Manuscript accepted on : 30-Sep-19
Published online on: 30-09-2019
Plagiarism Check: Yes
Reviewed by: varucha misra
Second Review by: Gabriel Godson AKUNNA
Niranjana. J, Kandasamy Arun Gandhi*, D. Sunmathi and P. Nanthavanan
Research Department of Biotechnology, Dr. N.G.P. Arts and Science College (Autonomous), Coimbatore, Tamilnadu, India
Corresponding Author E-mail: arungandhicbe@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2783
ABSTRACT: L-asparaginase has been a promising therapeutic agent in the treatment of acute lymphoblastic leukemia and lymphoma. In recent times, due to the side effects of commercially available bacterial L-asparaginase and its unavoidable importance, plants are being explored as the source of L-asparaginase. The enzyme L-asparaginase was partially purified from Arachis hypogaea L. The crude enzyme extract was subjected to different purification steps including ammonium sulphate precipitation, dialysis followed by separation on Sephadex G-100 gel filtration (size exclusion chromatography) to obtain partially pure form of L - asparaginase. The enzyme was partially purified to 118 folds and contained specific activity of 4686.86 U/mg with 9.85% yield. SDS-PAGE electrophoresis of the partially purified enzyme revealed that it was a single protein with molecular weight of 70 kDa. The study on physiochemical properties showed that L - asparaginase from Arachis hypogaea L. was potassium-dependent in nature, where its optimum pH of enzyme activity was found to be 8.0 and temperature as 40°/50°C with reaction time of 15 - 20 minutes. Also it was observed that the L-asparaginase activity increased with the presence of metal ions such as Na+, Mg++, making it an enzyme dependent on metal ions for its reaction. In addition to this, it was revealed that the enzyme was partially inhibited in presence of certain chelators. The specificity of L-asparaginase obtained from Arachis hypogaea L. with lack of urease activity and minimal glutaminase activity along with less cytotoxicity on human blood indicated it as an efficient chemotherapeutic agent that could be investigated further in future studies.
KEYWORDS: Arachis Hyogaea L; Cytotoxicity; Gel Filtration; Groundnut Plant; L-Asparaginase; Sephadex G-100; Size Exclusion Chromatography
Download this article as:| Copy the following to cite this article: Niranjana J, Gandhi K. A, Sunmathi D, Nanthavanan P. Extraction, Characterization and Partial Purification of L-Asparaginase from the Leaves of Arachis Hypogaea L. Biosci Biotech Res Asia 2019;16(3). |
| Copy the following to cite this URL: Niranjana J, Gandhi K. A, Sunmathi D, Nanthavanan P. Extraction, Characterization and Partial Purification of L-Asparaginase from the Leaves of Arachis Hypogaea L. Biosci Biotech Res Asia 2019;16(3). Available from: https://bit.ly/31LlfJC |
Introduction
L-asparaginase (EC 3.5.1.1) is an enzyme belonging to “Ntn-hydrolases” family, catalyzes the hydrolysis of L-asparagine producing L-aspartic acid and ammonia[1-2]. The inability of the leukemic cells to synthesize L-asparagine due to the absence of L-asparagine synthetase has made the enzyme L- asparaginase as a key therapeutic agent in the treatment of acute lymphoblastic leukemia (ALL) and lymphoma. As the leukemic cells requires high amount of L-asparagine, they rely on the exogenous sources for their proliferation and survival. Injection of L-asparaginase results in depletion of L-asparagine in blood, which will lead to starvation of tumor cells selectively whereas normal cells are protected from L-asparagine starvation since they are capable of synthesizing this amino acid which is essential for both protein synthesis and cell survival[3].
There are two formulations of L-asparaginase used clinically for ALL treatment, one from Erwinia chrysanthemi and other from Escherichia coli. However, its long term use was known to result in normal cell toxicity, rapid clearance of enzyme in blood stream and patient’s hypersensitivity[4]. The cell toxicity indicated that the L-asparaginase’s toxicity is due to enzyme glutaminase activity and bacterial endotoxins in enzyme preparations. Therefore in attempt of minimizing various side-effects, plants are explored for the production of L-asparaginase and are known to be less toxic compared to bacterial L-asparaginase[5]. In plants, L-asparagine is known to be nitrogen compound and may accumulate under stress conditions[6]. There are two types of plant L-asparaginase such as potassium- dependent and potassium- independent asparaginase. Although these enzymes have high relative sequence similarity, potassium independency of L-asparaginase is not well understood. This study is done in order to partially purify and characterize L-asparaginase from the leaves of Arachis hypogaea L. (groundnut plant) as a plant source.
Materials and Methods
Plant material
The plant was collected from the agricultural field at Puliampatti, Erode district. It was authenticated as Arachis hypogaea L. by Botanical Survey of India (BSI), Coimbatore, Tamil Nadu, India (No.: BSI/SRC/5/23/2018/Tech/2324).
Crude enzyme extract
The crude enzyme extract was prepared by homogenizing 1 gram of shade dried leaves of Arachis hypogaea L. in 0.1M Phosphate buffer with pH 7.5 at 3°C containing β-mercaptoethanol and 1mM EDTA. The process was carried out in chilled condition. The homogenate was centrifuged at 12,000 rpm for 30 minutes at 3°C. The supernatant was designated as crude enzyme extract.
Partial purification of L-asparaginase
Ammonium sulphate precipitation
The extracted crude enzyme was precipitated by adding ammonium sulphate salt with continuous stirring using magnetic stirrer providing 70% saturation. The precipitated enzyme was refrigerated for an hour and centrifuged at 10,000 rpm for 10 minutes at 4°C. The pellet obtained was re-suspended in 0.02M phosphate buffer (pH 6.9) and subjected to dialysis with phosphate buffered saline of pH 7.4. After series of dialysis, the sample was centrifuged at 13,000 rpm for 20 minutes at 4°C. The supernatant was collected and was subjected to next step of purification.
Sephadex G-100 (Gel filtration)
The concentrated enzyme preparation was added on the top of Sephadex G-100 column equilibrated with 100mM Phosphate buffer (pH 7.0) and eluted with the same buffer with the flow rate of 0.4ml/minute. The fraction showing high L-asparaginase activity was selected and the homogeneity was checked by SDS- PAGE.
Asparaginase activity Assay
The activity of L-asparaginase was measured by using Nessler’s reagent[7]. The L-asparaginase catalyzes L-asparagine to L-aspartic acid and ammonia and later react with Nessler’s reagent to produce pale yellow to orange color product. The reaction mixture consisted of 0.5 ml of 40 mM L-asparagine, 0.5 ml of 50 mM phosphate buffer (pH 8.0) and 0.1 ml of enzyme sample. The tubes were incubated for 15 minutes at 37°C. The reaction was stopped by adding 0.5 ml of 20% Trichloroacetic acid (TCA). The blank was prepared without the enzyme sample. The reaction mixture was centrifuged at 8000 rpm for 5 minutes. To 1 ml of supernatant, 3 ml of distilled water and 1 ml of Nessler’s reagent was added. The mixture was incubated for 20 minutes at room temperature and the absorbance was measured at 570 nm using Colorimeter (ELICO). The ammonia released in the reaction was determined based on the standard curve obtained with ammonium sulphate, where 1 µmole of ammonium sulphate is equal to 2 µmoles of ammonia liberated. One unit of L-asparaginase activity is defined as the amount of the enzyme that liberates 1 µmole of ammonia per minute at room temperature.
Protein Determination
Protein was quantified by using Nanophotometer (Implen N 60) – Protein UV (A260/ 280nm).
Characterization of L-asparaginase
Potassium dependency of L-asparaginase
To determine the nature of L-asparaginase from Arachis hypogaea L., the enzyme preparation was pre-incubated with potassium chloride of different concentration (20 mM, 40 mM, 60 mM, 80 mM and 100 mM) for 15 minutes prior to the addition of substrate. The enzyme activity was determined under assay condition and relative activity (ratio of enzyme activity in presence of potassium chloride and absence of potassium chloride) was calculated. The activity which was assayed on absence of L-asparagine was taken as 100%.
Effect of buffer pH
The effect of buffer pH on L-asparaginase activity was studied by incubating the enzyme preparation in the 50 mM phosphate buffer of various pH ranges (5.0, 6.0, 7.0, 8.0 and 9.0) with 40 mM L-asparagine for 15 minutes at room temperature. Then enzyme activity was determined and relative activity (ratio of enzyme activity of reaction mixture at different pH and enzyme activity of control at pH 8.0) was calculated. The activity which was assayed at pH 8.0 was taken as 100%.
Effect of reaction time
The effect of reaction time on L-asparaginase activity was studied by incubating the reaction mixture for different period of time (10, 15, 20, 25 and 30 minutes) at room temperature. Then enzyme activity was determined and relative activity (ratio of enzyme activity of reaction mixture at different reaction time and enzyme activity of control with specific reaction time). The activity which was assayed at 10 minutes reaction time was taken as 100%.
Effect of temperature
Optimum temperature for the L-asparaginase activity was determined by incubating the reaction mixture at different temperatures (20, 37, 40, 50, 60 °C). Then the enzyme activity was determined and relative activity (ratio of enzyme activity of reaction mixture at different temperature and enzyme activity of control at specific temperature) was calculated. The activity which was assayed at room temperature was taken as 100%.
Effect of substrate concentration
The effect of substrate concentration on L-asparaginase activity was determined by incubating the enzyme with different concentration (20 mM, 40 mM, 60 mM, 80 mM and 100 mM) of L-asparagine for 15 minutes. Then the enzyme activity was determined and relative activity (ratio of enzyme activity at different substrate concentration and enzyme activity with 40mM substrate concentration) was calculated. The activity which was assayed at 40 mM substrate concentration was taken as 100%.
Substrate specificity
To determine the specificity of L-asparaginase, the enzyme reaction mixture was made with 40 mM of each L-glutamine, L-glutamic acid, L-aspartic acid, DL-ornithine and urea as these are analogs of L-asparagine. The enzyme activity was determined under assay conditions and relative activity (ratio of enzyme activity in presence of different analogs as substrate and L-asparagine as substrate) was calculated. The activity which was assayed on presence of L-asparagine was taken as 100%.
Effect of metal ions
The effect of different metal ions on L-asparaginase activity was determined by pre-incubating enzyme alone with 100 mM metal ions such as Na+, Mg2+, Zn2+, Cu2+, Hg2+ for 15 minutes prior to adding the substrate. Then enzyme activity was determined and relative activity (ratio of enzyme activity in presence and absence of metal ions) was calculated. The activity which was assayed on absence of metal ions was taken as 100%
Effect of chelators and surfactant
The effect of different chelators and surfactant on L-asparaginase activity was determined by pre-incubating enzyme alone with 10 mM chelators such as EDTA, SDS, urea, β- mercaptoethanol and tween-20 for 15 minutes prior to adding the substrate. Then enzyme activity was determined and relative activity (ratio of enzyme activity in presence and absence of chelator) was calculated. The activity which was assayed on absence of metal ions was taken as 100%.
Cytotoxicity study
in-vitro hemolytic activity test
The cytotoxicity of the partially purified L-asparaginase was studied based on the lysis of RBCs. 5 ml of blood was collected in a polystyrene tube containing EDTA (anticoagulant). The blood sample was centrifuged at 800 rpm for 3 minutes. The supernatant containing plasma and buffy coat phase was discarded while the pellet/ cell suspension was washed with phosphate buffered saline (PBS) and centrifuged at 800 rpm for 3 minutes (this process was repeated thrice). 0.5 ml of cell suspension and 0.5 ml of partially purified enzyme was added in PBS. The mixture was incubated for 30 minutes at 37°C and was centrifuged at 1500 rpm for 10 minutes. The supernatant was added to round bottom 96 well microtitre plates and checked for its absorbance value (at 540 nm) using microplate reader ELISA Reader (BIORAD). The PBS containing blood sample acted as negative control whereas distilled water containing blood sample served as positive control. The hemolytic activity (%) was calculated.
Results and Discussion
The crude enzyme extract obtained from Arachis hypogaea L. was partially purified by a series of purification steps that included salt precipitation dialysis and size exclusion chromatography (gel filtration), where the purification fold increased after each purification step. The enzyme assay and protein determination was carried out after each step of purification. The dialyzed ammonium sulphate precipitate added to Sephadex G- 100 gel filtration column was eluted with 100 mM phosphate buffer (pH 7.0) at the flow rate of 0.4 ml/minute and the fraction showing highest total enzyme activity of 1392 U was selected for further characterization. The results of purification of L-asparaginase are summarized in Table 1. The partially purified L-asparaginase was subjected to SDS-PAGE and the molecular weight was found to be 70 kDa (Figure A).
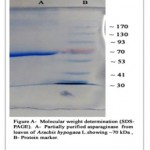 |
Figure A: Molecular Weight Determination (SDS-PAGE). A- Partially Purified Asparaginase Fom Leaves of Arachis Hypogaca L. Showing 70 Kda, B- Protein Marker. |
Table 1: Purification of L-asparaginase from leaves of Arachis hypogaea L.
|
Purification steps |
Collected volume of sample(ml) |
Total Enzyme activity (U) |
Total protein (mg) |
Specific activity (U/mg) |
Purification fold |
Yield (%) |
|
Crude extract |
14 |
14126 |
358.05 |
39.45 |
– |
100 |
|
Post-dialysis |
14 |
5650.4 |
84.21 |
67.09 |
1.7 |
40 |
|
Sephadex G-100 filtration |
3 |
1392 |
0.297 |
4686.86 |
118.80 |
9.85 |
In general, plant L-asparaginase is commenced into two categories such as potassium dependent and potassium non-dependent L-asparaginase. Investigation was made to determine the nature of partially purified L-asparaginase from Arachis hypogaea L. by incubating it in different concentrations of potassium chloride ranging from 20 mM to 100 mM concentration (Table 2, Figure 1).
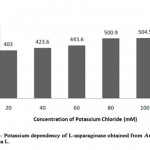 |
Figure 1: Potassium Dependency of L-Asparaginase Obtained from Arachis Hyopogaca L. |
Table 2: Relative activity (%) of L-asparaginase from Arachis hypogaea L. at different concentration of potassium chloride
|
Concentration of Potassium chloride (mM) |
Relative activity (%) |
|
Control
20 |
100
99.8 |
|
40 |
104 |
|
60 |
109 |
|
80 |
124 |
|
100 |
125 |
The enzyme activity was gradually increased from 403 U/ml to 504.5 U/ml showing that presence of potassium increased the L-asparaginase activity when compared to the control (enzyme in absence of potassium chloride) with minimum enzymatic activity of 403.6 U/ml (relative activity, 100%). Similarly, stimulation of L-asparaginase activity on addition of potassium was reported in Phaseolus multiflorus, Zea mays and Lupinus arboreus as well as potassium independency was reported in Lupinus angustifolius, where dependent form was found to be less stable[8].
The L- asparaginase was active over a broad range of pH where it exhibited pH optimum at 8.0 (Table 3, Figure 2). The enzyme activity increased gradually from pH 5.0 till pH 8 with the maximum enzyme activity 403.6 IU/ml (relative activity, 100%) However, there was decrease in the activity as the pH of buffer increased to 9.0 providing enzyme activity as 232 IU/ml (relative activity, 57.48%). The enzymes residual activity was higher at pH 9.0 while compared to relative activity at pH 5.0 with enzyme activity of 141.26 IU/ml. Thus, it is known that L- asparaginase from Arachis hypogaea L. can lose its activity at minimum and maximum pH range of buffer, being partially stable at pH 7.0 with enzyme activity 302.7 IU/ml (relative activity, 75%) and completely stable at pH 8.0 (relative activity, 100%).
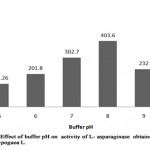 |
Figure 2: Effect of Buffer Ph on Activity of L-Asparaginase Obtained from Arachis Hypogaca L. |
Table 3: Relative activity (%) of L-asparaginase from Arachis hypogaea L. at different buffer pH
|
Buffer pH |
Relative activity (%) |
|
5 |
34.9 |
|
6 |
50 |
|
7 |
74.82 |
|
8 |
100 |
|
9 |
57.48 |
Similarly, highest enzyme activity of 399.5 IU/ml at pH 8.0 was reported in Pisum sativum[9], Spinacia oleracea[7] and in Phaseolus vulgaris seed[10]. The optimum pH was found to be comparative with L-asparaginase extracted from various microorganisms [11-12].
The effect of reaction time on the enzyme- substrate complex is necessary to determine the efficiency of an enzyme over a period of time. The L-asparaginase activity increased over the time of incubation up to 25 minutes (Table 4, Figure 3) and found to decrease as the time of incubation extended. The enzyme activity was maximum (504.516 U/ml; relative activity, 125%) at time period of 15 minutes and 20 minutes of incubation when compared to enzyme activity 464.2 U/ml; relative activity, 100% at 10 minutes. The enzyme activity was slightly decreased with incubation time of 25 minutes providing activity of 403.6U/ml, where the relative activity (99.8%) was higher when
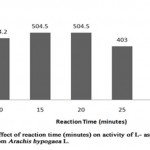 |
Figure 3: Effect of Reaction Time (Minutes) on Activity Of L-Asparaginase Obtained from Arachis Hypogaca L. |
Table 4: Relative activity (%) of L-asparaginase from Arachis hypogaea L. at varying reaction time (minutes)
|
Reaction time (minutes) |
Relative activity (%) |
|
10 |
100 |
|
15 |
125 |
|
20 |
125 |
|
25 |
99.8 |
|
30 |
32.4 |
compared to the relative activity (32.45%) at reaction time of 30 minutes showing that the activity of L-asparaginase from Arachis hypogaea L. decrease with increase in reaction time. Similar result was reported in Spinacia oleracea, showing increase in enzyme activity at reaction time of 15 minutes[7], whereas L-asparaginase from Pisum sativum was reported to have highest enzyme activity at 30 minutes[9]. However, L-asparaginase activity of Arcahis hypogaea L. had minimum enzyme activity (131 IU/ml) at the reaction time of 30 minutes.
The study of effect of temperature on enzyme activity was made in order to know the stability of enzyme (Table 5, Figure 4). The L-asparaginase activity was found to be minimum of 131 U/ml at 20°C (relative activity, 32.4%) where as highest enzyme activity of 504.5 U/ml was noted at 40°C and 50°C (relative activity, 125%). As the temperature was increased to 60°C, the enzyme activity was reduced to 302.6 U/ml (relative activity, 74.9%) showing 40°C/50°C as optimum temperature of L-asparaginase from Arachis hypogaea L. Similarly, L-asparaginase from Solanum nigrum, Spinacia oleracea and Phaseolus vulgaris seeds were found to be highest at 40°C and partially minimum at 50°C [13-14].
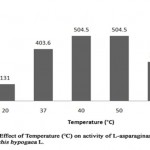 |
Figure 4: Effect of Temperature (°C) on Activity of L-Asparaginase Obtained from Arachis Hypogaca L. |
When L-asparaginase was incubated with different substrate concentration (Table 6, Figure 5), the maximum L-asparaginase activity of 504.5 U/ml (relative activity, 125%) was noted with 100 mM substrate concentration and minimum activity of 70.6 U/ml (relative activity, 17.5%) with 20 mM substrate concentration. The enzyme activity was found to be relative amongst three different concentrations (40 mM, 60 mM and 80 mM) with relative activity of 100%, 107.5 % and 110% respectively. This indicates the gradual increase of enzyme activity of L-asparaginase from Arachis hypogaea L. with increase in substrate concentration. However, it was reported that L-asparaginase activity of Pisum sativum was higher (332.7 U/ml) with 200 mM concentration and partial with 100 mM concentration of substrate exhibiting, enzyme activity of 140 U/ml[9].
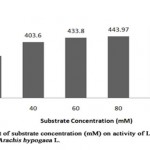 |
Figure 5: Effect of Substrate Concentration (Mm) on Activity of L-Asparaginase Obtained from Arachis Hypogaca L. |
Table 5: Relative activity (%) of L-asparaginase from Arachis hypogaea L. at different temperature (°C)
|
Temperature (°C) |
Relative activity (%) |
|
20 |
32.4 |
|
37 |
100 |
|
40 |
125 |
|
50 |
125 |
|
60 |
74.9 |
Table 6: Relative activity (%) of L-asparaginase from Arachis hypogaea L. at varying substrate (L-asparagine) concentrations
|
Substrate concentration (mM) |
Relative activity (%) |
|
20 |
75 |
|
40 |
100 |
|
60 |
107.5 |
|
80 |
110 |
|
100 |
125 |
The substrate specificity of an enzyme act as an important characteristic to determine the efficiency of it as a drug. When L-asparaginase was incubated with L-asparagine and its analogs such as L-glutamine, L-glutamic acid, DL-ornithine, L-aspartic acid and urea at concentration of 40mM, respectively (Table 7, Figure 6).The result showed that partially purified L-asparaginase from Arachis hypogaea L. had high specificity towards L-asparagine with enzyme activity of 403.6 U/ml (relative activity, 100%). However, 17% activity against L-glutamine showing enzyme activity of 70.6 U/ml was observed. No other substrate was found to interact with enzyme. Specifically, no interaction with urea indicates that L- asparaginase from Arachis hypogaea L. lacks urease activity. Similarly, substrate specificity analysis carried out with L-asparaginase of Phaselous vulgaris showed activity against different analogs with lesser than 2% of relative activity[10], whereas L-asparaginase from microbe had no specificity against the analogs expect L-glutamine (relative activity lesser than 10%)[15].
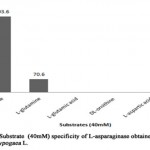 |
Figure 6: Substrate (40mm) Specificity of L-Asparaginase obtained From Arachis Hypogaca L. |
Table 7: Relative activity (%) of L-asparaginase from Arachis hypogaea L. in relation with substrate specificity
|
Substrate |
Relative activity (%) |
|
L-asparagine |
100 |
|
L-glutamine |
17 |
|
L-glutamic acid |
– |
|
DL-ornithine |
– |
|
L-aspartic acid |
– |
|
Urea |
– |
Metal ions are known to play an important role in maintaining the active configuration of enzyme at elevated temperature protecting it from thermal denaturation[16]. The effect of various metal ions was determined by incubating partially purified L-asparaginase with metal ions (100 mM) 15 minutes prior to the addition of substrate (Table 8, Figure 7). L- asparaginase activity was not affected by the metal ions such as Mg2+, Cu2+, Hg2+andNa+. It was evident that the enzyme activity increased on presence of Mg2+ and Na+ as enhancers showing relative activity of 115% (464 U/ml) and 107% (433.8 U/ml) respectively when compared to enzyme activity of 403.6 U/ml in absence of metal ions (relative activity, 100%). It suggests that these metal ions can act as co-factors, which can help to increase the enzymatic reaction. However, heavy metal ions like Hg2+, Cu2+ partially inhibited the L-asparaginase activity with relative activity of 62% and 82% respectively. The presence of Zn2+ completely inhibited the L-asparaginase activity from Arachis hypogaea L. The studies made on L-asparaginase from Pectobacterium carotovorum showed the similar partial inhibition of enzyme activity on presence of Hg2+ and Cu2+[12] whereas in Solanum nigrum, Na+ enhanced the enzyme activity but Hg2+ acted as a complete inhibitor[13].
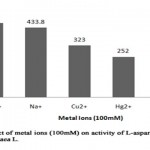 |
Figure 7: Effect of Metal Ions (100mm) on Activity of L-Asparaginase from Arachis Hypogaca L. |
Table 8: Relative activity (%) of L-asparaginase from Arachis hypogaea L. in presence of metal ions
|
Metal ions |
Relative activity (%) |
|
Mg2+ |
115 |
|
Na+ |
107 |
|
Cu2+ |
82 |
|
Hg2+ |
62 |
|
Zn2+ |
– |
When different chelators (10 mM) were incubated in the reaction mixture (Table 9, Figure 8), it was found that EDTA and β-mercaptoethanol partially inhibited the enzyme resulting in the enzyme activity of 302.6 IU/ml and 302.7 IU/ml respectively with 25 % of inhibitory action. The result was similar to the study, where EDTA partially inhibited the L-asparaginase activity from Phaseolus vulgaris[10]. SDS consisted of maximum inhibitory action (83%) on L-asparaginase from Arachis hypogaea L. providing enzymatic activity of 70.6U/ml whereas urea had no effect on L-asparaginase activity. However, results of certain studies showed urea as a strongest inhibitor[13]. Tween 20 was found to enhance the L-asparaginase activity (464.2 U/ml) with relative activity of 115%, where it suggests that surfactants appears to increase quick and direct contact of enzymes with substrate site. Similar result was reported in Streptomyces brollosae NEAE-115[16].
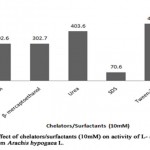 |
Figure 8: Effect of Chelators/Surfacants (10mm) on Activity of L-Asparaginase Obtained from Arachis Hypogaca L. |
Table 9: Relative activity (%) of L-asparaginase from Arachis hypogaea L. in the presence ofchelators and surfactant
|
Chelators/Surfactant |
Relative activity (%) |
|
EDTA |
75 |
|
β- mercaptoethanol |
75 |
|
Urea |
100 |
|
SDS |
17 |
|
Tween-20 |
115 |
Cytotoxicity of L-asparaginase from Arachis hypogaea L. was studied in-vitro viz., hemolytic activity assay. The cell suspension was incubated with 100µl of enzyme fraction with protein content of 99µg along with positive and negative control. On obtaining absorbance value (at 540 nm) and calculating the percentage of hemolytic activity, the test sample containing L-asparaginase provided 80% cell viability. This indicated that partially purified L-asparaginase from Arachis hypogaea L. is less toxic to healthy blood cells and can be administrated as drug with further purification. Similar result has been reported in L-asparaginase from Bacillus lichenformis[15].
Conclusion
This study clearly revealed that leaf extract of Arachis hypogaea L. is a potent source of L- asparaginase with the molecular weight of 70 kDa. The enzyme contained similar physiochemical properties of L- asparaginase produced earlier from other sources. The study on various environmental conditions such as pH, temperature showed that L- asparaginase from Arachis hypogaea L. can be stable at wide range of pH , showing highest enzyme activity at pH 8 and at temperature 40°C as well as 50°C. The excellent characteristic of this enzyme such as catalytic activity over range of pH, temperature and high substrate specificity makes it highly valuable to be exploited as a potent anti-cancer agent. As the partially purified L- asparaginase from Arachis hypogaea L. is in urease- free form and with minimal glutaminase activity, it can reduce the side effects during course of anti-cancer therapy. Moreover, it’s less toxicity against the anticoagulated blood supports its efficiency as a therapeutic agent and it can further investigated for its capacity as a potential candidate for treating acute lymphoblastic leukemia and lymphoma in future studies.
Acknowledgement
The authors thank the management, Principal and Deans of Dr. N.G.P. Arts and Science College (Autonomous), Coimbatore for providing the research facilities to pursue the research work (Communication No – DrNGPASC 2019–20 BS003). The authors are also thankful to DBT Star Scheme for the instrument facilities provided in this study.
Conflict of Interest
None
References
- Michalska K, Jaskolski M. “Structural aspects of L-asparaginases, their friends and relations.” Acta biochimica polonica-english edition, 2006; 53(4):627.
- Michalska K, Bujacz G and Jaskolski M. “Crystal structure of plant asparaginase.” Journal of Molecular Biology. 2006; 360(1):105-16.
- Narta, U.K., Kanwar, S.S. and Azmi, W. “Pharmacological and clinical evaluation of L-asparaginase in the treatment of leukemia.” Crit Rev Oncol Hematol, 2007; 61: 208-221.
- Khalaf AZ, et al. “Optimum conditions for asparaginase extraction from Pisum sativum subspp Jof.” Iraian J Plant Physiology, 2012; 6(4): 517-521.
- Oza VP, Parmar PP, Kumar S and Subramanian RB. “Anticancer properties of highly purified L-asparaginase from Withania somnifera L. against acute lymphoblastic leukemia.” Applied Biochemistry and Biotechnology, 2010; 160(6):1833-40.
- Bruneau L, Chapman R and Marsolais F. “Co-occurrence of both L-asparaginase subtypes in Arabidopsis: At3g16150 encodes a K+-dependent L-asparaginase.” Planta, 2006; 224(3):668-79.
- Khabade SP, Patel MS and Sowjanya R. “Extraction and characterization of L-asparaginase from Spinacea oleraceae.” International Research Journal of Biological Sciences, 2016; 5 (10):45-50.
- Chang KS and Farnden KJ. “Purification and properties of asparaginase from Lupinus arboreus and Lupinus angustifolius.” Archives of biochemistry and biophysics, 1981; 208(1):49-58.
- Khalaf AZ. “Extraction and Purification of Asparaginase enzyme from Pisum sativum plant and studying their cytotoxicity against L20B tumor cell line.” Msc. Theises.Al-Nahrain University, (2012).
- Mohamed SA et al. “Purification and characterization of asparaginase from Phaseolus vulgaris seeds.” Evidence-Based Complementary and Alternative Medicine (2015)
- Kamble VP, Rao RS and Borkar PS. “Purification of L-asparaginase from a bacteria Erwinia carotovora and effect of a dihydropyrimidine derivative on some of its kinetic parameters.” Indian Journal of Biochemistry and Biophysics, 2006; 4:391-394.
- Kumar S, Dasu VV and Pakshirajan K. “Purification and characterization of glutaminase-free L-asparaginase from Pectobacterium carotovorum MTCC 1428.” Bioresource technology, 2011; 102(2):2077-82.
- Kaur J, Kumar R and Kumar K. “Comparative characterization of L-asparaginase extracted from plant and microbial sources.” International Journal of Research in Ayurveda and Pharmacy, 2017; 8(5): 86-89.
- Khabade SP. “Purification and characterization of L-asparaginase enzyme from Spinacea oleracea.” World Journal of Pharmaceutical Research, 2017; 6(11): 709-717.
- Mahajan, R.V et al. “Purification and characterization of a novel and robust L-asparaginase having low-glutaminase activity from Bacillus licheniformis: in vitro evaluation of anti-cancerousproperties.” PLoOne, 2014; 9(6):p.e99037.
- El-Naggar, et al. “Purification, characterization and immunogenicity assessment of glutaminase free L-asparaginase from Streptomyces brollosae NEAE-115.” BMC Pharmacology and Toxicology, 2018; 19(1):51.

This work is licensed under a Creative Commons Attribution 4.0 International License.





