Manuscript accepted on : 21-12-2019
Published online on: --
Plagiarism Check: Yes
Reviewed by: Dr. Amr Salah
Second Review by: Dr Hossein Jahani-Azizabadi
Final Approval by: Dr. Eugene A. Silow
Production of Rumen-Protected Essential Amino Acids with Chemical Technique
Mitra Mazinani1 , Abas Ali Naserian2*, Brian Rude3, Reza Valizadeh2
, Abas Ali Naserian2*, Brian Rude3, Reza Valizadeh2  and Abdolmansur Tahmasbi2
and Abdolmansur Tahmasbi2
1Student of Ruminant Nutrition, Ferdowsi University of Mashhad, Mashhad, Iran
2Department of Animal Sciences, Faculty of Agriculture, Ferdowsi University of Mashhad, Mashhad, Iran
3Department of Animal and Dairy Sciences, Faculty of Agriculture, Mississippi State University, USA
Corresponding Author E-mail: naserian@um.ac.ir.
DOI : http://dx.doi.org/10.13005/bbra/2795
ABSTRACT: In this experiment, essential amino acid (Methionine) and two chemical compound, were used to make ligands that produce pH-sensitive amino acids that are stable in the rumen and absorbable in the post rumen part of the digestive tract. The treatments were made with heat and steer, reflux and ultrasound reaction at different times, temperatures and solvents to make new bonds and pH-sensitive amino acid ligands. During the first 8 hours of incubation (rumen phase) 55.42% of RPMet1 was released, during the 2 hours (abomasum phase) 91.00% of the compound had been released and during the final hours up to 35h (intestine phase) 93.21% had been released and 6.79% of this product was stable. About the RPMet2 figure shown that in first 8 hours of incubation (rumen phase) 49.25% of RPMet1 was released, in next 2 hours (abomasum phase) 87.93% of the compound was released and in the final hours up to 35h (intestine phase) 94.05% was released and 5.95% of this product was stable. Result shows that this chemical method increased retention time in rumen and the bond is reversible in lower pHs, similar to the abomasum.
KEYWORDS: Essential Amino Acids; Methionine; Rumen Protected
Download this article as:| Copy the following to cite this article: Mazinani M, Naserian A. A, Rude B, Valizadeh R, Tahmasbi A. Production of Rumen-Protected Essential Amino Acids with Chemical Technique. Biosci Biotech Res Asia 2019;16(4). |
| Copy the following to cite this URL: Mazinani M, Naserian A. A, Rude B, Valizadeh R, Tahmasbi A. Production of Rumen-Protected Essential Amino Acids with Chemical Technique. Biosci Biotech Res Asia 2019;16(4). Available from: https://www.biotech-asia.org/?p=34660 |
Introduction
It is well known that ruminants need bioavailable essential amino acids to perform as well as domesticated livestock (2,4). For this goal, if ruminants, like dairy cows, have not met their minimum requirements for essential amino acids such as lysine and methionine, they will not produce milk at optimum yield and generally, their health may be negatively impacted. Providing essential amino acids for ruminants is not simple. For example, bacteria in the rumen are known to routinely degrade amino acid, like lysine and methionine. This is done by bacteria in order to rumen metabolize the amino acids and thus “rob” the animal of the benefit of the amino acid. By the time the metabolized by-product passes from the rumen into the intestine, the amino acid is gone. Therefore, the challenge is to develop products which will allow amino acids to be stable in the rumen environment, but still capable of absorption when they pass from the rumen into the intestine. In other words, the essential amino acids, such as lysine and methionine, need to be bioavailable only in the intestine, and remain stable, and therefore, not metabolized in the rumen.
Previously, feed developers have used fats, minerals, carbohydrates, and binders to protect amino acids from rumen degradation. This technology involves a simple coating of the material in hopes that the coated amino acid is stable in the rumen. Recently, Rhone Poulenc (a French chemical and pharmaceutical company) has provided a pH-sensitive polymer coating. The theory of a pH-sensitive coating polymer for making the amino acid revolves around the pH difference between the rumen and the abomasum of ruminants. The rumen pH is usually 5.5 to 7.0, and the Abomasum pH is 2 to 3. Polymer-coated of essential amino acids (EAA) is a method of accomplishing this by profiting the AA in the rumen environment, but is soluble at lower pH’s of the post ruminal environment (pH 2 to 3), therefore it could be undegraded in the rumen, but be available in the intestine.
Technologies which have been used in the past, like coatings, and the more recently developed pH-sensitive polymers for coating, have had some limited success and some problems. The primary problem with these products is that they may become abraded during human handling and chewing by the animal. If the coat damages, amino acids will become available to rumen microbes and utilized by them, and therefore wasted and not available to the animal. Likewise, if animals abrade the coating with chewing, then it becomes available for rumen microbes to metabolize, and also wasted. Additionally, fat-protected or coated essential amino acids rely upon the fat resistance to enzymes in the rumen that are capable of digesting the protective fat coat, and, on the other hand, the ability of digestion by enzymes post-rumenally. However, if there is not a proper balance between resistance to attack in the rumen and ability to be digested in the intestine, then the potential EAA benefit for the animal may be decreased. From the above description, it can be seen that there is a real and continuing need for the development of products for delivery of EAAs to ruminant animals in a form that allows being stable in rumen, but after delivery to the intestine, highly absorbable and bioavailable. It was the primary objective of this study to improve products intestinal availability to fulfill this need in a safe, effective, efficient manner at a low cost.
History of rumen-protected AA in ruminant
In the past, Zinpro Corporation discovered that calcium or magnesium salts of some amino acids could be used as a bypass rumen product. This product takes a different approach to the problem with the specificity of improving the availability of essential amino acids in ruminants. Lysine and methionine are essential amino acids in the diet of dairy cows and other ruminants. This is because Lysine and methionine cannot be synthesized by mammals at on adequate rate for metabolic requirements so they must be supplied in their ration. Corn base diets are containing little lysine and lysine must be supplemented for both maintaining and health of an animal and also to achieve animal growth economically.
For the first time, Broderick et al. [1], fed an encapsulated Methionine (Met) to early-lactation Holstein cows in different amounts that provided 0, 5, 15, or 45 g/d of Met. The basal diet contained 15.3% CP, and the animals had ad libitum access to urea-treated corn silage and 8 to 11.5 kg/d of a mix concentration (including ground shelled corn, ground oats, soybean meal, urea, minerals, and vitamins), and 2.1 kg/d of legume-grass hay. There was no effect of this supplemented Met on feed intake, milk yield or percentages of milk component. Cow’s average milk was 26 kg per day. Based on increased plasma Met concentrations and plasma Met: Val ratios, it was clear that at least some Met from the supplement was being absorbed. Williams et al. [2] also observed no production benefits of feeding 12 g/d of Met from the same encapsulated Met supplement when cows were fed a basal ration with 60: 40 corn silage and concentrate (corn, oats, dehydrate alfalfa, bran, molasses, urea, minerals, and vitamins) contained 13 to 14% CP. Averaged fat-corrected milk (FCM) production was 18.1 kg/d. In contrast with Broderick et al. [1], Williams et al. [2] did not observe any increase in plasma concentrations of Met. Although there was no evidence that Met was limiting in either experiments. Broderick et al. [1] reported changes of AA concentrations in plasma could be evidence that Met may be the most limiting AA in lactating ruminants. Numerous studies have reported Met and Lys as two most limiting amino acids in lactating dairy cows when fed corn-based rations [3]. Other studies were conducted after the publication of NRC [3] and confirmed that Met and Lys have been implicated as limiting AA in many different feeding conditions ([4], [5], [6], [7], [8], [9], [10]). Other research has shown that Histidine could be the first limited AA in dairy cows fed high-forage grass or silage based rations with barley and oats supplemented ([11], [12]).
Table 1: Chemical and physical properties of two Met compound
| Comp. no | Wavelength Range | Melting point | Physical state | Molecular formula | Molecular weight | Yield | Elemental Analysis | ||||
| C | H | N | O | S | |||||||
| mbnz | 210-230 | 243 | White solid | C12H15NO2S | 237.08 | 91.61 | 60.73 | 6.37 | 5.90 | 13.48 | 13.51 |
| gm | 200-230 | 197 | Pale yellow solid | C15H26N2O4S2 | 362.16 | 77.09 | 49.70 | 7.23 | 7.73 | 17.65 | 17.69 |
Rumen-Protected AA
Interest to develop new rumen-protected amino acids started in the early 1960’s when it was observed that Met is the first limiting amino acid in sheep and some evidence indicated it could be first limiting AA in lactating dairy cows. Early products included Met supplements such as those produced by Sibbald et al. [13]. The Met product of Delmar Company had a core of 20% dl-Met and this core wrapped in a continuous film of tristearin. Although it was shown this product wasn’t effective [1], it also showed a limited intestinal digestibility. It was suggested that Met released from this product was not effective and it needs to be improved, possibly by replacing some of the coating ingredients with CaCO3. The resulting product was named Ketionin and contained 30% dl-Met (US Patent No. 3,959,493). Daugaard [14] concluded that 80% of this product could escape ruminal degradation and another 20% was lost in feces. Arambel et al. [15] reported similar findings when the Met product was fed to growing heifers: 72% escaped ruminal degradation and 19% was wasted in the feces. These encapsulated Met products resulted with increased in plasma Met when they were fed to ruminants [16]. These details about the history of rumen-protected amino acids are provided to highlight that it has been understood that producing products with both excellent protections from rumen degradation and high release in the intestinal environment has been difficult to accomplish. Therefore, considerable effort was made to find alternative methods for encapsulation by producing analogs (for example Met hydroxy analog) and its derivatives (such as isopropyldl- Met, tert-butyl-dl-Met, N-stearoyl-dl-Met) of Met. However, because of minimal success, attention was focused again on encapsulation methods.
Therefore, knowing these problems with AA products, this study objective was to create and evaluate a technology to produce protected Met, by forming compounds which are essentially immune to attack by microbes in the rumen but still can be digested and absorbed through the intestine wall to allow a highly bioavailable form of essential amino acids that are immune from rumen organism attack. The structure of these prepared compounds is centered on the amin of the essential amino acids.
Chemical methods to produce rumen-protected EAA
According to literature most of the methods that are used for protecting amino acids are physical methods. Although these products are not completely acceptable as their coated layers are still sensitive and could be damaged by chewing and physical or heat treatments that have been used in diet proportion. On the other hand, most of the chemical methods could also be used for protecting amino acids as they do not have any physical layers so won’t be damage by feed processing, therefore more work is needed to understand bioavailability of these products.
Materials and methods
Step 1- synthesis of rumen-protected Met: During to this experiment, essential amino acids and two chemical compound were used to make ligands that produce pH-sensitive amino acids. For achieving this goal Met was bonded with chemically with two chemical ingredients that makes RPM1 (rumen protected Met number 1) and RPM2 (rumen protected Met number 2). These two products are stable in the rumen and absorbable in the post rumen portion of the digestive tract. This part of the study was done in the chemistry faculty of Ferdowsi University of Mashhad. The treatments were produced with refluxing and ultrasound reactions at different times, temperatures and solvents to make new bonds and pH-sensitive amino acid ligands. The products in each method were dried and weighed. The melting point, mass spectrometric and physico-chemical characterization were determined.
Step 2- in vitro experiments: In the following stages, the products from step 1 were tested by modified three-step methods to determine the rate pH-sensitive amino acids were released in different pHs, composed of buffer solutions with enzymes and developed as a standardized method to evaluate rumen-protected Met products.
The ruminal phase was prepared by Modified McDougal’s buffer containing lipase (from porcine pancreas), pH was adjusted to 6.8 and Met samples were incubated for 8 hours in this phase.
The second phase was Abomasal during which Hydrochloride buffer containing pepsin (pH 2.0) and samples were incubated for 2 hours. The last part was the duodenal phase which included phosphate buffer containing pancreatin and gall powder (pH~7.9). Samples incubated for 24 hours. At each time point (0, 2, 4, 6, 8, 9, 11 and 35h) aliquot samples of solution were taken and read with UV-VIS in 210-230 wavelength (Vis 2100 SPECTROPHOTOMETER). The data were analyzed with IBM SPSS software and equation and R-square were given by excel software.
Results and Discussion
According to table 1, there is two rumen-protected methionine (RPMet). RPMet1 has a lighter molecular weight but its melting point is greater. The wavelength range of both RPMets is nearly similar which is in agreement with Nnenna et al, [17] estimating amino acid composition of plant samples with UV-VIS Spectrophotometric method and they reported that S-amino acids (like Cysteine) had a wavelength range around 204-220 nm. The yield of RPMet1 is 91.61 which is very good and RPMet2 is 77.09 which is acceptable.
In Figure 1, the regression equation and R-square of each RPMets. Is illustrated during the first 8 hours of incubation (rumen phase) 55.42% of RPMet1 was released, in next 2 hours (abomasum phase) 91.00% of the compound was released and in the final hours up to 35h intestine phase 93.21% was released and 6.79% of this product was stable. For RPMet2 figure shown that in first 8 hours of incubation (rumen phase) 49.25% of RPMet1 was released, in next 2 hours (abomasum phase) 87.93% of the compound was released and in the final hours up to 35h (intestine phase) 94.05% was released and 5.95% of this product was stable. Assuming the rapidly soluble fractions or protein fractions can be soluble in the first hour. In one experiment [18] free methionine incubated with rumen fluid disappeared rapidly. This was evident after1 hr of incubation and after 4 hours more than 95% of Met analog was disappeared. This was indicative that free methionine was decarboxylated rapidly or incorporated into bacterial protein and their finding was in agreement with other research [19, 20, 21, 22, 23]. Blasco [18] reported M-analog was more resistant to degradation in the rumen fluid than free methionine. In these tests, methionine degradation was approximately 45% after 4hour rumen incubation. Most of the chemical protected amino acids are analogs and derivatives of Met. Amino acid derivatives are free AA to which a chemical blocking group has been added to the alpha-amino group or in the acyl group. Some examples of these Met derivatives are isopropyl-DL-Met, butyl-DL-Met, and capryl-caproylic-DL-Met [16]. Escaping ruminal degradation of these products has a positive relation with chain length. Shorter chain rather longer chain would escape rapidly which is in agreement with this experiment as the RPMet2 with shorter chain length had better stability in the rumen. The isopropyl ester of 2-hydroxy-4-methylthio butanoic acid (HMB) has been shown to have a good replacement value for absorbed Met [24]. In one another study in early lactation dairy cows, Koenig et al. [25] reported that around 50% of HMB escaped ruminal degradation and became available for post ruminal absorption. Subsequent studies use of a dual effluent continuous culture system and indicated ruminal escape values were between 22 to 43% for HMB [26]. Smartamine™ is a commercial Met product that has one of the best efficacy as a source of Met. In situ studies, of Smartamine indicate ruminal stability exceeding 90% at 24 hours incubation and intestinal release values were approximately 90%. The intestinal result of Smartamine similar with the current study. According to the results of figure 2 and figure 3, the mass spectroscopy analyzed compound number 1 and compound number 2 was near to theoretical mass that obtains with ChemDraw software.
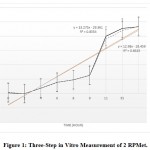 |
Figure 1: Three-Step in Vitro Measurement of 2 RPMet. |
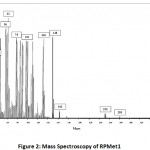 |
Figure 2: Mass Spectroscopy of RPMet1 |
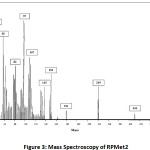 |
Figure 2: Mass Spectroscopy of RPMet1 |
Conclusions
According to the results, it may be concluded that chemical technics for protecting amino acids could be useful and decrease part of free amino acids in the rumen. Additionally, the modified three-step in vitro method used had benefit of not using cannulated cows and also can be done in normal laboratory environment and it is less expensive than in vivo and in situ methods. Although determining the exact response of animals and evaluating more parameters is needed.
References
- Abbasi, I.H.R., Abbasi, F., El-Hack, M.E.A., Swelum, A.A., Yao, J. and Cao, Y., 2018. Post-ruminal effects of rumen-protected methionine supplementation with low protein diet using long-term simulation and in vitro digestibility technique. AMB Express, 8(1), p.36.
- Ayyat, M.S., Al-Sagheer, A., Noreldin, A.E., Abd El-Hack, M.E., Khafaga, A.F., Abdel-Latif, M.A., Swelum, A.A., Arif, M. and Salem, A.Z., 2019. Beneficial effects of rumen-protected methionine on nitrogen-use efficiency, histological parameters, productivity and reproductive performance of ruminants. Animal biotechnology, pp.1-16.
- 2001. Nutrient Requirements of Dairy Cattle. 7th rev. ed. National Academy Press, Washington, DC.
- Noftsger, S., and N. R. St-Pierre. 2003. Supplementation of methionine and selection of highly digestible rumen undegradable protein to improve nitrogen efficiency for milk production. J. Dairy Sci. 86:958–969.
- Socha, M. T., D. E. Putnam, B. D. Garthwaite, N. L. Whitehouse, N. A. Kierstead, C. G. Schwab, G. A. Ducharme, and J. C. Robert. 2005. Improving intestinal amino acid supply of pre- and postpartum dairy cows with rumen-protected methionine and lysine. J. Dairy Sci. 88:1113–1126.
- Osorio, J. S., E. Trevisi, P. Ji, J. K. Drackley, D. Luchini, G. Bertoni, and J. J. Loor. 2014. Biomarkers of inflammation, metabolism, and oxidative stress in blood, liver, and milk reveal a better immunometabolic status in peripartal cows supplemented with Smartamine M or MetaSmart. J. Dairy Sci. 97:7437–7450.
- Osorio, J. S., P. Ji, J. K. Drackley, D. Luchini, and J. J. Loor. 2013. Supplemental Smartamine M or MetaSmart during the transition period benefits postpartal cow performance and neutrophil function. J. Dairy Sci. 96:6248–6263.
- Giallongo, F., M. T. Harper, J. Oh, J. C. Lopes, H. Lapierre, R. A. Patton, C. Parys, I. Shinzato, and A. N. Hristov. 2016. Effects of rumen-protected methionine, lysine, and histidine on lactation performance of dairy cows. J. Dairy Sci. 99:4437–4452.
- Zhou, Z., M. Vailati-Riboni, E. Trevisi, J. K. Drackley, D. N. Luchini, and J. J. Loor. 2016b. Better postpartal performance in dairy cows supplemented with rumen-protected methionine compared with choline during the peripartal period. J. Dairy Sci. 99:8716–8732.
- Zhou, Z., O. Bulgari, M. Vailati-Riboni, E. Trevisi, M. A. Ballou, F. C. Cardoso, D. N. Luchini, and J. J. Loor. 2016a. Rumen-protected methionine compared with rumen-protected choline improves immunometabolic status in dairy cows during the peripartal period. J. Dairy Sci. 99:8956–8969.
- Kim, C. H., T. G. Kim, J. J. Choung, and D. G. Chamberlain. 1999. Determination of the first limiting amino acid for milk production in dairy cows consuming a diet of grass silage and a cereal-based supplement containing feather meal. J. Sci. Food Agric. 79:1703–1708.
- Kim, C. H., T. G. Kim, J. J. Choung, and D. G. Chamberlain. 2000. Variability in the ranking of the three most-limiting amino acids for milk protein production in dairy cows consuming grass silage and a cereal-based supplement containing feather meal. J. Sci. Food Agric. 80:1386–1392.
- Stark, P.A., Zinpro Corp, 2019. Folic acid, metal complexes for rumen by-pass nutritional supplementation of ruminants. U.S. Patent 10,219,528.
- Süss, D., Iwersen, M., Schweinzer, V., Gusterer, E., Kanz, P., Krieger, S., Pothmann, H., Wagener, K., Hoelker, M., Tesfaye, D. and Schellander, K., 2019. Supplementing rumen‐protected methionine to lactating multiparous dairy cows did not improve reproductive performance. Reproduction in Domestic Animals.
- Haryanto, B., 2018. Manipulating protein degradability in the rumen to support higher ruminant production.
- Loerch, S. C., and B. O. Oke. 1989. Rumen protected amino acids in ruminant nutrition. Pages 187–200 in Absorption and Utilization of Amino Acids. Vol. III. M. Friedman, ed. CRC Press, Boca Raton, FL.
- Nnenna E. Okoronkwo, Kalu C. Mba, Innocent C. Nnorom. 2017. Estimation of Protein Content and Amino Acid Compositions in Selected Plant Samples Using UV-Vis Spectrophotometeric Method. American J. of Food Sci & Health. 3: 41-46.
- EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP), Rychen, G., Aquilina, G., Azimonti, G., Bampidis, V., de Lourdes Bastos, M., Bories, G., Chesson, A., Cocconcelli, P.S., Flachowsky, G. and Gropp, J., 2018. Safety and efficacy of hydroxy analogue of methionine and its calcium salt (ADRY+®) for all animal species. EFSA Journal, 16(3), p. e05198.
- Ríus, A.G., 2019. Invited Review: Adaptations of protein and amino acid metabolism to heat stress in dairy cows and other livestock species. Applied Animal Science, 35(1), pp.39-48.
- Lewis, T. R., and R. S. Emery. 1960. Amino acid metabolism by rumen microorganisms. Abstr. J. Animal Sci., 19: 1272.
- Looper, C. G., O. T. Stallcup, and F. E. Reed. 1959. Deamination of amino acids in vivo by rumen microorganisms. 3. Animal Sei, 18: 954.
- Jin, D., Zhao, S.G., Zheng, N., Bu, D.P., Beckers, Y. and Wang, J.Q., 2018. Urea nitrogen induces changes in rumen microbial and host metabolic profiles in dairy cows. Livestock science, 210, pp.104-110.
- Stallcup, O. T., C. G. Looper, and K. E. Kerr. 1966. Nitrogen metabolism in rumen of steers fed linseed oil meal, chicken litter, and various amino acids. J. Dairy Sci., 49: 452.
- Schwab, C. G., N. L. Whitehouse, A. M. McLaughlin, R. K. Kadariya, N. R. St- Pierre, B. K. Sloan, R. M. Gill, and J. C. Robert. 2001. Use of milk protein concentrations to estimate the “methionine bioavailability” of two forms of 2-hydroxy-4-methylthio butanoic acid (HMB) for lactating cows. J. Dairy Sci. 84(Suppl.1):146. (Abstr.)
- Koenig, K. M., L. M. Rode, C. D. Knight, and P. R. McCullough. 1999. Ruminal escape, gastrointestinal absorption, and response of serum methionine to supplementation of liquid methionine hydroxy analog in dairy cows. J. Dairy Sci. 82:355-361.
- Vazquez-Anon, M., T. Cassidy, P. McCullough, and G. A. Varga. 2001. Effects of Alimet on nutrient digestibility, bacterial protein synthesis, and ruminal disappearance during continuous culture. J. Dairy Sci. 84:159-166.
- Jacometo, C. B., Z. Zhou, D. Luchini, E. Trevisi, M. N. Correa, and J. J. Loor. 2016. Maternal rumen-protected methionine supplementation and its effect on blood and liver biomarkers of energy metabolism, inflammation, and oxidative stress in neonatal Holstein calves. J. Dairy Sci. 99:6753–6763.
- Schwab, C. G., G. A. Broderick., 2017. A 100-Year Review: Protein and amino acid nutrition in dairy cows. J. Dairy Sci. 100:10094–10112.
- St-Pierre, N. R., and J. T. Sylvester. 2005. Effects of 2-hydroxy-4-(methylthio) butanoic acid (HMB) and its isopropyl ester on milk production and composition by Holstein cows. J. Dairy Sci. 88:2487–2497.
- Cimerman, S. Miljanic and N. Galic, Croatica Chemica Acta, 2000, 73 (1), 81- 95.

This work is licensed under a Creative Commons Attribution 4.0 International License.





