-192.
CrossRef - Reid P.C., et al. Impacts of the oceans on climate change. In: Advances in marine biology (David WS, ed). vol. 56. Academic Press, 2009; pp 1–150.
- Havens K. E., Norman D. Y., Keller, W. Lake acidification: effects on crustacean zooplankton populations. Sci. Technol. 1993;27:1621-1624.
CrossRef - Halsband C., Kurihara H. Potential acidification impacts on zooplankton in CCS leakage scenarios. Pollut. Bull. 2013;73(2):495-503.
CrossRef - Rose C. D., Williams W. G., Hollister T. A., Parrish, P. R. Method for determining acute toxicity of an acid waste and limiting permissible concentration at boundaries of an oceanic mixing zone. Sci. Technol. 1977;11(4):367-371.
CrossRef - Weydmann A., Søreide J. E., Kwasniewski S., Widdicombe S. Influence of CO2-induced acidification on the reproduction of a key Arctic copepod Calanus glacialis. Exp. Mar. Biol. Ecol. 2012;428:39-42.
CrossRef - Fabry V. J., Seibel B. A., Feely R. A., Orr, J. C. Impacts of ocean acidification on marine fauna and ecosystem processes. ICES J. Mar. Sci. 2008;65(3):414-432.
CrossRef - McGinty, N., Barton, A. D., Record, N. R., Finkel, Z. V., Johns, D. G., Stock, C. A., Irwin, A. J. Anthropogenic climate change impacts on copepod trait biogeography. Chang. Biol. 2021;27(7):1431-1442.
CrossRef - Yan N. D., Keller W., Scully N. M., Lean D. R., Dillon P. J. Increased UV-B penetration in a lake owing to drought-induced acidification. Nature. 1996;381(6578):141-143.
CrossRef - Perovich D. K., Govoni J. W. Absorption coefficients of ice from 250 to 400 nm. Res. Lett. 1991;18(7):1233-1235.
CrossRef - De Stasio Jr. B. T., Hill D. K., Kleinhans J. M., Nibbelink N. P., Magnuson J. J. Potential effects of global climate change on small north‐temperate lakes: Physics, fish, and plankton. Oceanogr. 1996;41(5):1136-1149.
CrossRef - Magnuson J. J. Historical trends in lake and river ice cover in the Northern Hemisphere. Science. 2000;289(5485):1743-1746.
CrossRef - Vinnikov K. Y., et al. Global warming and Northern Hemisphere sea ice extent. Science. 1999;286(5446):1934-1937.
CrossRef - Morris D. P., Hargreaves B. R. The role of photochemical degradation of dissolved organic carbon in regulating the UV transparency of three lakes on the Pocono Plateau. Oceanogr. 1997;42(2):239-249.
CrossRef - Grad G., Williamson C. E., Karapelou D. M. Zooplankton survival and reproduction responses to damaging UV radiation: A test of reciprocity and photoenzymatic repair. Oceanogr.2001;46:584–591.
CrossRef - Williamson C. E., Grad G., De Lange H. J., Gilroy S., Karapelou D. M. Temperature‐dependent ultraviolet responses in zooplankton: Implications of climate change. Oceanogr. 2002;47(6):1844-1848.
CrossRef - Rose, K. C., Williamson, C. E., Fischer, J. M., Connelly, S. J., Olson, M., Tucker, A. J., Noe, D. A. The role of ultraviolet radiation and fish in regulating the vertical distribution of Daphnia. Oceanogr.2012;57(6):1867-1876.
CrossRef - Hylander, S., Hansson, L. A. Vertical distribution and pigmentation of Antarctic zooplankton determined by a blend of UV radiation, predation and food availability. Ecol. 2013;47(4):467-480.
CrossRef - Lampert, W. The adaptive significance of diel vertical migration of zooplankton. Ecol. 1989;3:21-27.
CrossRef - Rhode, S. C., Pawlowski, M., Tollrian, R. The impact of ultraviolet radiation on the vertical distribution of zooplankton of the genus Daphnia. Nature. 2001;412(6842):69-72.
CrossRef - Williamson, C. E., Olson, O. G., Lott, S. E., Walker, N. D., Engstrom, D. R., Hargreaves, B. R. Ultraviolet radiation and zooplankton community structure following deglaciation in Glacier Bay, Alaska. Ecology. 2001;82(6):1748-1760.
CrossRef - Hylander, S., Hansson, L. A. Vertical migration mitigates UV effects on zooplankton community composition. Plankton Res. 2010;32(7):971-980.
CrossRef - Lampert, W., Taylor, B. E. Zooplankton grazing in a eutrophic lake: implications of diel vertical migration. Ecology. 1985;66(1): 68-82.
CrossRef - Reichwaldt, E. S., Wolf, I. D., Stibor, H. The effect of different zooplankton grazing patterns resulting from diel vertical migration on phytoplankton growth and composition: a laboratory experiment. Oecologia. 2004;141(3): 411-419.
CrossRef - Wolf, R., Heuschele, J. Water browning influences the behavioral effects of ultraviolet radiation on zooplankton. Ecol. Evol. 2018;6:26.
CrossRef - Smith, K. C., Macagno, E. R. UV photoreceptors in the compound eye of Daphnia magna (Crustacea, Branchiopoda). A fourth spectral class in single ommatidia. Comp. Physiol. A. 1990;166(5):597-606.
CrossRef - Leech, D. M., Williamson, C. E. In situ exposure to ultraviolet radiation alters the depth distribution of Daphnia. Oceanogr. 2001;46(2):416-420.
CrossRef - Fischer, J. M., Nicolai, J. L., Williamson, C. E., Persaud, A. D., Lockwood, R. S. Effects of ultraviolet radiation on diel vertical migration of crustacean zooplankton: an in situ mesocosm experiment. Hydrobiologia. 2006;563(1):217-224.
CrossRef - Williamson, C. E., Fischer, J. M., Bollens, S. M., Overholt, E. P., Breckenridge, J. K. Toward a more comprehensive theory of zooplankton diel vertical migration: Integrating ultraviolet radiation and water transparency into the biotic paradigm. Oceanogr. 2011;56(5):1603-1623.
CrossRef - Häder, D. P., Sinha, R. P. Solar ultraviolet radiation-induced DNA damage in aquatic organisms: potential environmental impact. Res-Fund. Mol. M. 2005;571(1-2):221-233.
CrossRef - Heyland, A., Moroz, L. L. Signaling mechanisms underlying metamorphic transitions in animals. Comp. Biol., 2006;46(6):743-759.
CrossRef - Wolinski, L., Souza, M. S., Modenutti, B., Balseiro, E. Effect of chronic UVR exposure on zooplankton molting and growth. Pollut. 2020;267:115448.
CrossRef - Balseiro, E., Souza, M. S., Modenutti, B., Reissig, M. Living in transparent lakes: Low food P:C ratio decreases antioxidant response to ultraviolet radiation in Daphnia. Oceanogr. 2008;53(6):2383-2390.
CrossRef - Tollrian, R., Heibl, C. Phenotypic plasticity in pigmentation in Daphnia induced by UV radiation and fish kairomones. Ecol. 2004;18(4):497-502.
CrossRef - Connelly, S. J., Moeller, R. E., Sanchez, G., Mitchell, D. L. Temperature effects on survival and DNA repair in four freshwater cladoceranDaphnia species exposed to UV radiation. Photobiol. 2009;85(1):144-152.
CrossRef - Wolinski, L., Modenutti, B., Souza, M. S., Balseiro, E. Interactive effects of temperature, ultraviolet radiation and food quality on zooplankton alkaline phosphatase activity. Pollut. 2016;213:135-142.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





