Manuscript accepted on : 22-02-2021
Published online on: 26-02-2021
Plagiarism Check: Yes
Reviewed by: Dr. Ana Cláudia Coelho
![]()
Second Review by: Dr. Waquar Ansari
Final Approval by: Dr. Muhammad Hamayun
![]()
Kawther Aabed* , Abeer Almutairi, Alaa Al-shwuair, Amal Al-otaibi, Arwa Alhazzani, Areej Al-shbi, Hind Al-moegelth, Lama Al-assaf, and Sultanah Al-omri
, Abeer Almutairi, Alaa Al-shwuair, Amal Al-otaibi, Arwa Alhazzani, Areej Al-shbi, Hind Al-moegelth, Lama Al-assaf, and Sultanah Al-omri
Department of Biology, Faculty of Sciences, Princess Nourah Bint Abdulrahman University, 84428 Riyadh, Saudi Arabia
Corresponding Author E-mail : dr.kaabed@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2904
ABSTRACT:
Soil bacteria play an essential function in different biogeochemical cycles. The present study aimed to estimate microbial diversity in five natural environments of Saudi Arabia by isolating and identifying thermophiles and thermo-tolerant bacteria. The collected soil samples were analyzed physically, chemically, and microbiologically. Biochemical and molecular techniques identified many bacteria, including Enterobacter ludwigii, Enterobacter sp., Enterobacteriaceae bacterium, Bacillus sp., Bacillus subtilis, Bacillus licheniformis, Paenibacillus sp., Paenibacillusdendritiformis, Paenibacillus lactis, Pseudomonas aeruginosa, Pseudoalteromonas sp., Staphylococcus sp., and Brevibacillus borstelensis. This is the first report of the Enterobacter ludwigii isolate from the soil samples collected in Saudi Arabia to the best of the authors' knowledge, which is part of plant growth-promoting rhizobacteria (PGPR) can influence the composition of the rhizosphere soils, root tissues, and enhanced plant outgrowth. The presence of these bacteria could be utilized to promote agricultural practices in the deserts in Saudi Arabia.
KEYWORDS: Hot Desert; Soil; Saudi Arabia; Thermophiles; Thermo-tolerant bacteria; 16S rRNA
Download this article as:| Copy the following to cite this article: Aabed K, Almutairi A, Al-shwuair A, Al-otaibi A, Alhazzani A, Al-shbi A, Al-moegelth H, Al-assaf L, Al-omri s. Diversity and Distribution of Thermophiles and Thermo-Tolerant Bacteria in the Soil Samples Obtained from Different Regions in Saudi Arabia. Biosci Biotech Res Asia 2021;18(1). |
| Copy the following to cite this URL: Aabed K, Almutairi A, Al-shwuair A, Al-otaibi A, Alhazzani A, Al-shbi A, Al-moegelth H, Al-assaf L, Al-omri s. Diversity and Distribution of Thermophiles and Thermo-Tolerant Bacteria in the Soil Samples Obtained from Different Regions in Saudi Arabia. Biosci Biotech Res Asia 2021;18(1). Available from: https://bit.ly/3aTB0Gf |
Introduction
Growth and adaptation of organisms in the hosting environment are affected by many factors, temperature in particular (Brooks et al.,2011). For organisms classified as thermo-tolerant, the mesophilic range (30−37 °C) is the most optimal; however, they are also able to grow in high-temperature environments in the philic range. On the other hand, the optimal growth temperature of organisms classified as thermophiles is much higher (60 °C), even though they were found to thrive in much hotter environments, such as terrestrial volcanic sites (Nazina et al., 2008). Microorganisms, specifically bacteria, are increasinglyfound to thrive in extreme conditions, such as those characterizing deserts, namely low nutrient status, extreme temperature fluctuations, high levels of UV radiation, and strong winds (Chamizo et al., 2012; Lester et al., 2007; Stomeo et al., 2013). Temperature is the dominant factor controlling the growth ofmicrobial species in the desert soil (Brooks et al., 2011). In the pertinent literature, a variety of thermophilic bacteria are described, which were extracted from various regions in theworld, including China (Lau et al., 2009), Turkey (Gul-Guven et al., 2008), Bulgaria (Derekova et al., 2008), India (Sharma et al., 2008), Greece (Sievert et al., 2000), Italy(Maugeri et al., 2001), Iceland (Takacs et al., 2001), and Saudi Arabia (SarhanandAlamrri 2014). Desert soils, irrespective of the locationfrom which they are obtained, typically comprise a number of universal phyla,including Proteobacteria, Bacteroidetes, and Actinobacteria (Chanal et al., 2006; Connon et al., 2007; Fierer et al., 2009; Lester et al., 2007).On theother hand, Cyanobacteria, Gemmatimonadetes, and Firmicutes (Bahl et al., 2011; Lacap et al., 2011; Makhalanyane et al., 2013; Richer et al., 2015) may be relatively more abundant in desert soils than in other biomes (Fierer et al., 2012). A wide range of molecular biology techniques can be employed in microorganism identification, such as 16S rRNA sequencing, rep-PCR profiling, and fatty acid methyl ester, which can be used in microorganism characterization at both species and subspecies levels (Adiguzel, 2006; Nazina et al., 2008; Zaliha et al., 2007). These techniques are also valuable for studying ecosystem diversity, in particular for analyzing the phylogenetic relation between strains, and discriminatingmicroorganisms that are genetically close to each other (Adiguzel, 2006).
The majority of research in microorganisms of the desert ecosystem was limited to few desert sites, particularly, in America and Australia. Consequently, significant effort is needed to expand to these studies to other regions, e.g., Asian and Africa. The more diverse data about microbial communities we have, the batter we are able to predict their impact on climate and land-use change (Makhalanyane et al., 2015). Subsequently, this study seeks to investigate microbial communities in hot deserts in Saudi Arabia.
The aim of this study was to identify and characterize thermophiles and thermo-tolerant bacteria isolated from soil in Saudi Arabia. In order to ensure diversity in soil samples, these were collected from Riyadh (central region), Dammam (eastern region), Hail (northern region), Abha (southern region), and AlmadinaAlmonawara (western region) via phenotypic and genotypic methods.
Materials and Methods
Sample Collection
Soil samples were obtained from natural ecosystems by collecting specimens of 20–40 g wet weight from the top 50 cm layer aseptically, which wereplaced in sterile glass containers and boxes kept at about 4 oC during transport. To ensure diversity, samples were collected from several sites, is illustrated in Figure 1,located in northern, southern, eastern, western, and central regions of Saudi Arabia.
 |
Figure 1: Map of regions of Saudi Arabia. |
Chemical Analysis of Soil
pH of the soils was determined by using an electronic pH meter (EckertandSims, 2011), and total salt concentration was determined by using Mehlich 3 method (Wolf and Beegle, 2011). Particle Size Analysis was conducted using Hydrometer Method (Gavlak,et al., 2005).The obtained results were presented as mg/l of dry soil.
Microbial Analysis
Each soil samplewas grown in 250ml flasks using 10g / 90ml of liquid media for enrichment,namely nutrient broth (v/v) for bacteria.The flasks were incubated at 45oC for 6 days. The Bacteria in the samples was enriched and isolated using the solid medium. Inoculum with adequate turbidity was transferred to three agar media, namely blood, nutrient, and MacConkey agar for bacteria. Each bacterial sample was incubated at four different temperatures (45°C, 50°C, 55°C, and 60°C) for 48−72 h. For all species, purification was carried out by applying the streaking plate method. Bacterial colonies were identified using microscopic examination, morphological analyses, and a biochemical kit.
DNA Extraction and 16S Ribosomal RNA-PCR Analysis
Bacterial DNA isolates was taken from 5 ml bacterial cultures grown overnight using DNeasy Blood & Tissue Kit (Qiagen, cat. #69504) for DNA isolation. Samples were processed as per the instructions in the kit.DNA amplification reactions were carried in Veriti® Thermal Cycler (AB, Applied Biosystems). The small-subunit rRNA (16S rDNA) were amplified by primers targeted to universal regions. The primers had the following sequences: universal forward primer Bac27F (5′-AGAGTTTGGATCMTGGCTCAG-3′) and universal reverse primer Bac1492R (5′- CGGTTACCTTGTTACGACTT-3′), used to amplify bacterial 16S rRNA. PCR amplifications were put according to the protocol described earlier (Flanagan et al., 2007).The PCR product was analyzed on 2.0% agarose gel with 0.5 μg/ml ethidium bromide and was imaged using Bio-Rad Gel Documentation System 2000.
16S rRNA Sequence Analysis
The 16S rRNA gene of the isolates was sequenced using ABI 3700 DNA Analyzer (Applied Biosystems, USA).BLAST algorithm in Gen Bank was used for analyzed homology of the 16S rRNA gene sequence of the isolates based on the available reference 16S rRNA sequences. MEGA version 7.0 software (Kumar et al., 2016) was employed when conducting phylogenetic and molecular evolutionary analyses.
Results
Physical and Chemical Characteristics of Soil from Different Regions
Five locations were prospected in Saudi Arabia, whereby the soil samples were obtained from Riyadh (central region), Dammam (eastern region), Hail (northern region), Abha (southern region) and AlmadinaAlmonawara (western region).The results of physical and chemical analyses performed on the fivesoil types are summarized in Table 1. During September 2014, temperaturesin Saudi Arabia were in the 31−43 °C range.The analyses revealed that all soil samplescontained slightly alkaline water (pH = 7.9–8.5), as well as high potassium concentrations.In addition, soil samples collected from the Dammam and Almadina locations had high concentrations of sodium,phosphate,and calcium chloride.
Table 1: Physiochemical Characteristics of Soil Samples.
| Characteristics | Soil Sample Location | ||||
| East | Centre | North | South | West | |
| Texture | L | LS | LS | LS | LiS |
| Clay % | 16.8 | 6.8 | 4.3 | 6.8 | 24.3 |
| Silt % | 35 | 5 | 1.25 | 7.5 | 60 |
| Sand % | 48.2 | 88.2 | 94.45 | 85.7 | 15.7 |
| pH | 8.12 | 8.2 | 8.45 | 7.94 | 8.38 |
| EC ms/cm | 3.83 | 0.38 | 1.21 | 2.02 | 1.99 |
| Na (ppm) | 178 | 49 | 63 | 35 | 840 |
| P (ppm) | 8.4 | 7 | 9.8 | 30 | 8.4 |
| K (ppm) | 156 | 96 | 103 | 90 | 305 |
| CaCl2 % | 10.2 | 2.29 | 2.9 | 2.6 | 13.03 |
Isolation, Selection, and Identification of Isolates
Among the 57 isolates of thermophilic and thermo-tolerant bacteria that grew on different agar media at 45−60°C, 16(28.1%)were obtained from the eastern region, 11(19.3%) from the central region, 7 (12.2%) from the northern region, 11 (19.3%) from the southern region, and 12 (21.1%) from the western region.
Further analyses confirmed that nine bacterial genera were identified across the soil sampling sites, with Enterobacter genera being the most dominant, andClostridium and Cedeceabeing the least prevalent, as indicated in Table 2. Other bacterial genera included Pseudomonas, Staphylococcus, Bacillus, Paenibacillus, andBrevibacillus. Moreover, three species were found to be thermophiles, Clostridiumsporognes, Paenibacillusdendritiformis, and Paenibacillus lactis, as they only grow under temperature condition of 60 °C. Whereas, all other isolates were found to be thermo-tolerant, as they grow under temperature conditions of 45-60 °C.
Table 2: Nine Bacterial Genera Identified in the Soil Samples.
| Temp. | Location | ||||
|
55 °C |
East | Centre | North | South | West |
| Clostridium sporogenes | Enterobacter sp. | Paenibacillusdendritiformis | Paenibacillussp | Paenibacillus lactis | |
| Enterobacter ludwigii | Bacillus sp. | Paenibacillusdendritiformis | Pseudomonas aeruginosa | ||
| Enterobacter ludwigii | – | Paenibacillusdendritiformis | Enterobacter sp. | Paenibacillus lactis | |
| Enterobacter ludwigii | – | – | – | Paenibacillussp. | |
| Brevibacillusborstelensis | |||||
| Paenibacillus sp. | |||||
| Enterobacter sp. | |||||
| Paenibacillussp | Paenibacillussp. | Bacillus subtilis | Enterobacter ludwigii | Bacillus subtilis | |
|
50 °C |
Enterobacter sp. | Staphylococcus sp. | Staphylococcus sp. | Cedeceadavisae | Bacillus licheniformis |
| Bacillus cereus | Pseudomonas aeruginosa | Pseudomonas aeruginosa | |||
| Pseudomonas aeruginosa | Enterobacter sp. | ||||
| Enterobacter ludwigii | Bacillus subtilis | Enterobacter ludwigii | Enterobacter ludwigii | Enterobacter ludwigii | |
| – | Bacillus licheniformis | Enterobacter ludwigii | Pseudoalteromonassp. | ||
|
45 °C |
Enterobacter hormaechei | Enterobacter ludwigii | Enterobacter sp. | Escherichia sp. | Pseudomonas aeruginosa |
| Enterobacter ludwigii | Enterobacter ludwigii | Enterobacter ludwigii | Enterobacter sp. | ||
| Enterobacter ludwigii | Bacillus subtilis subsp. | Pseudomonas aeruginosa | Bacillus sp. | ||
| Brevibacillusborstelensis | – | – | – | Enterobacter ludwigii | |
16S rRNA Sequence Analysis
Species level confirmations of 27 isolates were performed by 16S rRNA sequencing. Based on the findings yielded by the BLAST search analysis of the sequences, the isolates showed maximum identity (99%). The isolate sequences have been deposited in GenBank, as follows:Enterobacter ludwigiiwith accession numbers MF682065, MF682066, MF682067, MF682068, MF682071, MF682073, MF682074, MF682077, MF682078, MF682079, MF682080, MF682083, MF682084, MF682085, MF682086, MF682088, and MF682091;Enterobacter sp. with accession numbers MF682069, MF682070, MF682075, MF682081, MF682082, and MF682089;Enterobacter hormaecheiwith the accession number MF682072;Bacillus sp. with accession numbers MF682076 and MF682090; and Paenibacillus sp. with the accession number MF682087 (Table 3).
Table 3: Identity of the 27 Thermophilic and Thermo-tolerant Bacterial Isolates Based on BLAST Searches.
| Code (accession number) | Identity Based on BLAST Searches | Max Identity (%) | GenBank Accession No. | E-value (Query Coverage %) |
| KF1 (MF682065) | Enterobacter ludwigii | 99% | KM077046.1 | 0.0 (100) |
| KF2 (MF682066) | Enterobacter ludwigii | 99% | KM077046.1 | 0.0 (100) |
| KF3 (MF682067) | Enterobacter ludwigii | 99% | KM077046.1 | 0.0 (100) |
| KF4 (MF682068) | Enterobacter ludwigii | 97% | KM077046.1 | 0.0 (99) |
| KF5 (MF682069) | Enterobacter sp. | 99% | KR856429.1 | 0.0 (100) |
| KF6 (MF682070) | Enterobacter sp. | 99% | KC342873.1 | 0.0 (100) |
| KF7 (MF682071) | Enterobacter ludwigii | 99% | KM077046.1 | 0.0 (100) |
| KF8 (MF682072) | Enterobacter hormaechei | 96% | KU312822.1 | 0.0 (100) |
| KF9 (MF682073) | Enterobacter ludwigii | 96% | KM077046.1 | 0.0 (98) |
| KF10(MF682074) | Enterobacter ludwigii | 98% | KM077046.1 | 0.0 (99) |
| KF11(MF682075) | Enterobacter sp. | 99% | KR856429.1 | 0.0 (100) |
| KF12(MF682076) | Bacillus sp. | 99% | HM566879.1 | 0.0 (95) |
| KF13(MF682077) | Enterobacter ludwigii | 99% | KX024731.1 | 0.0 (100) |
| KF14(MF682078) | Enterobacter ludwigii | 99% | KM077046.1 | 0.0 (99) |
| KF15 MF682079 | Enterobacter ludwigii | 98% | KM077046.1 | 0.0 (99) |
| KF17(MF682080) | Enterobacter ludwigii | 99% | KM077046.1 | 0.0 (100) |
| KF18(MF682081) | Enterobacter sp. | 98% | KC342873.1 | 0.0 (100) |
| KF19(MF682082) | Enterobacter sp. | 98% | MF125281.1 | 0.0 (99) |
| KF20 (MF682083) | Enterobacter ludwigii | 99% | KM077046.1 | 0.0 (100) |
| KF22 (MF682084) | Enterobacter ludwigii | 99% | KM077046.1 | 0.0 (100) |
| KF23 (MF682085) | Enterobacter ludwigii | 99% | KM077046.1 | 0.0 (100) |
| KF24 (MF682086) | Enterobacter ludwigii | 99% | KM077046.1 | 0.0 (100) |
| KF25 (MF682087) | Paenibacillus sp. | 99% | KR364781.1 | 0.0 (96) |
| KF26 (MF682088) | Enterobacter ludwigii | 99% | KM077046.1 | 0.0 (100) |
| KF27 (MF682089) | Enterobacter sp. | 98% | MF125281.1 | 0.0 (100) |
| KF28 (MF682090) | Bacillus sp. | 99% | KF217252.1 | 0.0 (100) |
| KF29 (MF682091) | Enterobacter ludwigii | 98% | KM077046.1 | 0.0 (100) |
The phylogenetic analyses of the 27 thermophilic and thermo-tolerantbacterial isolates and closely related species were conducted using the neighbor-joining tree method, as shown in Figure2. The generated dendrogram revealed two clades supported by high bootstrap values. These clades are represented by two major lineages, namely Proteobacteria (89%) consisting mainly of the generaEnterobacter, and Firmicutes consisting of the genusBacillus (11%).
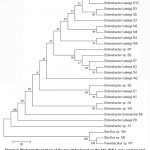 |
Figure 2: Phylogenetic analysis of the two clades based on the 16S rRNA gene quences and neighbor-joining tree method analysis results. |
We found that the Enterobacterludwigiiwas the most dominant species of the identified genera Enterobacter (71%). The phylogenetic tree of the 17Enterobacterludwigiistrains and the closest NCBI (BLASTn) strains—KM077046.1 and KX024731.1—based on the 16S rRNA gene sequences (neighbor-joining tree method) is illustrated in Figure3. A high similarity (~ 99%) with the reference strains available in the GenBank databases was identified (Table 3). Thirty-five percent of the 17 Enterobacterludwigii strains were found in the Eastern region of the country. To the best of the authors’ knowledge, this is the first report of the Enterobacterludwigiiisolate from the soil samples collected in Saudi Arabia.
 |
Figure 3: Phylogenetic tree of the Enterobacterludwigii strain and the closest NCBI (BLASTn) strains based on the 16S rRNA gene sequences (neighbor-joining tree method). |
Discussion
In this study we have isolated and identified different bacteria that grow and survive at high temperatures from 5 five different regions in the Kingdom of Saudi Arabia. We identified four genera of thermophilic and thermo-tolerantbacteria isolated from soil samples.This study shows that Proteobacteria and Firmicutes were the dominant phyla in the microbiota of the Soil Samples. Interestingly, these two phyla were also found to be dominant in hot springs (Lee et al., 2018). Analyses also revealed the presence of 57 thermophilic isolates pertaining toEnterobacter, Bacillus, Paenibacillus, and Pseudomonas.Other studies have identified Bacillus genus, specifically the Bacillus licheniformis, in hot springs, deserts and salt marshes in Morocco and in hot spring in Jorden (Aanniz et al., 2015; Mohammad et al., 2017; Al-Shammary et al., 2017;Bahkaliand Khiyami, 2008; Khalil, 2011). In addition, a study in the Northern region of the Kingdom of Saudi Arabia, Hail, identified Bacillus and Staphylococcusin soil isolates similar to our findings of the northern soil samples (Al-Shammary et al., 2017). A nation-wide study on thermophilic organism soil isolates has also identified all the reported genus in this study (Bahkaliand Khiyami, 2008). Bacillus and Brevibacillusgenus were identified in hot springs in the Kingdom of Saudi Arabia (Khalil, 2011). In another investigation, Alotaibi et al. 2020 have proven a wide variety of microbial communities in various areas that varied in physiochemical soil features in Saudi Arabia. Also, they have proven that higher fungal diversity than bacterial was isolated from desert areas and Sabkha. Also,Murgia et al. 2019 have showed significant fungal biodiversity in the Middle East desert soil.
The highest and lowestpercentages of bacterial species wasnoted in the samples collected from in the eastern and northern region, respectively. On the other hand, we observed similar percentages were found in the central and southern regions of Saudi Arabia.Enterobacter ludwigii was the most common bacterial species, followed by Enterobacter sp. and Bacillussp.To our knowledge, we are the first to identify this species of Enterobacter in the Kingdom of Saudi Arabia.
Conclusion
The resultsyielded by the present study indicate that numerous thermophilic and thermo-tolerant bacteria species thrive in different regions of Saudi Arabia.Moreover, although samples were collected from different regions to ensure diversity and comprehensive geographic coverage, the isolated bacteria species were generally similar. The abundance of bacteria in this study was typical of the environment with functional diversity and high species richness.Consequently, the findings of this study will provide invaluable information to microbial ecologists, as a diverse set of microbial communities of hot deserts in Saudi Arabia was identified. In addition, identification of thermophilic bacteria could be later used for biotechnological industry. In our future studies, the aim will be to determine the genetic variance among isolated thermophilic and thermo-tolerant bacteria and affected downstream proteins.
Data Availability
The sequencing data generated in this paper have been deposited in the GenBank repository with accession codes provided in Table 3.
Acknowledgements
We would like to express our gratitude to Prof. Mohammad Al ahdaal, Dr. Ahmed AlQahtani, and MashaelAlanazi at King Faisal Specialist Hospital & Research Center, Department of Infection and Immunity, for providing us access to their facilities.
Conflicts of Interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
References
- Brooks, A. N.,Turkarslan, S., Beer, K. D., Lo, F. Y., and Baliga,N. S. 2011. Adaptation of cells to new environments. Wiley Interdisciplinary Reviews Systems Biology and Medicine 3(5), 544-561.
CrossRef - Nazina, T. N., Sokolova, D. S., Grigoryan, A. A., Shestakova, N. M., Mikhailova, E. M., Oshima T., Moriya T. 2008. A preliminary analysis of microbial and biochemical properties of high-temperature compost. Annals of the New York Academy of Sciences 1125, 338-344.
CrossRef - Chamizo, S., Canto ́n, Y., Miralles, I. 2012. Biological soil crust development affects physicochemical characteristics of soil surface in semiarid ecosystems. Soil Biology and Biochemistry 49, 96-105.
CrossRef - Lester, E. D., Satomi, M., Ponce, A. 2007. Micro ora of extreme arid Atacama Desert soils. Soil biology and biochemistry 39, 704-708.
CrossRef - Stomeo, F., Valverde, A., Pointing, S. B. 2013. Hypolithic and soil microbial community assembly along an aridity gradient in the Namib Desert. Extremophiles17, 329-337.
CrossRef - Lau, M. C., Aitchison, J. C., Pointing, S. B. 2009. Bacterial community composition in thermophilic microbial mats from five hot springs in central Tibet. Extremophiles13, 139-149.
CrossRef - Gul-Guven, R., Guven, K., Poli, A., Nicolaus, B.2008; Anoxybacilluskamchatkensis subsp. asaccharedens subsp. nov., a thermophilic bacterium isolated from a hot spring in Batman. Journal of General and Applied Microbiology 54, 327-334.
CrossRef - Derekova, A., Mandeva, R.,Kambourova, M. 2008. Phylogenetic diversity of thermophilic carbohydrate degrading bacilli from Bulgarian hot springs. World Journal of Microbiology and Biotechnology24, 1697-1702.
CrossRef - Sharma, A., Pandey, A., Shouche, Y. S., Kumar, B.,Kulkarni, G. 2008.Characterization and identification of Geobacillus spp. isolated from Soldharhot spring site of Garhwal Himalaya, India. Journal of Basic Microbiology48, 1-8.
- Sievert, S. M., Ziebis, W., Kuever, J., Sahm, K. 2000. Relative abundance of Archaea and Bacteria along a thermal gradient of a shallow water hydrothermal vent quantified by rRNA slot-blot hybridization. Microbiology146, 1287-1293.
CrossRef - Maugeri, T. L., Gugliandolo, C., Caccamo, D., Stackebrandt, E. 2001. A polyphasic taxonomic study of thermophilic bacilli from shallow, marine vents. Systematic and Applied Microbiology24, 572-587.
CrossRef - Takacs, C. D., Ehringer, M., Favre, R., Cermola, M., Eggertsson, G., Palsdottir, A., Reysenbach, A. 2001. Phylogenetic characterization of the blue filamentous bacterial community from an Icelandic geothermal spring. Federation of European Microbiological Societies Microbiology Ecology35, 123-128.
CrossRef - Sarhan, M. A., Alamrri, S. 2014. Characterization and Identification of Moderately Thermophilic Bacteria Isolated from Jazan Hot Springs in Saudi Arabia, Egypt. Academic Journal of Biological Sciences, G, Microbiology6(1), 67-75.
CrossRef - Chanal, A., Chapon, V., Benzerara, K. 2006. The desert of Tataouine: an extreme environment that hosts a wide diversity of microorganisms and radiotolerant bacteria. Environmental Microbiology 8, 514-525.
CrossRef - Connon, S. A., Lester, E. D., Shafaat, H. S. 2007. Bacterial diversity in hyperarid Atacama Desert soils. Journal of Geophysical Research 112, G04S17.
CrossRef - Fierer, N., Strickland, M. S., Liptzin, D. 2009. Global patterns in below-ground communities. Ecology Letters 12, 1238-1249.
CrossRef - Bahl, J., Lau, M. C., Smith, G. J. 2011. Ancient origins determine global biogeography of hot and cold desert cyanobacteria. Nature Communications2: 163-168.
CrossRef - Lacap, D. C., Warren-Rhodes, K. A., McKay, C. P. 2011. Cyanobacteria and chloroexi-dominated hypolithic colonization of quartz at the hyper-arid core of the Atacama Desert, Chile. Extremophiles15, 31-38.
CrossRef - Makhalanyane, T. P., Valverde, A., Lacap, D.C. 2013. Evidence of species recruitment and development of hot desert hypolithic communities. Environmental Microbiology Reports,5, 219-224.
CrossRef - Richer, R., Banack, S. A., Metcalf, J. S. 2015. The persistence of cyanobacterial toxins in desert soils. Journal of Arid Environments, Part B112, 134-139.
CrossRef - Fierer, N., Leff, J. W., Adams, B. J. 2012. Cross-biome metagenomic analyses of soil microbial communities and their functional attributes. Proceedings of the National Academy of Sciences109, 21390-21395.
CrossRef - Adiguzel, A. Molecular characterization of thermophilic bacteria isolated from water samples taken from various thermal plants Ph.D. Thesis,Atatürk University, Graduate School at Natural and Applied Sciences, Erzurum, Turkey2006.
- Zaliha, R. N., Rahman, R. A., Leow, T. C., Salleh, A. B., Basri, M. 2007. Geobacilluszalihae sp. nov., a thermophilic lipolytic bacterium isolated from palm oil mill effluent in Malaysia. BMC Microbiology7, 77-87.
CrossRef - Makhalanyane, T. P., Valverde, A., Gunnigle, E., Frossard, A., Ramond, J. Cowan, A. P.2015. Microbial ecology of hot desert edaphic systems. FEMS Microbiology Reviews39, 203–221.
CrossRef - Eckert, D. Sims, J.T. Recommended soil pH and lime requirement tests(ed). Recommended soil testing procedures for the northeastern United States. Northeast regional bulletin # 493. 3rd edn. Agricultural Experiment Station, University of Delaware, NewYark, DE. 2011, pp 19 -25.
- Wolf, A.M. Beegle, D.B. Recommended soil pH and lime requirement tests (ed). Recommended soil testing procedures for the northeastern United States. Northeast regional bulletin # 493. 3rd edn. Agricultural Experiment Station, University of Delaware, Newark, DE. 2011; 19 -25.
- Gavlak, R., D. Horneck, and R. Miller. Plant, soil and water reference methods for the Western Region. Western Regional Extension Publication (WREP), 2005; 125, WERA-103 Technical Committee.
- Flanagan J.L., Brodie E.L., Weng L., Lynch S.V., Garcia O., Brown R. et al. 2007. Loss of bacterial diversity during antibiotic treatment of intubated patients colonized with Pseudomonas aeruginosa. Journal of Bacteriology45, 1954–1962.
CrossRef - Kumar, S., Stecher, G., Tamura, K. 2016. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Molecular Biology and Evolution33, 1870-1874.
CrossRef - Lee Li., Goh Ki., Chan Ch., Tan Ge., Yin Wa., Chong Ch., Chan Ko. 2018. Microbial diversity of thermophiles with biomass deconstruction potential in a foliage-rich hot spring. MicrobiologyOpen 7, 615.
CrossRef - Aanniz, T., Ouadghiri, M., Melloul, M., Swings, J., Elfahime, E., Ibijbijen, J., Amar, M. 2015. Thermophilic bacteria in Moroccan hot springs, salt marshes and desert soils. Brazilian Journal of Microbiology46(2), 443-453.
CrossRef - Mohammad B., Al Daghistani H., Jaouani A, Abdel-Latif S., Kennes C. 2017. Isolation and Characterization of Thermophilic Bacteria from Jordanian Hot Springs: Bacillus licheniformis and Thermomonashydrothermalis Isolates as Potential Producers of Thermostable Enzymes. International Journal of Microbiology 12.
CrossRef - Al-Shammary, A., Sulieman, A., Abdelmageed, A., Veettil, V. Microbiological Study of the Soil in Hail industrial Zone, Kingdom of Saudi Arabia. Journal of Microbiology Research7(1), 8-13.
- Bahkali, A.,Khiyami, M. Isolation of thermophiles (>45 oC) and hyperthermophiles (>80 oC) bacteria and evaluation their enzymes and biosurfactants. Kingdom of Saudi Arabia, King Abdulaziz City for Science and Technology General Directorate of Research Grants Programs, 2008.
- Khalil, A. 2011. Isolation and characterization of three thermophilic bacterial strains (lipase, cellulose and amylase producers) from hot springs in Saudi Arabia. African Journal of Biotechnology10(44): 8834-8839.
CrossRef - Alotaibia, H. Sonbola, H. Alwakeela, S. Suliman R. Fod, R. S.AbuJaffal, A. AlOthman, N. Mohammeda, A. 2020. Microbial Diversity of Some Sabkha and Desert Sites in Saudi Arabia. Saudi Journal of Biological Sciences2778-2789.
CrossRef - Murgia, M. Fiamma, M. Barac, A. Deligios, M. Mazzarello, V. Paglietti, B. Cappuccinelli, P. Al‐Qahtani, A. Squartini, A. Rubino, S. Al‐Ahdal, M. 2019. Biodiversity of fungi in hot desert sands, MicrobiologyOpen8:1-10.
CrossRef
(Visited 635 times, 1 visits today)

This work is licensed under a Creative Commons Attribution 4.0 International License.





