Manuscript accepted on : 16-03-2021
Published online on: 26-03-2021
Plagiarism Check: Yes
Reviewed by: Dr. Pinalysa Cosma
![]()
Second Review by: Dr. Thaigarajan A/L Parumasivam 
Final Approval by: Dr. Everaldo Silvino dos Santos
Hanan S. Alyahya1, Mohammed A. Alkuriji2, Lina Soror1, Nada Ghazal1, Khulud Alghannam1 and Fekri M. Shaher2,3*
1Department of Biology Sciences, Faculty of Sciences, King Abdulaziz University, Jeddah, Saudi Arabia.
2National Center of Agricultural Technology, Life Science & Environmental Research Institute, King Abdulaziz City for Science and Technology (KACST), Riyadh, Saudi Arabia.
3Hodeidah University, Hodeidah, Republic of Yemen.
Corresponding Author E-mail: fikry1978@yahoo.com
DOI : http://dx.doi.org/10.13005/bbra/2909
ABSTRACT:
Severe human diseases are spread by mosquitoes, causing millions of deaths every year. Many well-known and severe problems have been caused by the indiscriminate use of synthetic chemical insecticides, such as the residual insecticides for humans and environment and high operating cost in addition to the possibility of developing insect resistance.The larvicidal and delayed effects of the body wall extract of Holothuria scabra and leaves extract of Acalypha fruticosa against 4th instar larvae of mosquito, Aedes aegypti were evaluated. Ethanolic extract of H. scabra recorded more larvicidal efficiency(LC50, 79.31 ppm) than A. fruticosa leaves extract (152.86 ppm) by about 1.93 folds.Morphological features showed abnormalities on the larval and pupal stages with H. scabra and to less extent with A. fruticosa.
Therefore, it is possible to build on the results of this study to use these two extracts to control of A. aegypti mosquitoes and in line with recent trends in adopting combat methods that are safe on humans and the environment.
KEYWORDS: Aedes Aegypti; Acalypha Fruticosa; Dengue Fever; Holothuria scabra; Natural larvicides
Download this article as:| Copy the following to cite this article: Alyahya H. S, Alkuriji M. A, Soror L, Ghazal N, Alghannam K, Shaher F. M. Natural Extracts as Eco-Friendly Larvicides Against Aedes Aegypti Mosquito, Vector of Dengue Fever Virus in Jeddah Governorate. Biosci Biotech Res Asia 2021;17(4). |
| Copy the following to cite this URL: Alyahya H. S, Alkuriji M. A, Soror L, Ghazal N, Alghannam K, Shaher F. M. Natural Extracts as Eco-Friendly Larvicides Against Aedes Aegypti Mosquito, Vector of Dengue Fever Virus in Jeddah Governorate. Biosci Biotech Res Asia 2021;17(4). Available from: https://bit.ly/3d5PeE1 |
Introduction
Mosquitoes belong to (Diptera: Culicidae) are a category of insects that pose the greatest threat to human and veterinary health as vectors of diseases, more than any other insect group. The mosquito Aedes aegypti is one of this category that shares a similar ecological niche with human.Globally, mosquitoes represent a major public health problem. They are estimated to spread diseases to more than 700 million individuals annually and are currently expected to be responsible for the deaths of a round one person in 17 people1.
Aedes aegypti is the principal transmitter of Dengue, Chikungunya, Yellow fever and Zika viruses. This mosquito species is well suited to humankind. Their females get blood meals through biting the mammals and digesting it inside their bodies to obtain the eggs. The control of A. aegypti is difficult task because they able to lay their eggs in many places even those of low quantity of water.The eggs have the ability to survive months in the dry conditions and they hatch as soon as water is available. Furthermore, they have been developed resistance against commonly used insecticides2,3,4.
Extensive application of pyrethroid and organophosphate insecticides to monitor all types of mosquito has resulted in an acceleration of the level of resistance created. In addition to resistance, other adverse effects may be associated with certain mosquiticides, including harmful effects against non-targeet species, environmental problems and human health concerns5.
Surveys of appropriate alternatives to conventional insecticides have shown that phytochemicals are a good choice in terms of relative protection, global availability and low cost. Therefore, screening for locally available medicinal plants for mosquito control may be an option that is cheaper than costly imported products to improve local jobs and public health6.
Chemicals extracted from natural sources have recently been predicted to be weapons of potential mosquito control programs as they are shown to be eco-friendly and largely non-toxic to humans and other mammals and biodegradable7.
These natural metabolites exhibit significant bioactivities including antidiabetic, antioxidant, antibacterial, anticancer and anti inflammatory activities 8,9 and several of these natural products can be used for derive a unique drug agents 10.
The marine ecosystem is an enormous and incredibly rich source of biological and chemicals products of natural origin.Many of these substances have beneficial medical and pharmaceutical properties.A large number of marine bioactive metabolites with specific properties have recently been isolated and characterized 11. Natural biologically active constituents such as steroids, terpenoids, alkaloids, sterols and other metabolites are formed by the marine biota 12. Recently, bioassay work was designed to evaluate the larvicidal effect of methanol, chloroform, ethyl acetate and aqueous extracts of macroalgae Codium edule against Aedes aegypti larvae. The findings indicated that Chloroform fraction exhibited the most larvicidal activity with LC50 value of 19.54 ppm13. In this manner the present work aimed to study the possibility of control of Aedes aegypti by extracts of Holothuria scabra as a marine animal and of Acalypha fruticosa as a terrestrial plant under the laboratory conditions.
Materials and Methods
Mosquito colony
The study requires a sufficient number of larvae to carry out the biological evaluation experiments and for this purpose, The mosquito Aedes aegypti strain was brought from the Jeddah Municipality laboratory to combat public health pests, Kingdom of Saudi Arabia. The mosquito colony was established under laboratory controlled conditions (temperature 27±2 °C, Relative humidity 75± 5 % and 10-14 light-dark periods) in the dengue research unit at King Abdulaziz University, where adult insects were kept in Canvas cages and daily provided with 10% sucrose solution. The females mosquitoes were fed with adequate blood meals from a pigeon and then mosquito eggs were obtained, which were immersed in ceramic dishes with sizes of 20-30 cm half-filled with dechlorinated water.Mosquitoes raring continued for a number of generations according to the method of (Mahyoub, 2013)14. The hatching larvae were fed daily by mixture of yeast powder: dry bread powder: skimmed milk powder in 1:1:1 proportions15.
Sea cucumber and plant materials
Sea cucumber Holothuria scabra was collected from AL-kharrar lagoon, Red Sea (west of Saudi Arabia) during July 2019. The animal was identified by marine biology department-King Abdulaziz university. The fresh leaves of plant Acalypha fruticosa were collected from AL-Baha region (south of Saudi Arabia) during August 2019 and the plant was classified by taxonomist, department of biology, faculty of Science, King Abdulaziz university. The H. scabra body wall and the leaves of A. fruticosa were washed by water and left in shade area at room temperature until become dry, then grinded using electrical blender to obtain fine powder.
Preparation of extracts
The extraction was performed by soaking 100 gm fine powder of both organisms in sufficient amount of 70 % ethanol in shaking machine for overnight. The supernatant was filtered using what man filter paper, the extraction was repeated three times and the combined solvent was subjected to rotary evaporator at 45 °C. The dry extract (6.45 gm of H. scabra and 8.67 gm of A. fruticosa) were kept under -4°C until start the experiments16.
The bioassay experiments
The mosquito fourth instars larvae were treated with a series of concentrations of both plant and sea cucumber extracts under laboratory conditions at a temperature of 27 ± 2 ºC and a relative humidity of 75 ± 5%. Standard method of immersion was used 1. The tests were carried out in a beaker containing 100 ml of water and five replicates were used for each concentration where each single contains 20 larvae in addition to five replicates for control. Taking into account the supply of larvae with food to avoid starvation factor. Mortality rate were recorded during the period from the start of the experiment until pupation and adult emergence.
Statistical analysis
The mortality percentages of larvae were calculated for each concentration. The results were analyzed using LDP line software to derive statistical values and constants 17 at 95% confidence intervals and a significant level of 0.05 based on the degree of probability Probit analysis and calculating LC50 and LC90 together with the lower and upper confident limits and the inclination of toxicity line and Chi square according to Finney 197118.
Record of deformities
The morphological features of treated larvae have been compared to control media during the course of lethal experiments. Any noticeable difference in appearance between control and treated was reported as deformity. The deformities were described according to their resemblance to those previously seen in the literature19.
Results and Discussion
Bioactive products from natural origin with insecticidal properties have been used in the recent pest control of different insect pests and vectors20. The mosquito-human relationship is related to many diseases. Serious diseases such as malaria, arboviral encephalitis, dengue fever, chikungunia fever, west Nile virus and yellow fever are transmitted by mosquitoes. These diseases cause significant mortality in human and livestock around the globe16.
Results of toxicity test of H. scabra and A. fruticosa extracts on the 4th larval instar of A. aegypti were listed in Table (1 & 2) and Figure (1, 2 and 3). It is clear that the mortality percent increased with increasing extracts concentrations. H. scabra extract was found to be more bioactive than A. fruticosa extract where LC50 values were (79.31, 152.89) ppm and LC90 values (314.12, 782.34) ppm, respectively.
Table 1: Effect of sea cucumber H. scabra and plant A. fruticosa on Aedes aegypti 4th instar larvae.
| Conc. (ppm) | Mortality
(%) |
LC50 (ppm)
(LCL-UCL) |
LC90 (ppm)
(LCL-UCL) |
(Chi)2 | slope |
| H. scabra
|
|||||
| 50 | 35 | 79.31
(65.4 -92.6) |
314.12
(260.4 -402.6) |
2.2 | 2.144 |
| 100 | 58 | ||||
| 200 | 79 | ||||
| 300 | 87 | ||||
| 500 | 98 | ||||
| A. fruticosa
|
|||||
| 50 | 18 | 152.86
(130.3-177.7) |
782.34
(588.3-1174.2) |
0.367 | 1.807 |
| 100 | 39 | ||||
| 200 | 57 | ||||
| 300 | 71 | ||||
| 500 | 82 | ||||
Table 2: Index compared Holothuria scabra with Acalypha fruticosa.
| Line name | LC50 | Lower limit | Upper limit | RR |
| Holothuria scabra | 79.31 | 65.49 | 92.61 | 1.927 |
| Acalypha fruticosa | 152.86 | 130.30 | 177.73 |
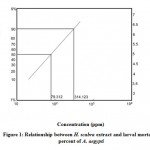 |
Figure 1: Relationship between H. scabra extract and larval mortality percent of A. aegypti. |
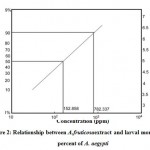 |
Figure 2: Relationship between A. fruticosa extract and larval mortality percent of A. aegypti. |
 |
Figure 3: Relationship between H. scabra and A. fruticosa extracts and larval mortality percent of A. aegypti. |
Both extracts caused reduction in the pupation percentage.The pupation decreased as the extract concentration increased. Moreover, the toxic effect of H. scabra had been extended to the pupae and adults and this effect also observed with A. fruticosa but to less extent.These effects of both extracts manifested in deformities noticed under microscope (Figure 4&5).In addition, the extracts caused decline in the emergence of adults and this reduction depended on the extract concentration.Such findings are consistent to some extent with previous outcomes of (Sharma et al., 2006)21.
 |
Figure 4: (a) larvae shows dark melanization (b) dwarf of larval body (c) Normal larvae (larvae treated with A. fruticosa). |
 |
Figure 5: (a, b) albino case (c) normal pupae (d, e) partly emerged adult with attached pupal case (larvae treated with H. scabra). |
Strong activity of H. scabra may be attributable to their high saponin content. High larvicidal activity of saponin extract has been proven in earlier study with LC50 11.7 ppm against Culex pipiens 20.Also dose of 35 ppm of saponin extract of Balanites aegyptiaca fruit mesocarp inhibited fifty percent of the population of treated larvae, this would definitely help to significantly decrease the mosquito density22.Earlier reports suggested the bioactivity of saponins as a naturally mosquito larvicidal agent; however, no investigation has been done on saponins regarding their effect on mosquito adult emergence 23.
These observed and reported deformations on the larvae treated with the sea cucumber extract may be due to the fact that it contains many valuable valid active chemical constituents such as saponins (mentioned above) and terpenes, this results are in line with previous study on Sea cucumber Holothuria atra extract against A. aegypti 24. On the other hand phytochemicals found in A.fruticosa can contribute collectively or independently in larvicidal action. In general, the death of treated larvae could be attributable to the failure of the moulting bodies to swallow adequate volume of air during ecdysis to break the old cuticle and extend the new one, or to the effect of the plant extract to inhibit larvae body metamorphosis which may be based on the hormone control disturbance and lead to an imbalance of the growth processes and larval death 19,25.
Acknowledgement
This work was funded by Mawakeb Alajer Association, Jeddah, Saudi Arabia through the Science Research and Innovation Unit at the Faculty of Science, King Abdulaziz University, Jeddah, Saudi Arabia under research number (scigriu41/11).The authors also are thankful to the staff of Dengue fever unit, King Abdulaziz University for their valuable technical cooperation in achievement the experimental part.
Conflict of interest
The authors declare no conflict of interest
References
- World Health Organization WHO (2005) Prevention and control of dengue and dengue hemorrhagic fever. WHO, Regional Publication, searl No.29, 134.
- Lima, E.P., Paiva, M.H.S., Paula de Araújo, A., da Saliva, U. M. da Saliva, de Oliveira, L. N., et al. (2011)Insecticide resistant in Aedes aegypti populations from ceara, Brazil. Parasites & Vectors, 4(1), 5.
CrossRef - Marcombe, S., Mathieu, R.B., Pocquet, N., Riaz, M.-A, Poupardin, R., Selior, S., et al. (2012) Insecticide resistant in the dengue vector Aedes aegypti from Matrinique: Distribution, mechanisms and relations with environmental factors, PLoS One, 7 (2), Article e30989.
CrossRef - Alkuriji, M. A., Al-Fageeh, M. B., Shaher, F. M. and Almutairi B. F. (2020) Dengue Vector Control: A Review for Wolbachia-based Strategies, BIOSCIENCES BIOTECHNOLOGY RESEARCH ASIA, 17(3): 507-515.
CrossRef - Rawani, A., Haldar, K.M., Ghosh, A., Chandra, G.(2009). Larvicidal activities of three plants against filarial vector Culex quinquefasciatus Say (Diptera: Culicidae). Parasitol. Res. 105(5) 1411-1417.
CrossRef - Bowers, W. S., Sener, B., Evans, P. H., Bingol, F., Erdogan, I. (1995). Activity of Turkish medicinal plants against mosquitoes Aedes aegypti and Anopheles gambiae. International Journal of Tropical Insect Science16, 339-341
CrossRef - Rahuman AA, Bagavan A, Kamaraj C, Saravanan E, Zahir AA, Elango G. (2009) Efficacy of larvicidal botanical extracts against Culex quinquefasciatusSay (Diptera: Culicidae). Res., 104(6), 1365-1372.
CrossRef - Fernando I.S., Kim M., Son K.T., Jeong Y. and Jeon, Y. J. (2016) Antioxidant activity of marine algal polyphenolic compounds: a mechanistic J. Med. Food. 19 (7) 615-628.
CrossRef - Wang H. D., Li X. C., Lee D. J. and Chang J. S. (2017) Potential biomedical applications of marine algae. Bioresource technology, 244, 1407-1415.
CrossRef - Benelli G., Pavela, R., Maggi F., Petrelli, R., Nicoletti (2017) Commentary: making green pesticides greener? The potential of plant products for nanosynthesis and pest control, J. Clust. Sci. 28, 3-10
CrossRef - Rania A. E. , Amany I., Eman H., Amir w., Haidy K., Manal A., Hashim H. and Safwat A. (2017) review of natural products from marine organisms in the Red Sea, IJPSR, (3): 940-974.
- Firn RD and Jones CG. (2003) Natural products: a simple model to explain chemical diversity. Natural Product Reports; 20: 382-391.
CrossRef - Alkuriji, M. A., Al-Fageeh, M. B., Shaher, F. M., Alorf M. S and Almazyad, H. F. (2020) Larvicidal Effect of Seaweed Codium Edule extracts on Aedes Aegypti mosquito. Research Journal of Pharmaceutical, Biological and Chemical Sciences (RJPBCS), 11(5): 82-89.
- Mahyoub J. A. (2013) Evaluation of the IGRs alsystin and pyriproxyfen as well as the plant extract jojoba oil against the mosquito Aedes aegypti . J. of Pure and Applied Microbiology I ; 7(04).
- Mahyoub Jazem A. (2019) USE OF METHOPRENE AND DIFLUBENZURON FOR LONG-TERM CONTROL OF AEDES AEGYPTI, THE VECTOR OF DENGUE FEVER IN JEDDAH GOVERNORATE, January–February, RJPBCS 10(1) Page No. 1
- El-Sheikh, T. M., Al-Fifi, Z. I., Alabboud, M. A. (2016) Larvicidal and repellent effect of some Tribulus terrestris L.,(Zygophyllaceae) extracts against the dengue fever mosquito, Aedes aegypti (Diptera: Culicidae). Journal of Saudi Chemical Society20, 13-19.
CrossRef - Hanan AS, Jazem MA, Hamed GA, Alhag SK. (2018) Larvicidal Activity of Synthesized Silver Nanoparticles using Rhazya stricta Leaf Extract against Mosquito Vectors Aedes Aegypti. Res J Biotechnol., 13(10).
- Finney, D. J. (1971). Statistical Method in Biological Assay, 3 rdedn. Griffn, London.
- Saranya M., Mohanraj R. S. and Dhanakkodi B. (2013) Larvicidal, pupicidal activities and morphological deformities of Spathodeacampanulata aqueous leaf extract against the dengue vector Aedes aegypti, European Journal of Experimental Biology 3(2):205-213.
- Djeghader, N. E.-H., Aïssaoui, L., Amira, K., Boudjelida, H. (2018)Toxicity evaluation and effects on the development of a plant extract, the Saponin, on the domestic mosquito, Culex pipiens. International Journal of Mosquito Research5, 01-05.
- Sharma P., Mohan L. and Srivastava C. N. (2006) Growth inhibitory nature of Artemisia annuaextract against Culex autnauetesctetus (Say), J. Asia-Pacific Entomol., 9 (4), pp. 389-395.
CrossRef - Chapagain B. P. and Wiesman Z. (2005) Larvicidal Activity of the Fruit Mesocarp Extract of Balanites aegyptiaca and its Saponin Fractions against Aedes aegypti, Dengue Bulletin, Vol 29, 203-207
- Wiesman Z and Chapagain B. (2003) Laboratory evaluation of natural saponin as a bioactive agent against Aedes aegypti and Culex pipiens. Dengue Bull. 27: 168-173.
- Mahyoub, J. A., Hawas U. W., Al-Ghamdi K. M., Aljameeli, M. M., Shaher F. M., Bamakhrama M. A. and Alkenani N. A. (2016) The Biological Effects of Some Marine Extracts Against Aedes aegypti (L.) Mosquito Vector of the Dengue Fever in Jeddah Governorate, Saudi Arabia, JOURNAL OF PURE AND APPLIED MICROBIOLOGY, 10(3): 1-8.
- Grzybowski, A., Tiboni, M., Silva, M. A., Chitolina, R. F., Passos, M., & Fontana, J. D. (2013) Synergistic larvicidal effect and morphological alterations induced by ethanolic extracts of Annona muricata and Piper nigrum against the dengue fever vector Aedes aegypti., Pest management science,2013; 69(5): 589-601.þ
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





