Manuscript accepted on : 14-01-2021
Published online on: 21-01-2021
Plagiarism Check: Yes
Reviewed by: Dr. Mahmoud Elalfy
Second Review by: Dr. Saeid Taghavi
Final Approval by: Dr. Everaldo Silvino dos Santos![]()

Biosynthesis and Characterization of Silver Nanoparticles using Ziziphus mauritiana Leaf Extract
Neeshat Fathima1 , Shaistha Afreen1
, Shaistha Afreen1 ,Thirumavalavan Muniyan1
,Thirumavalavan Muniyan1 , Venkatesa Prabhu Sundramurthy2*
, Venkatesa Prabhu Sundramurthy2* and Nahom Daniel2
and Nahom Daniel2
1Department of Biotechnology, Sreenidhi Institute of Science and Technology, Hyderabad, Telangana, India.
2Department of Chemical Engineering, Addis Ababa Science and Technology University, Addis Ababa, Ethiopia
Corresponding Author Email: venkatchemdata@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2873
ABSTRACT:
In the area of Nano technological research, green synthesis of Nanoparticles (NPs) has pulled in a ton of interest in light of the fact that the green-synthesized Ag NPs show more prominent antimicrobial and inhibitory qualities, in perspective on which they could be utilized in various applications in the areas of medical and drug delivery. It might be the most appropriate option for the conventional techniques that are commonly conflicting and exert dangerous impacts on the earth. In this research, green synthesis of silver NPs using Ziziphus mauritiana leaf extract was carried out. The synthesized Ag NPs were characterized using UV–V is spectrophotometry, scanning electron microscopy (SEM), Fourier-transform infrared spectroscopy (FTIR), and X-ray diffraction (XRD) techniques. The consequences of UV-Vis spectroscopy showed plasma resonance peaks around 413 nm, that exhibited the existence of Ag NPs. The result observedfromSEM demonstrated thatNPs werefound inspherical and in the 4–96 nm range. The practical gatherings forNPs synthesis using organic compounds with minimized procedure of biosynthesis and stabilization of silver NPs were studied with FTIR and were observed to be phenols, alcohols, primary amines, and alkenes. The XRD pattern demonstrated the FCC structure of AgNO3 and average particle was observed to be 12.0 nm.
KEYWORDS: FTIR; Plasma Resonance; Silver Nanoparticles; SEM; X-Ray Diffraction
Download this article as:| Copy the following to cite this article: Fathima N, Afreen S, Muniyan T, Sundramurthy V. P, Daniel N. Biosynthesis and Characterization of Silver Nanoparticles using Ziziphus mauritiana Leaf Extract.Biosci Biotech Res Asia 2020;17(4). |
| Copy the following to cite this URL: Fathima N, Afreen S, Muniyan T, Sundramurthy V. P, Daniel N. Biosynthesis and Characterization of Silver Nanoparticles using Ziziphus mauritiana Leaf Extract.Biosci Biotech Res Asia 2020;17(4). Available from: https://bit.ly/2Mbsclr |
Introduction
Nanotechnology has turned into a prominent zone of the revolutionary interdisciplinary research that manages structure, synthesis, and manipulation of nano sized particles. It has an immense potential for use in various applications such as drug gene delivery, biomedical sciences, and mechanics (Shanmuganathanet al., 2018). However, research on nanoparticles (NPs) still gainingimmense interest towards different synthesis methods and applications (Ghiassiet al., 2018). Traditionally, chemical and physical syntheses of NPs yield byproducts that are dangerous and unsafe to environment; also, these methods are not efficient (Mancuso et al., 2020). Keeping this view, researchers focus to use‘green engineering science’ that incorporates a wide scope of potential applicationswith ecologically friendly.It also provides procedure that lessen squander items and pollution (Bankaret al., 2010; Begumet al., 2018; Farzanehet al., 2020). Therefore, green synthesis gains an immense interest, which includes the utilization of ecofriendly compatible materials such as bacteria, fungi, and plants (Ahmad et al., 2010;Farzanehet al., 2020). In this manner, green synthesis forproduction of NPs is developing rapidly that fills as a critical strategy for growing clear, safe, and eco-accommodating methodology for the synthesis metal NPs (Yallappaet al., 2013). Benefits of green synthesis of NPs over conventional methods include being economical and easy to regulate. Besides it produces less wastage, is an energy-efficient procedure, has decreased rates of fewer accidents, yields safe products, is competitive, and contributes to healthier workplaces and communities (Kumar et al., 2016). Nature has structured various strategies for the synthesis of nano- and micro-sized inorganic materials. They help being developed of a novel and uninvestigated area zone of research dependent on the green synthesis of NPs (Mandalet al., 2018). Numerous works have been distributed on the green synthesis of NPs utilizing microorganisms such as fungi, bacteria, and plants, because of their diminishing qualities that lead to the decrease of metal compounds to respective NPs (Mittal et al., 2017). Among many NP biosynthesis methods, microbe-mediated synthesis is not thought to be feasible on industrial scale as it requires high aseptic conditions and a considerable maintenance (Sinha et al., 2015). Accordingly, plant extract uses for this reason is conceivably valuable over microbes because of the simplicity of the less biohazard and opportunity from expand procedure of keeping up cell cultures (Pambuket al., 2019; Setegnet al., 2020). In such a way, plant extracts provide a better platform for synthesis of NPs as it skipsthe use of harmful chemicals and yields natural capping agents (Obaid et al., 2015; Sasikalaet al., 2015; Ganjkhanlu and Sara, 2019). Among the different important NPs, silver NPs are becoming a quite significant product as they have gained huge interest because of their novel characteristics like chemical stability, excellent conductivity, catalytic activity, and antifungal, antibacterial, and antiviral properties (Parmar et al., 2011; Sureshet al., 2020). On account of their good anti-inflammatory activities, silver NPs can be consolidated into various applications, for example, cryogenic superconducting materials and composite fibers, into cosmetics, and into electronic parts as well. They exhibit extensive biocidal action against microorganisms by disrupting their unicellular membrane (Sutradhar and Saha, 2015). As a consequence, it has huge potential in biomedical applications such as topical creams, antiseptic sprays, fabrics, and wound dressings. A few investigations have been led on the utilization of plant concentrates to blend silver NPs. Several phytocompounds acquired from leaf extracts of Solidago altissima(Kumar et al., 2016), Acalypha indica (Krishnarajet al., 2010; Sorbiunet al., 2018),Murrayakoenigii (Christensen et al., 2011, Al-Quwaieet al., 2020), Xanthium strumariumL. (Mittal et al., 2017), seed extricate of Acacia farnesiana(Yallappaet al., 2013), Ocimum sanctum (Ahmad et al., 2010), root extracts of Trianthemadecandra(Geethalakshmi and Sarada, 2012), Macrotyloma uniflorum (Vidhu et al., 2011), fruit extricate of Musa paradisiaca peels (Bankaret al., 2010), Carica papaya (Jain et al., 2009), and stem extracts of O. sanctum (Ahmad et al., 2010; Farzanehet al., 2021) to fill in as diminishing or/and topping specialists reaction with silver nitrate (AgNO3) as precursor have been studied. In any case, plausibility of plants to be utilized as organic materials for synthesizing NPs has not been totally considered.
Among the members of the family Rhamnaceae, plants of genus Ziziphus have been utilized for a long time due to their medicinal and nutritive properties (Sutradhar and Saha, 2015, Khataket al., 2020). They are common plants that are mostly available throughout the world. There are about 40 species available in genus Ziziphus. Among them, Z. mauritiana is the one that mostly grows in dry places and has abundant amount of starch, sugar, carbohydrate, mucilage, proteins, and vitamins (Parmar et al., 2011). The dried ripe fruit of this plant is a mild laxative and fruits are used for treating depression, diabetes, and ulcers (Lopez et al., 2018). It is utilized as a medicine in fevers and the leaves of this plant are useful in liver problem and asthma. Also, the powder of its leaves is applied on wounds. It is appeared to have antioxidant, antimicrobial, antitumor, and anticancer activities, consequently demonstrated to be one among the most encouraging plants to be utilized for phytomedicinal applications (Parmar et al., 2011).Recently, Z. mauritianaleaves were used for biosynthesis of copper oxide (CuO) andgold NPs.Using this plant material, silver NPs were not yet synthesized by biological method using aqueous extract of Ziziphus mauritianaL. leaves as a reducing and stabilizing agent. In this study, Z. mauritianaleaves was used as plant source for biosynthesis of silver NPs. Further, integrated NPs were portrayed by various techniques.
Methodology
Preparation of Plant Extract
Every single fine chemical from Merck (Mumbai, India)and solvents, and media used in this investigation were acquired from HiMedia (Mumbai, Maharashtra, India) and of AR grade. Every one of the arrangements were made in sterile Milli-Q water. Commonly, a plant-extract-mediated biosynthesis of NPs incorporates blending the watery plant separate with a fluid solution of the suitable metal salt. Here, Z. mauritiana leaf concentrate was utilized to get ready silver NPs thinking about simplicity of availability, cost-effectiveness, and medicinal properties. Z. mauritianaleaves are alternate and elliptic. Flowers are small and bisexual. The leaves are about 2.5 – 3.2 cm long. Fresh leaves were collected from Hussain Sagar Road, Hyderabad district, Telangana, India, in the month of January. They were surface-cleaned by tap water followed by double-distilled water to remove contaminated organic contents and other debris. Additionally, they were parched at room temperature and turned into a fine powder utilizing an electric blender. About 10 g of the powder was overflowed with 150 mL double-distilled water for 30 min at 70-80 °C and was hatched overnight. The extract was chilled off and sifted with Whatman Filter Paper Number 1. The filtrate was centrifuged at 13000 rpm for 3 minutes and the supernatant was utilized for the further explores.
Biosynthesis of Silver NPs
Initially, 500 mL, 1 mM (0.08 g silver nitrate was disintegrated in 500 mL distilled water to prepare 1 mM solution of AgNO3) solution of silver nitrate was set up in an Erlenmeyer flask. Thereafter, 50 mL aqueous extract of Z. mauritiana leaves was mixed with 500 mL AgNO3 solution. The solution mixture was heated on a mantle at 70-80 °C for 30 min. To maintain a strategic distance from photo-activation, the solution was brooded in a dim chamber at room temperature overnight. Decrease of Ago from Ag+was affirmed by the adjustment in color of the solution, from colorless to brown. After medium-term incubation, the mixture was centrifuged at 12000 rpm for 4 minutes. Supernatant was isolated and the pellets were washed two times with twofold refined water. Further, particles were secluded by presenting to centrifugation again at 12000 rpm for 3 min. The washed pellets were collected in a watch glass and left to dry in a hot-air oven at 30-42 °C. After totally drying out, the AgNPs, which seemed dim dark colored, were scratched utilizing a surgical tool and put away in a cool dry spot.
Characterization of Silver NPs
Fundamentally, the silver NPs synthesized from the Z.mauritiana leaf extract were portrayed by UV–Vis spectral analysis, which was completed by using a UV–Vis spectrophotometer (UV-1800 Model; Shimadzu, Japan) with goals of 1 nm somewhere in the scope of 300 and 600 nm with de-ionized water as clear. At that point, 1 mL sample was lay hold of test tube and in this way dissected at room temperature. Dynamic light dispersing (Spectroscatter 201) was done to choose the ordinary size of blended silver NPs. Fourier-transform infrared spectroscopy (FTIR) was utilized to distinguish the conceivable biomolecules present that are in charge of keeping up the dependability, capping, and formation of silver NPs in the Z. mauritiana aqueous leaf extract. It likewise decided the potential physiochemical connections among the components of the extract. The estimations were taken for the synthesized silver NPs after 24 h incubation using an FTIR spectrophotometer (8400S; Shimadzu) with a wavelength scope of 4000–500 cm–1 and resolution of 4 cm−1. The examples were joined into KBr pellets to gain the spectra. The got outcomes were looked at for computing shift in functional peaks of critical value. The morphology of the synthesized silver NPs was contemplated by a SEM (S-3700N; Hitachi, Japan). The crystalline reality of the synthesized silver NPs was investigated using an X-ray diffractometer (XRD700 Model; Shimadzu) with K-beta filter, using monochromatic Cu Kα radiation of wavelength 1.5418 Å. The X-ray generator was worked at 30 mA and 40 KV, and the checking mode was nonstop with scanning range (2θ) from 4 o to 90o.
Results and Discussion
Visual Monitoring and UV-Vis Spectroscopy
It is notable that silver NPs display a solid retention band in the obvious region. In this analysis, expansion of plant extract of Z. mauritiana to aqueous solution of silver nitrate prompted the adjustment in the color of the blend from yellowish to rosy dark colored (reddish brown). The imaged properties of silver NPs were examined by UV–Vis assimilation spectroscopy. The Plasmon resonance reverberation band distinguished at 400-430 nm is practically like that revealed by Obaid et al. in 2015, and is delineated in Figure 1. The slight varieties in the estimations of absorbance imply that there is adjustment in particle size. The reddish brown color showed up because of the excitation of the surface plasmon resonance (SPR). Silver NPs having absorbance esteems in the visible scope of 410-448 nm have been accounted for somewhere else. The examined tests displayed a strong and steady noticeable ingestion spire in the scope of 413 nm (Figure 2) because of excitation of SPR. The results are near the reports introduced in literature demonstrating absorbance crest at 413 nm for silver NPs combined by Cochlospermumreligiosum extricate (Sasikalaet al., 2015) and by Pithophoraoedogonia extract (Sinha et al., 2015).
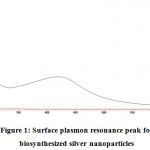 |
Figure 1: Surface plasmon resonance peak for biosynthesized silver nanoparticles |
X-ray Diffraction Analysis
So as to check the aftereffects of the UV–V is spectral analysis, the examples of the synthesized silver NPs were analyzed by X-ray diffraction (XRD). Figure 2 outlines the XRD pattern for silver NPs. The diffractogram comprised of four distinct reflections at 38.01° (1 1 1), 44.35° (2 0 0), 64.39° (2 2 0), and 77.37° (3 1 1). Consequently, the XRD result affirmed the crystalline nature of the sample. The XRD pattern additionally demonstrated a peak at 31.67°. This might be because of the presence of other organic compounds of the leaf extract or crystalline impurities present on the surface of silver NPs. For example, a few investigations describing green synthesis of silver NPs using plant extracts by XRD analysis have likewise revealed the presence of comparable additional peaks in the XRD pattern of silver NPs.
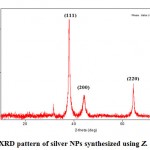 |
Figure 2: XRD pattern of silver NPs synthesized using Z. mauritiana. |
Table 1: Values of diffraction peaks in the range of 2θ and FWHM
| Sl. No. | 2θ (deg) | FWHM (deg) |
| 1 | 31.673 (8) | 0.10 (3) |
| 2 | 38.014 (14) | 0.563 (16) |
| 3 | 44.35 (4) | 1.15 (4) |
| 4 | 64.39 (2) | 0.47 (4) |
| 5 | 77.377 (15) | 0.44 (4) |
Scanning Electron Microscope Studies
Figure 3 demonstrates the images of SEM synthesized silver NPs, which uncovers that a large portion of the silver NPs are predominately round with smooth surface, and the particles are in the scope of 4-96 nm. Additionally, the examination utilizing SEM showed that the totals of silver NPs were very much scattered in a balanced out structure as they were in indirect contact with each other because of plant-capping agents. Using software, ImageJ, it was discovered that the averageparticle size was about 13.25 nm.
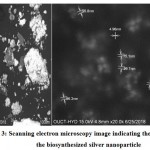 |
Figure 3: Scanning electron microscopy image indicating the size of the biosynthesized silver nanoparticle |
FTIR Spectroscopy Studies
The results of the synthesized NPs from FTIR were looked at for figuring the move in functional peaks of critical value. The spectrum is spoken to in Figure 4. The FTIR spectrum studies of the Z.mauritiana leaf extract shows various bands, which uncovers a perplexing nature of the leaf extract. An aggregate of 41 pinnacles were watched, among which 3 sharp peaks were seen at 3417.98, 3444.98, and 1629.90 cm–1 relating to the O-H stretch of the alcohol, N-H stretch of amides, ArO-H H-bonded phenol compound, and Ar-CH=CHR compound of alkenes, as appeared in Figure 4. Likewise, the outcomes were analyzed using IRPal2.0 software. Peak value and the functional groups present in leaf extracts of Z. mauritiana obtained by FTIR studies are shown in Table 2. They demonstrate the presence of natural mixes containing carbon and oxygen, along these lines proposing that silver may be topped by organic components of plant extract, stabilizing them and further upgrading their antimicrobial activity. The shifting of bands at 3444.12 and 1629.90 implies the presence of organic components such as phenols, alcohols, alkenes, and primarily primary amines of the plant associated with the decrease procedure for arrangement of silver NPs. Carboxyl groups, a middle formation of phenolic gatherings, proteins, and carbohydrates of Z. mauritiana leaf extract are associated with the decrease forms for synthesis of silver NPs.Silver NPs are built up as antimicrobial agents, while the presence of plant bioorganic capping material on the silver NPs gives them improved antibacterial movement and potential to be utilized as antioxidant agents.
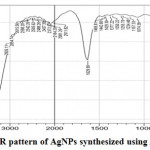 |
Figure 4: FTIR pattern of AgNPs synthesized using Z. mauritiana |
Table 2: FTIR peak value and its functional groups present in leaf extracts of Z. mauritiana
| Peak | Range | Bond | Compound | Structure |
| 3444 | 3600-3400 | O–H stretch | Alcohols | RCH2OH |
| 3600-3400 | O–H stretch | Alcohols | R2CHOH | |
| 3600-3400 | O–H stretch | Alcohols | R3COH | |
| 3445-3435 | NH- stretch | Amides | RCONHR | |
| 3500-3200 | ArO–H H-bonded | Phenols | ArO–H bonded | |
| 3417 | 3600-3400 | O – H stretch | Alcohols | RCH2OH |
| 3600-3400 | O – H stretch | Alcohols | R2CHOH | |
| 3600-3400 | O – H stretch | Alcohols | R3COH | |
| 3500-3200 | ArO–H H-bonded | Phenols | ArO–H bonded | |
| 1629 | 1640-1600 | NH out of plane | Amides | RCONH2 |
| 1630-1620 | Ar–CH=CHR | Alkenes | Ar–CH=CHR | |
| 1700-1615 | C=N | – | C=N |
Conclusion
The improvement of cost-efficient and eco-friendly methods for synthesis of nanomaterials still stays a scientific logical challenge. In this study, Z. mauritiana plant leaf extract was effectively used as a diminishing and balancing out agent for consistent and quick synthesis of silver NPs. Silver NPs were synthesized by a clean, nontoxic, low-cost, and eco-friendly method. The green blend of silver NPs was performed by mixing Z. mauritiana leaf extract with 1 mM AgNO3 at 70-80 °C for 30 minutes. The spectroscopic portrayals from UV–Vis, FTIR, SEM, and XRD bolster the development and dependability of the biosynthesized silver NPs. The results of UV–Vis qualitative analysis confirmed the existence of silver as a result of the actual peak in the 400–430 nm region.The SEM analysis affirmed spherical and uniform silver NPs with broadness varying from 4 to 96 nm. This is a simple, proficient, and quick technique for green synthesis of silver NPs that can be utilized in various biomedical and biotechnological applications. Such sort of studies, for instance, production of NPs using plant extracts, which are used for mediating the NPs for rapid single-step protocol, can beat a several environmental issues and can give another dimension to green synthesis of silver NPs.
Acknowledgement
Authors are thankful to Sreenidhi Institute of Science and Technology, Hyderabad, and Addis Ababa Science and Technology University, Ethiopia for providinglaboratory support.
Conflict of Interest
Authors assure to disclose there is no conflict of interest including honorarium, grants, membership, employment, ownership of stock or non‐financial interest.
References
- Abdelghany T. M, Al-Rajhi A. M. H, Al-Abboud M. A, Alawlaqi M. M, Magdah A. G, Helmy E. A. M, Mabrouk A. S. Recent advances in green synthesis of silver nanoparticles and their applications: about future directions. A review. Nano Science. 2018;8(3):5-12.
CrossRef - Ahmad N, Sharma S, Alam M. K, Singh V, Shamsi S, Mehta B, and Fatma A. Rapid synthesis of silver nanoparticles using dried medicinal plant of basil. Colloids and Surfaces B: Biointerfaces. 2010;81(1):81.
CrossRef - Al-Quwaie D. A. H. Extracorporeal Circuit Device for Camel Antibodies Production from Blood Using Magnetic Nanoparticles. Biosci. Biotech. Res. Asia. 2020;17(1): 127-132
CrossRef - Bankar A, Joshi B, Kumar A. R, Zinjarde S. Banana peel extract mediated novel route for the synthesis of silver nanoparticles. Surf. A. 2010;368(1):58.
CrossRef - Begum S. R, Rao D. M, Reddy P. D. S. Role of Green Route Synthesized Silver Nanoparticles in Medicinal Applications with Special Reference to Cancer Therapy. Biosci. Biotech. Res. Asia. 2018;15(4): 783-790
CrossRef - Christensen L, Vivekanandhan S, Misra M, Mohanty A. K. Biosynthesis of silver nanoparticles using Murrayakoenigii (curry leaf): an investigation on the effect of broth concentration in reduction mechanism and particle size. Adv. Mater. Lett. 2011;2(6):429.
CrossRef - Farzaneh M, Saeid T. F, Ali R, Samira O, Ilnaz A. Green sol–gel synthesis of CoMnCrO4 spinel nanoparticles and their photocatalytic application. Micro & Nano Letters. 2020;15(10);674.
CrossRef - Farzaneh M, Saeid T. F,Ali R,Vinod K. G. Green synthesis of recyclable MgFeCrO4 spinel nanoparticles for rapid photodegradation of direct black 122 dye. Photochem. Photobiol. A. 2020;392(1):1
CrossRef - FarzanehM,SaeidT. F,Ali R.B,Sang W,Rajender S.V. Magnetic Mg0.5Zn0.5FeMnO4 nanoparticles: Green sol-gel synthesis, characterization, and photocatalytic applications, J. Clean. Prod, 2021;288(3):125632
CrossRef - Ganjkhanlu, Sara. Biosynthesis of MgFe2O4 magnetic nanoparticles and its application in photo-degradation of malachite green dye and kinetic study, Nanochemistry Research. 2019;4(1): 86-93.
- Geethalakshmi R, Sarada D. Gold and silver nanoparticles from Trianthemadecandra: synthesis, characterization, and antimicrobial properties. J. Nanomedicine. 2012;7(6):5375.
CrossRef - Ghiassi S, Sedaghat S, Mokhtary M, Kefayati H. Plant-mediated bio–synthesis of silver-montmorillonite nanocomposite and antibacterial effects on gram-positive and negative bacteria. Nanostructure. Chem. 2018;8(3):353.
CrossRef - Jain D, Daima H. K, Kachhwaha S, Kothari S. L. Synthesis of plant-mediated silver nanoparticles using papaya fruit extract and evaluation of their anti-microbial activities. J. Nanomater. Bios. 2009;4(3):557.
- Kathiravan V.Green synthesis of silver nanoparticles using different volumes of Trichodesma indicum leaf extract and their antibacterial and photocatalytic activities. Chem. Intermediat. 2018;44(9):4999.
CrossRef - Khatak S, Wadhwa N, Jain P. Probiotics: An Alternative Therapeutic Strategy for Covid-19. Biosci. Biotech. Res. Asia. 2020;17(4): 674.
CrossRef - Krishnaraj C, Jagan E. G, Rajasekar S, Selvakumar P, Kalaichelvan P. T, Mohan N. Synthesis of silver nanoparticles using Acalypha indica leaf extracts and its antibacterial activity against water borne pathogens. Surf. B. 2010;76(1):50.
CrossRef - Kumar V. A, Uchida T, Mizuki T, Nakajima Y, Katsube Y, Hanajiri T, Maekawa T. Synthesis of nanoparticles composed of silver and silver chloride for a plasmonic photocatalyst using an extract from a weed Solidago altissima (goldenrod). Nat. Sci-Nanosci. 2016;7(1):0150.
CrossRef - Lopez-Miranda L. J, Vazquez Gonzalez M. A, Mares-Briones F, Cervantes-Chavez J. A, Esparza R, Rosas G, Perez R. Catalytic and antibacterial evaluation of silver nanoparticles synthesized by a green approach. Chem. Intermediat. 2108;44(12):7479.
CrossRef - Mancuso L, Manis C, Murgia A, Isola M, Salis A, Piras F, Caboni P, Cao G. Effect of Zno Nanoparticles on Human Bone Marrow Mesenchymal Stem Cells: Viability, Morphology, Particles Uptake, Cell Cycle and Metabolites. Biosci. Biotech. Res. Asia. 2018;15(4).
CrossRef - MandalP, GhoshS. Green synthesis of poly (vinyl alcohol)–silver nanoparticles hybrid using Palash (Butea monosperma) flower extract and investigation of antibacterial activity. Bull. 2018;75(5):1949.
CrossRef - Milorad C, Glisic S, Cvetkovic D, Miroslav C, Ljiljana S, Bojana D,Katarina C. Green synthesis, characterization and antimicrobial activity of silver nanoparticles produced from Fumaria officinalis L.plant extract. J. 2018;80(2):803.
CrossRef - Mittal J, Jain R, Sharma M. M. Phytofabrication of silver nanoparticles using aqueous leaf extract of Xanthium strumerium L. and their bactericidal efficacy. Nat. Sci-nanosci. 2017; 8(2):1.
CrossRef - Obaid A. Y, Al-Thabaiti S. A, Al-Harbi L. M, Khan Z. Extracellular bio-synthesis of silver nanoparticles. Global Advanced Res. J. of Microb. 2015;3(8):11.
- Pambuk C. I. A, Muhammad F. M. Nanoparticles in Medicine: Applications and Hope. Biosci. Biotech. Res. Asia. 2019;16(3).
CrossRef - Parmar P, Bhatt S, Dhyani S, Jain A. Phytochemical studies of the secondary metabolites of Ziziphus mauritiana Lam. leaves. J. Curr. Pharm. Res. 2011;4(3):153.
- Sasikala A, Linga Rao M, Savithramma N, Prasad T. N. V. K. V. Synthesis of silver nanoparticles from stem bark of Cochlospermumreligiosum (L.) Alston: an important medicinal plant and evaluation of their antimicrobial efficacy. Nanosci. 2015;5(7):827.
CrossRef - Setegn G. A, Fedlu K. S, Dinsefa M. A, Osman A. Z.Green synthesis of p-Co3O4/n-ZnO composite catalyst with Eichhornia Crassipes plant extract mediated for methylene blue degradation under visible light irradiation. Mater. Res. Express. 2020;7(2):95508
CrossRef - Shaikh A. E, Satardekar K. V, Khan R. R, Tarte N. A. Silver nanoparticles: green synthesis using Phoenix dactylifera fruit extract, characterization, and anti-oxidant and anti-microbial activities. Nanosci. 2018;8(3):407.
CrossRef - Shanmuganathan R, Ali D. M, Prabakar D, Muthukumar H, Thajuddin N, Kumar S. S, Pugazhendhi, A. An enhancement of antimicrobial efficacy of biogenic and ceftriaxone-conjugated silver nanoparticles: green approach. Sci. Pollut. Res. 2018;25(11):10362.
CrossRef - Sinha S. N, Paul D, Halder N, Sengupta D, Patra S. K. Green synthesis of silver nanoparticles using fresh water green alga Pithophoraoedogonia (Mont.) Wittrock and evaluation of their antibacterial activity. Nanosci. 2015;5(6):703.
CrossRef - Sorbiun M, Mehr E. S, Ramazani A, Fardood S. T. Green synthesis of zinc oxide and copper oxide nanoparticles using aqueous extract of oak fruit hull (Jaft) and comparing their photocatalytic degradation of basic violet 3. J. Environ. Res. 2018;12(1):29.
CrossRef - Suresh G, Trupti P, Shreyas P, Rajeshwari O, Dhananjay M, Prashant K. Plant Extract Assisted Eco-benevolent Synthesis of Selenium Nanoparticles- A Review on Plant Parts Involved, Characterization and Their Recent Applications, J.Chem.Rev. 2020;2(3):157.
- Sutradhar P, Saha M. Size-controlled synthesis of silver nanoparticles using Zizyphusmauritiana fruit extract. Main Group Chem. 2015;15(1):47.
CrossRef - Vanlalveni C, Rajkumari K, Biswas A, Adhikari P. P, Lalfakzuala R, Lalthazuala R. Green synthesis of silver nanoparticles using Nostoclinckia and its antimicrobial activity: a novel biological approach. Bionanoscience. 2018;8(2):624.
CrossRef - Vidhu V, Aromal S. A, Philip D. Green synthesis of silver nanoparticles using Macrotyloma uniflorum. Acta.A. 2011;83(1):392.
CrossRef - Yallappa S, Manjanna J, Peethambar S, Rajeshwara A, Satyanarayan N. Green synthesis of silver nanoparticles using Acacia farnesiana (sweet acacia) seed extract under microwave irradiation and their biological assessment. Clust sci. 2013;24(4):1081.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





