Manuscript accepted on : 8 December
Published online on: --
Plagiarism Check: Yes
Reviewed by: Dr. Avinash Cau 
Second Review by: Dr. Rasha Hadi
![]()
Final Approval by: Dr. Chateen Izaddin Ali Pambuk![]()
Department of Zoology Asansol Girls’ College, Asansol, 713304, West Bengal, India.
Corresponding Author Email-ray.supriya@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2872
ABSTRACT: Introduction: Mastacembelusarmatus, an indigenous fish species of southern Asia, also resides in Indian subcontinent. This fish species is facing an alarming declining in their number in the last decade. Due to its moderate cost, it is mainly taken by the lower income group of people of the society. Reproductive care, by artificial breeding, has been taken for those fish species having a high cost in the market or becoming less in number in nature for business purposes or preserving the biodiversity, respectively. Aim of the study: The present study was undertaken to understand the structure of hypothalamic nulceiof M. armatus, because these are ultimately responsible for the maintenance of pituitary-gonadal endocrine cascade. Material and methods:This work had been done purely on histological techniques.Hypothalamic region with the brain was first dissected out then followed by fixation, embedding in paraffin wax, sectioning, staining and microphotography. Results: In the present investigation the nucleus preopticus (NPO) are paired, eachnuclear area being situated on either side of the third ventricle. The NPO iselongated in structure and the differentiated zones, the pars magnocellularis andpars parvocellularis. The neurosecretory nuclei ofnucleus lateralis tuberis (NLT) are very prominent and occupy a position nearer to the pituitary gland. The cells of the NLT aredivided into two subgroups. The comparatively larger α – cells are located anterior end of lateral wall of the hypothalamus and the β – cells are located above the pituitary gland. Conclusion: Understanding the hypothalamic architecture and cell types for this fish species is of immense importance to save this indigenous variety by artificial breeding.
KEYWORDS: Mastacembelusarmatus; Nucleus Preopticus; Nucleus Lateralis Tuberis
Download this article as:| Copy the following to cite this article: Ray S. Characterization of hypothalamic Nuclei in Indian Fresh Water Spiny Eel Mastacembelusarmatus (Lacepede). Biosci Biotech Res Asia 2020;17(4). |
| Copy the following to cite this URL: Ray S. Characterization of hypothalamic Nuclei in Indian Fresh Water Spiny Eel Mastacembelusarmatus (Lacepede). Biosci Biotech Res Asia 2020;17(4). Available from: https://bit.ly/2JZOOVd |
Introduction
Management and conservation of fish together with its breeding biology areessential for successful culture and mobilization of seed resources. Bothenvironmental and hormonal factors are extremely important in regulatingreproductive behavior and spawning in fishes. Various central mechanismstranslate environmental cues into chemical messengers which function to activateand maintain the reproductive organs. In this regard the functional relationshipbetween the hypothalamus and pituitary gland is important, and the pineal glandplays a positive role in regulating sexual maturation. Therefore environment,hypothalamus, pituitary and gonad are the four principle factors which areinterrelated and behave together (Malhotra and Gupta, 1985; Lal and Pandey,1998). The function of pituitary is mostly controlled by the hypothalamus through the synthesis and release of gonadotropin-releasing hormone(GnRH), therefore,acting as a major initiator of the hormonal cascade controlling the reproductiveaxis. Pituitary gonadotrophic hormones and GnRH are important in implicatingthese hormones in gonadal maturation and sex steroid production which plays avery important role in gametogenesis, final maturation of oocytes and spermiation(Parharet al., 2003; Lethimonieret al., 2004). Gonadal activities in teleost fishesprimarily depend on the function of pituitary gonadotrophs and that the pituitary and the gonads exist in a mutual state of excitation and inhibition (Farbridgeetal.,1985; Kaneko et al., 1986). The hypothalamo-hypophyseal complex invertebrates with their neurosecretory nuclei and long axons, is a coordination pointin the vertebrate brain and is known to involve in a complex interaction of a varietyof neurotransmitters which modulate the influence of several trophic hormones bycontrolling their active secretion by releasing or inhibiting hormones within thehypophysis itself (Peter et al., 1991).
Materials and methods
Adult male (average length 15.2 to 15.8 cm) and mean body weight (50g to 75g)and female (average length 17.5 to 17.7 cm) and mean body weight (55g to 70g) ofM. armatuswere procured fortnightly throughout the consecutive years fromparticular pond of Asansol in order to avoid ecological variations than can affectdevelopment of hypothalamus, pituitary and gonads. The fishes were collectedduring the second week of every month from January 2019 to December 2019.As the pituitary gland of M. armatuslodged inside sella turcica, it is difficultto dissect out the pituitary intact along with the brain. The entire brain was exposedby dissection from the dorsal aspect and subsequently immersed in 10% neutralformalin for hardening at the fish collection site. After 45 minutes, the brain including the hypothalamusand the pituitary gland were carefully dissected out from the cranium andsubsequently fixed in Bouin’s fixative, Zenker’s fluid and Eltman fixatives.After proper fixation, pituitary gland throughout the year were placed in 70%ethanol for overnight and subsequently dehydrated through ascending ethanolseries followed by acetone and then cleared in benzene. Tissues were thenembedded in paraffin wax (560C-580C melting point). Mid sagittal section andfrontal section of pituitary gland along with hypothalamus were cut at 4 μmthickness using a Leica RM 2125 RT microtome. Deparaffinized sections ofpituitary and hypothalamus were stained by techniques which areas follows: a)Chrome alum haematoxylinPhloxin (CAHP) (Gomori 1941). b)Aldehyde Fuchsin (AF) (Gabe, 1953).
Results
The cells of the NPO are situated above the optic chiasma in an oblique planeand lie on either side of the ventricle. The cells of the NPO in M. armatusshowconsiderable variation in morphological features and staining reactions (Fig.1).They may be divided into two groups viz., the pars magnocellularis (PMC) andpars parvocellularis (PPC). The PMC occupies the dorsal part of the nucleus and isgenerally composed of relatively larger cells measuring 16.2 μm to 20.5 μm indiameter. The nuclei are 7.5 μm to 9.2 μm. The nuclei of the cells of PMC take up deeper stain probably due to the presence of large amount of intranuclear granulesaround the nucleus (Fig.2). The PPC constitutes ventral part of the NPO. Itcomprises generally smaller cells measuring 12.5 μm to 14.2 μm in diameter(Fig.2). The cells of PMC and PPC are oval. The cytoplasm and nuclei of PMCand PPC varying in abundance and tinctorial intensity during different months ofthe year. The nuclei and surrounding areas of the PMC and PPC cells take up bluish purple colour in chrome alum haematoxylinphloxin stain (Fig.1) and deepaldehyde fuchsin stain (Fig.2) probably due to the presence of large amounts ofintranuclear granules around the nucleus.
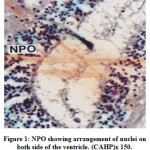 |
Figure 1: NPO showing arrangement of nuclei on both side of the ventricle. (CAHP)x 150. |
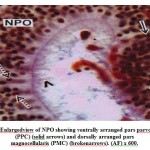 |
Figure 2: Enlargedview of NPO showing ventrally arranged pars parvocellularis (PPC) (solid arrows) and dorsally arranged pars magnocellularis (PMC) (brokenarrows). (AF) x 600. |
The NLT extends longitudinally as far as plane corresponding to the pituitarygland (Fig.3). The cells of the NLT may be of two types viz., the larger cells or α– cells and the smaller or β – cells. This region is highly vascular. The cells of theNLT are paired and occupy nearer to the pituitary gland. The cells of the NLT areconnected by axonal pathway with the pituitary (Fig.3). The α – cells havedistinct nuclei with abundant cytoplasm and generally vary in size from 11.8 μm to14.6 μm. The nuclei generally range from 5.6 μm to 7.8 μm in diameter. The comparatively smaller cells or β – cells occupying a position lateral to α – cellswith scanty cytoplasm. The size varies from 9.2 μm to 11.6 μm and the nucleigenerally range from 3.8 μm to 5.0 μm. The cells of the NLT take reddish purplecolour in aldehyde fuchsin stain (Fig.4).
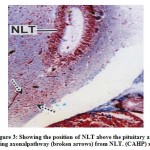 |
Figure 3: Showing the position of NLT above the pituitary andshowing axonalpathway (broken arrows) from NLT. (CAHP) x 100. |
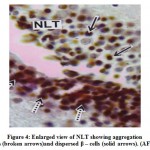 |
Figure 4: Enlarged view of NLT showing aggregation α – cells (broken arrows)and dispersed β – cells (solid arrows). (AF) x 600. |
Discussion
In the present investigation the nucleus preopticus (NPO) are paired, eachnuclear area being situated on either side of the third ventricle. The NPO iselongated in structure and the differentiated zones, the pars magnocellularis andpars parvocellularis. The shape of NPO in fishes has been reported to vary.Chandrasekhar and Khosa (1972) reported that in Ophiocephalus punctatus theNPO is located anteriorly at the point of emergence of the optic nerve while inClariasbatrachusand Heteropneustesfossilisthey occupy a position posterior to it. In the present study the cells of magnocellularis and parvocellularis are AF andCAHP positive.Anterior parvocelular preoptic (PPa) neurons exhibit very staining than neurons from magnocelular preoptic (PM) neurons (Laura Rincón et al., 2017), thus exhibits a close agreement with the author. A similar observation has also been identified in the preopticnuclei of certain teleosts (Sathyanesan and Haider, 1970; Sathyanesan, 1973;Rizkalla, 1976; Bose and Chakrabarti, 2018). Belsare (1967) opined that inOphiocephalus punctatus the occurrence of vacuoles in the cytoplasm and colloiddroplets in the vicinity of blood vessels indicate the state of secretory activity ofthe nucleus preopticus.At posterior diencephalic area, neurons form ventral hypothalamic area, located around diencephalic ventricle do show round and strongly stained nuclei, with scarce cytoplasm (Camilo R. Q et. al., 2019), also superimposed with present findings.
In the present observation, the neurosecretory nuclei of NLT are veryprominent and occupy a position nearer to the pituitary gland. The cells of the NLTas observed in the present study, may be divided into two subgroups. The comparatively larger α – cells are located anterior end of lateral wall of thehypothalamus and the β – cells are located above the pituitary gland. The nuclei ofα – cells and β – cells respond to CAHP and AF staining. Samuelsson et al., (1968)suggested that the groups of nerve fibre cells situated in the infundibular region ofthe teleost hypothalamus constitute the paired nucleus lateralis tuberis (NLT). Thedivision of NLT cells into two subgroups have been suggested by Desai andAkhunji (1971) in Pampus argenteus and Sathyanesan (1973) in Catlacatla. Desaiand Akhunji further reported AF negative and CAHP positive NLT cells in twospecies of Hilsa and Pampusrespectively. On the contrary, Jose and Sathyanesan(1977) reported that in Labeorohitathe ventromedian component of the NLT is AF positive whereas the anterolateral neurons are AF negative. This studyindicates that the cells of NLT vary in their staining reactions in fishes. In M.armatusaxons arising from NLT cells are traceable during the maturation andspawning periods when they come in close contact with blood capillaries. Theaccumulation of neurosecretory materials (nsm) occurs in the subterminal area andnsm are found to accumulate around the blood capillaries. The nsm play pivotal role in maintaining the hypothalamo – pituitary – gonadal cascade. Up-regulated transcription of brain FSHβ and LHβ along with ovarian ERα, FSHR and LHR suggested positive feedback regulation in the HPGL-axis (Jie Hou,2016).Kasuga and Takahashi(1971), Sathyanesan and Jose (1975) have also made similar observations in otherteleosts. There is some relation between secretory phenomena in the NLT and thematuration of gonocytes (Belsare, 1967). In M. armatusit has been observed thatthe probable passages of neurosecretory materials from the NLT cells are along theaxonal routes as well as blood capillaries. The cells of the NLT undergo seasonalcyclical changes which appear to correspond with quantitative variations inpituitary gonadotrophin.Existence of a hypothalamic neurosecretory control over pituitary function that occurs in teleost fish was histologically demonstrated by Adina Popescu et.al. (2020).
Conclusion
In MastacembelusarmatusNPO are paired, each nuclear area being situated on either side of the third ventricle. The NPO is elongated in structure and the differentiated zones, the pars magnocellularis and pars parvocellularis. Both nuclei are CAHP and AF positive. The NLT are very prominent and occupy a position nearer to the pituitary gland. The cells of the NLT as observed in the present study, may be divided into two subgroups. The comparatively larger α – cells are located anterior end of lateral wall of the hypothalamus and the β – cells are located above the pituitary gland. The nuclei of α – cells and β – cells respond to CAHP and AF staining.Understanding the pituitary architecture and cell types for this fish species is of immense importance to save this indigenous variety by artificial breeding.
Acknowledgement
I would like to acknowledge Prof. (Retd.) P. Chakrabarti, Department of Zoology, The University of Burdwan and Mainak Banerjee, research scholar Department of Zoology, The University of Burdwan, for guiding me during the time of preparation of the manuscript.
Conflict of interest
Author does not have any conflict of interest.
References
- Adina Popescu, Daniela Cristina Ibanescu, FanicaBalanescu, ‘’Pituitary – lobulation and seasonal changes of the basophil pituitary in cyprinids’’. Animal Science. LXIII, (1), pp. 247-252, 2020.
CrossRef - Bose S. and P. Chakrabarti,‘‘Changes in the hypothalamus in relation totesticular cells during growth, maturation and spawning phases in thebrackishwater teleost Liza parsia(Ham.)’’. Indian J.Fish,65(1) , pp. 40-46, 2018.
- BelsareD.K. ‘‘Periodic activity of the pituitary gland and nucleuspreopticus in Channa punctatus’’. Zool. Polonica, 17, pp. 273-285, 1967.
- Camilo Riaño-Quintero, Edwin Gómez-Ramírez and Hernán Hurtado-Giraldo.Glyphosate commercial formulation effects on preoptic area and hypothalamus of Cardinal Neon Paracheirodonaxelrodi (Characiformes: Characidae). Neotropical Ichthyology, 17(4), e190025, 2019.
CrossRef - Chandrasekhar, K. and D. Khosa, ‘‘Histomorphological studies on theneurosecretory system of three genera of freshwater teleotean fishes’’. Proc. Ind.Acad. Sci., Sec. B, springer, 75(6),pp. 257 – 265. 1972.
CrossRef - Desai K. and U. U. Akhunji. ‘‘Histological studies on the hypothalamoneurohypophyseal complex of Pampus argenteus’’. Zool. Jap, 44 (3),pp. 161-169, 1971.
- M.G. Burke and J.K. Leatherland. ‘‘Seasonal changes in thestructure of the adenohypophysis of the brown bull head, Ictalurus nebulosus(Leusuer)’’. Cytobios, 44,pp. 49-66, 1985.
- Gabe M. ‘‘Sur quelques applications de la coloration par la fuchsine paraldehyde’’.Bull. Micr. Appl, Paris, 3,pp. 153-162, 1953.
- ‘‘Observations with differential stains on human islets of Langerhans’’. Amer. J. Path, 17(3), pp. 395, 1941.
- Jie Hou, Li Li , Ning Wu, YujingSu, Wang Lin, Guangyu Li, “Reproduction impairment and endocrine disruption in female zebrafish after long – term exposure to MC – LR : A life cycle assessment’’ Environmental Pollution, 208 (B), pp 477 – 485, 2016.
CrossRef - 11. Jose T.M. and A.G. Sathyanesan. ‘‘Pituitary cytology of the Indian carp Labeorohita(Ham.)’’. Anat. Anz, 142(4), pp. 410-423,1977.
CrossRef - Kaneko T. K. Aida and J. Haryu. ‘‘Ultrastructural changes in thepituitary gonadotrophs during the annual reproductive cycle of the female chichibugoby, Triclenfiger obscurus’’. Cell Tissue Res, 246, pp. 137-144, 1986.
CrossRef - Kasuga, S. and H. Takahashi. ‘‘The preoptico-hypophysial neurosecretory system of the medaka, Oryziaslatipesand its changes in relation to the annual reproductive cycle under natural conditions’’. Bull. Fac. Hakkaido Univ,21(4),pp.259-268, 1971.
CrossRef - Lal K.K. and A.K. Pandey. ‘‘Hypothalamo-neurosecretory system of thefemale seabass, Lates calcarifer (Bloch), with special reference to gonadalmaturation’’. Indian J. Fish, 45, pp. 51-60, 1998.
CrossRef - Laura Rincón1, Martha J. Obando2, Mario O. Tovar, MatíasPandolfi and Hernan Hurtado, “Topological and histological description of preoptic area and hypothalamus in cardinal tetra Paracheirodonaxelrodi” (Characiformes: Characidae), Neotropical Ichthyology, 15(1), e160145, 2017.
CrossRef - Lethimonier, C., T. Madigou, J.A. plunoz-cueto, J.J. Lareyre and O. Kah. ‘‘Evolutionary aspects of GnRHs, GnRH neuronal systems and GnRHreceptors in teleost fish’’. Gen. comp. endocrinol, 135 (1), pp. 1-16, 2004.
CrossRef - Malhotra, Y.R. and K.Gupta, ‘‘Histophysiology of hypothalamohypophysealsystem in relation with reproductive cycle in Puntius sophore(Ham.)’’Zool. Orient, 2, pp. 59-65, 1985.
CrossRef - Parhar, I.S., T. Soga, S. Ogawa and Y. Sakuma. ‘‘FSH and LH-β subunitsin the preoptic nucleus: Ontogenic expression in teleost’’. Gen. Comp. Endocrinol,132(3), pp. 369-378, 2003.
CrossRef - Peter, R.E., V.L. Trudeau and B.D. Sloley.‘‘Brainregulation of reproduction in teleosts’’. Bull. Inst. Zool., Acad. Sinica (Monograph),16, pp. 89-118, 1991.
- Rizkalla. W. ‘The hypothalamic neurosecretory system of the marine teleost fish, Mugil auratus’. Risso. Acta Biol. Acad. Sci., Hung, 27(2-3),pp.163-170,1976.
- Samuelsson, B., B. Ernholm and G. Fridberg. ‘‘Light microscopic studies on the Nucleus Lateralis Tuberis and the pituitary of the perch, Leuciscus rutilus with reference to the nucleus-pituitary relationship’’. Acta Zoologica, 49(1-2),pp. 141- 153, 1968.
CrossRef - Sathyanesan, A.G. and S. Haider. ‘‘Hypothalamo-neurohypophysialcomplex of the teleost, Heteropneustesfossilis(Bl.) with some experimentalevidence on the regeneration of the neurosecretory tract’’. Ind. J. Exp. Biol, 8(3),pp. 174-178,1970.
- Sathyanesan, A.G. ‘‘Hypothalamo-hypophyseal neurosecretory system ofthe freshwater teleost, Catlacatla(Ham.)’’. Zool. Beitr, 19(2),pp. 163-178, 1973.
- Sathyanesan, A.G. and T.N. Jose. ‘‘Hypothalamo-hypophysial vascular and neurosecretory link in the teleost, Bagariusbagarius(Ham.)’’.Cell Tissue Organs, 93(3), pp. 387-398, 1975.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





