Manuscript accepted on : 05-Aug-19
Published online on: 05-09-2019
Plagiarism Check: Yes
Suwarni1, Joeharnani Tresnati1  , Sharifuddin Bin Andy Omar1
, Sharifuddin Bin Andy Omar1  and Ambo Tuwo2
and Ambo Tuwo2 
1Department of Fisheries, Faculty of Marine Sciences and Fisheries, Hasanuddin University, Makassar, Indonesia.
2Department of Marine Sciences, Faculty of Marine Sciences and Fisheries, Hasanuddin University, Makassar, Indonesia.
Corresponding Author’s E-mail: suwarnifishery@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2777
ABSTRACT: The rabbitfish Siganus canaliculatus population has been exploited intensively in the Jeneponto Regency South Sulawesi by fishermen used non selective fishing gear, throughout the year even the spawning season. The intensive fishing without management policy can caused decreasing of the rabbit fish population, and if it continues population will be collapse. This study was conducted to investigate some of the reproductive biological study of this species. A total of 1821 specimens of S. canaliculatus consisting of 1436 males and 385 females were randomly collected on a monthly from fishers in the coastal waters of the Jeneponto, South Sulawesi. The fecundity and gonad stage were studied for 39 female individuals varied between 85 and 284 mm total length (TL). Egg diameters were determined using the microscope. The overall sex ratio (Males: Females) ranged from 1.7: 1 to 8.2:1 The estimation of fecundity was between 5416 and 130760 eggs.ind-1, and increased with fish length, body weight and gonad weight. Egg diameter of S. canaliculatus in this study ranged from 0.1-0.5 of stage III. 0.35-0.45 of stage IV, 0.1-0.55 of stage V, and 0.35-0.55 of stage VI. Egg diameters increased with increased fish length. Egg diameters increased with increased fish length.
KEYWORDS: Egg Diameter; Fecundity; Rabbitfish; Sex Ratio
Download this article as:| Copy the following to cite this article: Suwarni, Tresnati J, Omar S. B. A, Tuwo A. Some Reproductive Biology Studies of Rabbitfish Siganus canaliculatus (Park,1797) from the Southern Coastal Waters of Jeneponto, South Sulawesi, Indonesia. Biosci Biotech Res Asia 2019;16(3). |
| Copy the following to cite this URL: Suwarni, Tresnati J, Omar S. B. A, Tuwo A. Some Reproductive Biology Studies of Rabbitfish Siganus canaliculatus (Park,1797) from the Southern Coastal Waters of Jeneponto, South Sulawesi, Indonesia. Biosci Biotech Res Asia 2019;16(3). Available from: https://bit.ly/2kkqUHh |
Introduction
The white-spotted spinefoot, Siganus canaliculatus is distributed throughout the Indo-Pacific from the Arabian Gulf to the Indo-Malay region, Western Australia and north to Hong Kong and Taiwan (Randall, 1995). One of the fisheries resources in the waters of the Flores Sea of South Sulawesi is white-spotted rabbit fish (Siganus canaliculatus, (Park 1797). This fish can be developed as an activity of economy of fishermen communities and also as source of district revenue. Although Siganus fishes have relatively small sizes, they have a taste delicious and much demand in the markets, especially is South Sulawesi, Indonesia.
This rabbit fish population has been exploited intensively in the Jeneponto Regency South Sulawesi by fishermen used non selective fishing gear, throughout the year especially even the spawning season. This condition is due to the demand for Siganus canaliculatus in south Sulawesi has increased continuously over the years. The intensive fishing without management policy can caused decreasing of the rabbit fish population, and if it continues population will be collapse.
Some biological study of S. canaliculatus has been undertaken by some experts from over the world. Biological Studies and Gonadal Development of S. canaliculatus in Mid Arabian Gulf has been dealt with (Wassef and Hady, 2001), Population biology and assessment in the southern Arabian Gulf (Grandcourt et al., 2007). Variations in size and catch distribution at Luwu District, South Sulawesi, Indonesia. (Suardi et al., 2016). Reproductive Biology in the Gulf of Mannar region, India. (Anand and Reddy, 2017), Fecundity and Oocyte Development in Palompon, Leyte, Eastern Visayas, Philippines (Paraboles and Campos. 2018). On the otherhand, there is no detailed biological study of S. canaliculatus in South Sulawesi Indonesia has been made.
The present study was undertaken on some of the reproductive aspects of S. canaliculatus such as fecundity and egg sizes in relation to female size along the southern coast of Jenepotno, South Sulawesi. The findings on the study will provide to assess the status of the fishery for useful inputs in the formulation of suitable management plans and policies for sustainable harvest of the resource and to avoid collapse of the stock in Jeneponto and similar waters in the Indonesia.
Materials and Methods
The marine waters of South Sulawesi consists of three fishing area including Makassar Strait, Flores Sea and Bone Bay (Musbir et al., 2017). The study was conducted from February 2017 to January 2018 in southern Jeneponto coastal waters, Flores Sea, South Sulawesi, Indonesia (Fig. 1).
 |
Figure 1: Positions of the major landing beaches of rabbit fish (Siganus canaliculatus) along the southern coastal of Jeneponto, South Sulaweesi. |
Samples Rabbit fish (Siganus canaliculatus) were collected monthly. A total of 1821 specimens of S. canaliculatus consisting of 1436 males and 385 females were randomly collected on a monthly from fishers in the coastal waters of the Jeneponto, South Sulawesi.
The specimens were brought to the laboratory then washed. Each specimen was measured for total length (TL) using a fish measuring board to the nearest 1 mm and the total wet weight (TW) using an electronic balance to the nearest 1 g. Afther that, each specimen was cut open for determination of the sex and maturity stage.
The male and female fishes were assessed according to testes and eggs in the body cavity by macroscopic examination. The female maturity stages were assessed according to the macroscopic development of eggs in the body cavity. The gonad developmental stages were identified following the criteria described by Al-Marzouqi et al., (2011). Six stages of maturity (I-Immature; II-Maturing 1; III-Maturing 2; IV-Mature; V Ripe/Running and VI-Spent).
The eggs were removed and weighed using an electronic balance to the nearest 0.01 g. Mature eggs of were preserved in 10% formalin solution for determination of fecundity. To determine the sex distribution in relation to fish size.
Sex ratio from the expected 1:1 ratio was tested by using the Chi-square test (Wooton, 1998). The fecundity of Siganus canaliculatus in the present study was determined from the examination of 49 fishes with a total length range of 85 to 284 mm. Ovaries of the stages III, IV, V, and VI (ripe and spent) were only used for fecundity estimation. Estimates of the fecundity of each individual female was obtained from the average of the three subsamples of eggs from different parts of the ovary (Murua et al., 2003):
![]()
Cn = counted number of eggs in sub-sample n, O = ovary weight, Wn = subsample weight and n = number of subsamples.
The linear regression of fecundity on both length and weight was calculated. Egg diameters were determined by taking pictures of randomly selected eggs in each ovary under low magnification (100X) in a compound microscope
Result and Discussion
Sex Ratio
The overall sex ratio (Males: Females) of the present result during the year was from 1.7: 1 to 8.2:1 (Table 1 and 2). The sex ratio (Males: Females) of the rabbitfish in southern coastal waters of South Sulawesi shows a high n in February, May, October and November. The number of males shows a rapid increase.
Table 1: Monthly sex ratio of Rabbit Fish (Siganus canaliculatus Park,1797) from southern coastal waters of Jeneponto, South Sulaweesi.
| Months | Male ((%) (N)) | Female ((%) (N) | Total (N) | Sex Ratio |
| February | 89.2 (116) | 10.8 (14) | 130 | 8.2:1 |
| March | 74.1 (89) | 27.9 (31) | 120 | 2.8:1 |
| April | 68.2 (84) | 31.8 (39) | 123 | 2.1:1 |
| May | 89.1 (90) | 10.9 (11) | 101 | 8.1:1 |
| June | 73.7 (104) | 26.3 (37) | 141 | 2.8:1 |
| July | 75.2 (85) | 24.8 (28) | 113 | 3.0:1 |
| August | 78.1 (104) | 21.9 (29) | 133 | 3.5:1 |
| September | 63.4 (80) | 36.6 (46) | 126 | 1.7:1 |
| October | 84.7 (195) | 15.3 (35) | 230 | 5.5:1 |
| November | 86.1 (106) | 13.9 (17) | 123 | 6.2:1 |
| December | 76.6 (190) | 13.4 (58) | 248 | 3.2:1 |
| January | 82.8 (193) | 17.2 (40) | 233 | 4.8:1 |
| Total | 78.7 (1436) | 21.3 (385) | 1821 | 3.7:1 |
Table 2: Sex ratio of Rabbit Fish (Siganus canaliculatus Park,1797) based on gonadal maturity stages from southern coastal waters of of Jeneponto, South Sulawesi.
| Stage of maturity | Male ((%) (N)) | Female ((%) (N)) | Total (N) | Sex Ratio |
| I | 79.1 (982) | 20.9 (259) | 1241 | 3.7:1 |
| II | 78.8 (325) | 21.(287) | 412 | 1.1:1 |
| III | 91.3 (84) | 8.7 (8) | 92 | 10.5:1 |
| IV | 85.1 (23) | 14.9 (4) | 27 | 5.7:1 |
| V | 47.7 (21) | 52.3 (23) | 44 | 1:1.09 |
| VI | 20 (1) | 80 (4) | 5 | 1:4 |
| Total | 78.7 (1436) | 21.3 (385) | 1821 | 3.7:1 |
Chi-Square analysis shows a comparison between the males and females each month was significantly different from the condition 1:1 (X2 = 57,302; P<0.001).. It was also (Table 2), Chi-Square analysis shows a comparison between the males and females each gonadal stage was significantly different from the condition 1:1 (X2 = 46,5916 P<0.001).
The sex ratio ratio of this species agrees with other on the other species Black-Banded Seabream, Mylio bifasciatus in Qatar that was found to be male:female ratio was from 2:1 to 8:1.( El-Sayed and Bary. 1994). On the other hand, there was a predominance of females amongst the the fish. The sex ratio of of rabbitfish Siganus canaliculatus (Siganidae) in mid Arabian Gulf was 1.4:1 to 2.3:1 (Wassef and Hady, 1997). The overall sex ratio (Males: Females) of of the rabbit fish Siganus canaliculatus in Saudi Arabia. was 1: 1.13 (Tharwat, 2004). There was 1:1.8 male to female sex ratio of Siganus canaliculatus, in the southern Arabian Gulf (Grandcourt et al., 2007). The annual male to female ratio the White-spotted Rabbitfish, Siganus canaliculatus (Park, 1797) in the Arabian Sea coast of Oman was 1:0.81 during 2005-2006 and 1:1.15 during 2006-2007, (Al-Marzouqi et al., 2011). The overall sex ratio (Males: Females) of Siganus rivulatus Inhabits Bitter Lakes in Egypt during the 201 was 1: 1.079 and did not differ significantly from 1:1. (El-Drawany, 2015).
Fecundity
In this study that the total lengths of individuals used for the observation of fecundity varied between 85 and 284 mm, and their weights were 98.4-284.6. The egg count in S. canaliculatus ranged from 5 x 103 to 130 x 103 eggs (Table 3). Its fecundity increased with increase in length and weight. The hghest fecundity was 130.760 occurred in September with total length 284 m and body weight 283 g. The lowest fecundity was 6215 eggs occurred in February with total length 132 mm and body weight 109 g.
Tablel 3: Monthly fecundity of rabbit fish (Siganus canaliculatus Park,1797) from southern coastal waters of of Jeneponto, South Sulawesi.
| Month | Total length (mm) | N | Body Weigth (g) | Fecundity | Average Fecundity |
| February | 132–142 | 2 | 109.01-119.39 | 6215–11068 | 8641± 432 |
| March | 156–194 | 2 | 105.84-121.51 | 70056–71221 | 7063±824 |
| April | 185 | 1 | 146.45 | 56024 | 56024 |
| July | 234 | 1 | 118.36 | 90723 | 90723 |
| August | 122–116 | 2 | 110.42-122.43 | 26790–50181 | 38486± 16540 |
| September | 85–284 | 21 | 98.45-284.63 | 5416–130760 | 64313±40766 |
| December | 186 | 1 | 150 | 77196 | 77196 |
| January | 194–245 | 9 | 106.61–181 | 10549–69751 | 49322± 21350 |
The fecundity in this study is lower than those reported for other stocks in other tropical countries (Jayasankar 1990; Wassef and Hadey 1997; Tharwat 2004; Marzouqi et al. 2011). Fecundity in S. canaliculalus from the Gulf of Mannar India varied from 33,711 to 284,516 measuring total length from 164 to 250 mm. (Jayasankar, 1990). Eggs per female Siganus canaliculatus in mid Arabian Gulf ranging from 42,253 to more than one million, for fish between 17 and 41 cm long (Wassef and Hady. 1997). The egg count in S. canaliculatus in the Arabian Sea coast of Oman ranged from 242 x 103 with total length 265 mm and body weight 294 g to 608 x 103 eggs with total length 375 mm and body weight 1025 g (Marzouqi et al., 2011). Fecundity of S.canaliculalus in Palompon, Leyte, Eastern Visayas, Philippines ranged from 18,350–306,850 in fish ranging in size from 7.1–15.5 cm SL (Paraboles and Campos, 2018).
Fecundity in S. canaliculalus from the Gulf of Mannar India varied from 33,711 to 284,516 measuring total length from 164 to 250 mm. (Jayasankar, 1990). Eggs per female Siganus canaliculatus in mid Arabian Gulf ranging from 42,253 to more than one million, for fish between 17 and 41 cm long (Wassef and Hady. 1997).
In the present study, Highest fecundity of S. canaliculalus occurred in September but lowest in January. The appearance of the fish with larger sizes (284 mm) in the September catch may suggest that older fish move inshore to spawn. On the other hand, studys were conducted by by Wassef and Hady (1997) that the appearance of larger sizes of fish with highest fecundity occurred in the spring (March-May).
Table 4: Fecundity of rabbit fish (Siganus canaliculatus Park,1797) based on gonadal maturity stages from southern coastal waters of of Jeneponto, South Sulawesi.
| Stage of Maturity | Range of Total Length (mm) | N | Range of Weight (g) | Range of Fecundity | Average Fecundity |
| III | 85–142 | 8 | 98.45-122.43 | 5 416-50 181 | 20 265±15 242 |
| IV | 111–234 | 4 | 118.36-145.05 | 17 258–90 723 | 41 407±33 421 |
| V | 125–284 | 23 | 105.84-283.63 | 10 549–130 760 | 72 363±32 740 |
| VI | 209–245 | 4 | 111.70-181.00 | 61 252–69 751 | 65 463±4 578 |
Four of the six gonad maturity stages (I-Immature; II-Maturing; III-Maturing; IV-Mature; V-Ripe/Running and VI-Spent) could be established in male and female fish based on macroscopic observations (Table 4). The lowest total eggs was 130 760 eggs occurred in stage V. The highest total eggs was 5416 eggs occurred in stage III.
Relationship between fecundity and total length, body weight, and ovary weight of Siganus canaliculatus in southern coastal waters of South Sulawesi presented in Fig. 3,4,5
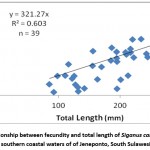 |
Figure 2: Relationship between fecundity and total length of Siganus canaliculatus from southern coastal waters of of Jeneponto, South Sulawesi. |
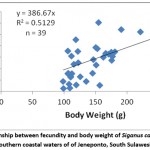 |
Figure 3: Relationship between fecundity and body weight of Siganus canaliculatus from southern coastal waters of of Jeneponto, South Sulawesi. |
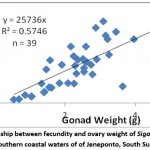 |
Figure 4: Relationship between fecundity and ovary weight of Siganus canaliculatus from southern coastal waters of of Jeneponto, South Sulawesi. |
Regression analysis of the relationship between fecundity and total length with coefficient of determination (R2 = 0.603) showed a significant (Fig. 3). Analysis of regression showed also that there were significant relationship between the fecundity with body weight (Fig. 4) and ovary weight (Fig. 5) as coefficient of determination R2 =0.512 and 0.574. The results indicated that the number of eggs per female increased with increasing length, body weight and ovary weight.
Similar findings was also reported by by Jayasankar (1990). that the fecundity Siganus canaliculatus from The Gulf of Mannar. India varied irrespective of the length or body weight of the fish and increased with ovary weight. It was also observed by Wassef and Hady (1997) that both absolute and relative fecundities of Siganus canaliculatus in mid Arabian Gulf increased with the increase in fish length. It was also (Paraboles and Campos, 2018) showed that the fecundity increased significantly with increased standard length, body weight and ovary weight and it increased significantly.
Egg Diameter
Egg diameter of S. canaliculatus in this study ranged from 0.1-0.5 of stage III. 0.35-0.45 of stage IV, 0.1-0.55 of stage V, and 0.35-0.55 of stage VI (fig. 5).
 |
Figure 5: Distribution of egg diameter of rabbit fish (Siganus canaliculatus Park,1797) based on gonad stage from southern coastal waters of of Jeneponto, South Sulawesi. |
The highest frequency (31.74%) of diameter of female Gonad stage III was range between 0.30 to 0.35 with 857 eggs. The highest frequency (98.42%). of diameter of female Gonad stage IV was range between 0.35–0.40 with 1181 eggs. The hghest frequency (40.16%). of diameter of female Gonad stage V was range between 0.40–0.45 with 4960 eggs. The hghest frequency (54.48%) of diameter of female Gonad stage VI was range between 0.45–050 with 1471 eggs (fig. 5).
Egg diameter of S. canaliculatus in this study was not different from other studies. Mean egg diameter of S. canaliculatus in Palau. was 0.475-0.530 mm (Hasse et al., 1977). The ripe eggs of Siganus canaliculatus in the Arabian Gulf Saudi Arabia ranging from 0,30-0.40 mm and the smaller eggs ranging from 0,10-0.25 both constitute about 88 % and 12 % from the total eggs in the ripe ovaries in the Arabian Gulf Saudi Arabia (Tharwat, A.A. 2004).
Egg diameters in the present study increased with increased fish length as a mature egg diameter increased significantly with increased standard length, in Palompon, Leyte, Eastern Visayas, Philippines (Paraboles and Campos, 2018).
Conclusions
A total of 1821 specimens of S. canaliculatus consisting of 1436 males and 385 females were randomly collected on a monthly during a year. The overall sex ratio (Males: Females) was from 1.7: 1 to 8.2:1. The estimatrion of fecundity was between 5 416 and 130 760 eggs.ind-1, and increased with fish length, body weight and gonad weight. Egg diameter of S. canaliculatus in this study ranged from 0.1-0.5 of stage III. 0.35-0.45 of stage IV, 0.1-0.55 of stage V, and 0.35-0.55 of stage VI. Egg diameters increased with increased fish length.
Acknowledgements
The authors thank the Indonesian Ministry of Risearch Technology and Higher Education for the supporting on this research. We thank also all field data enumerators and fishers who participated in the survey.
References
- Al-Marzouqi, N. Jayabalan, A. Al-Nahdi and I. Al-Anbory. 2011. Reproductive Biology of the White-spotted Rabbitfish, Siganus canaliculatus (Park, 1797) in the Arabian Sea coast of Oman. 2011. Western Indian Ocean J. Mar. Sci. Vol. 10, No. 1, pp. 73-82.
- Anand, M. and Reddy, P.S.R. 2017. Reproductive Biology of rabbitfish, Siganus canaliculatus in the Gulf of Mannar region, India. Indiaj Journal of Geo Marine Science, Vol. 46 (01), 131-140.
- El-Drawany. 2015. On the Biology of Siganus rivulatus Inhabits Bitter Lakes in Egypt. Journal of Aquaculture Research & Development. 6:6: 1-6.
- El-Sayed, A.F.M. and K.A. Bary. 1994. Population Biology of Sparid Fishes in Qatar Waters 3. Reproduction cycle and fecundity of Black-Banded Seabream, Mylio bifasciatus (Forsska). Qatar Univ. Sci. J. (1994), 14 (1): 212-216.
- Grandcourt, E., T. Al Abdessalaam, F. Francis and A. Al Shamsi. 2007. Population biology and assessment of the white-spotted spinefoot, Siganus canaliculatus (Park, 1797), in the southern Arabian Gulf. J. Appl. Ichthyol. 23 (2007), 53–59.
CrossRef - Hasse, J.J. B. B. Madraisau, and J. P.Mcvey. 1977. Some Aspects of the Life History of Siganus canaliculatus (Park) (Pisces: Siganidae) in Palau. Micronesica 13(2): 297-312.
- Jayasankar, P., 1990. Some Aspects of Biology of the White-spotted spine-Foot Rabbitfish, Siganus canaliculatus (Park, 1797) from The Gulf Of Mannar. Indian ]. Fish., 37 (1): 9-14.
- Murua, H., Kraus, G., Sabarido-Rey, F., Witthames, P.R., Thorsen, A. and Junquera, S., 2003. Procedures to estimate fecundity of marine fish species in relation to their reproductive strategy. Journal of the Northwest Atlantic Fishery Science, 33, 33–54.
CrossRef - Musbir, Sudirman, A. Mallawa, R. Bohari. 2017. Egg quantity of wild breeders of spiny lobster (Panulirus ornatus) caught from southern coastal waters of Bulukumba, South Sulawesi, Indonesia . AACL Bioflux, 2018, Volume 11, Issue 1, 295-300.
- Paraboles, L.C. and W.L. Campos. 2018. Fecundity and Oocyte Development of the White-spotted Rabbitfish Siganus canaliculatus (Park 1797) in Palompon, Leyte, Eastern Visayas, Philippines. Asian Fisheries Science 31 (2018):245–251.
- Randall, J. E.; Allen, G. R.; Steene, R. C., 1997: Fishes of the Great Barrier Reef and Coral Sea. University of Hawaii Press, Honolulu, HI, 507 p.
- Suardi, Budy Wiryawan, Mochammad Riyanto, A. Azbas Taurusman, Joko Santoso. 2016. Variations in size and catch distribution of white spotted rabbit fish (Siganus canaliculatus) on bioFADs from spatially and temporary point of view, at Luwu District, South Sulawesi, Indonesia. AACL Bioflux, 2016, Volume 9, Issue 6. 1220-1232.
- Tharwat, A.A. 2004. Reproductive cycle and mariculture potential of the rabbit fish Siganus canaliculatus in Saudi Arabia. Egypt. J. AtfiuiL BioL & Fish. VoL8t So.4: 123 – 143.
CrossRef
- Wassef, E.A. and H.A. Hady. 1997. Breeding biology of rabbitfish Siganus canaliculatus (Park, 1797) in Mid Arabian Gulf. Fisheries Research 33:159–166.
CrossRef - Wassef, E.A. and H.A. Hady. 2001. Some Biological Studies and Gonadal Development of Rabbitfish Siganus canaliculatus (Park) and Siganus spinus L. (F: Siganidae) from the Gulf Waters off Saudi Arabia. Some Biological Studies… J. KAU: Mar. Sci., vol. 12, Special Issue, pp. 189-17.
CrossRef - Wootton, R. J., 1998. Ecology of teleost fishes, Second Edition. Kluwer Academic Publishers, London. 386P.

This work is licensed under a Creative Commons Attribution 4.0 International License.





