Manuscript accepted on : 21-Aug-19
Published online on: 30-09-2019
Plagiarism Check: Yes
Diabetes and Hypercholesterolemia Impair the Cytological Structure of the Anterior Pituitary Gland
Ahmed MR Abdo3, Mohamed E El-Beeh1, 2 ![]() , Sameer H. Qari2
, Sameer H. Qari2 
![]() , Dina A El-badry1 and Hassan IH El-Sayyad1*
, Dina A El-badry1 and Hassan IH El-Sayyad1*
1Department of Zoology, Faculty of Science, Mansoura University, Mansoura, Egypt
2Biology Department, El-Jumum University College, Umm Al-Qura University, KSA
3Biology Department, Faculty of Science and Art, Baha University, KSA
Corresponding Author’s E-mail: elsayyad@mans.edu.eg
DOI : http://dx.doi.org/10.13005/bbra/2780
ABSTRACT: Increase consumption of high fat diet was found to alter blood sugar level similar to diabetes and contributed to the development of obesity and affected the reproductive function of both sexes. The study aimed to clarify the influence of diabetes and or hypercholesterolemia on the cytological picture of cells of the anterior lobe of pituitary gland of male albino rats. Eighteen male albino rats weighing approximately 120 gram body weight were divided into three main groups; control, diabetes, hypercholesterolemia, diabetes (single i.p. 40 mg streptozotocin/kg B.wt plus 100mg. nicotinamide /kg body weight) and hypercholesterolemia (diet containing 3% cholesterol). Dietary feeding on cholesterol and diabetes were carried out for 12 weeks. At the end of treatment, animals were sacrificed, and pituitary glands were separated and their anterior lobe was processed for cytological investigations by transmission electron microscopy. The present study revealed that the rats subjected to experimental diabetes and/ or hypercholesterolemia exhibited a decrease of the secretory granules within the gonadotroph cells somatotroph and corticotrophin cells. There was a detected intracellular accumulation of fat globules in both the gonado- and sommatotroph cells. The authors reported that the altered cytological structures of the secretory function of the anterior pituitary gland led to marked impairment of the male hormonal level and causing infertility.
KEYWORDS: Biochemical Markers; Diabetes; Hypercholesterolemia; Pituitary; TEM
Download this article as:| Copy the following to cite this article: Abdo A. MR, El-Beeh M. E, Qari S. H, El-badry D. A, El-Sayyad H. I. Diabetes and Hypercholesterolemia Impair the Cytological Structure of the Anterior Pituitary Gland. Biosci Biotech Res Asia 2019;16(3). |
| Copy the following to cite this URL: Abdo A. MR, El-Beeh M. E, Qari S. H, El-badry D. A, El-Sayyad H. I. Diabetes and Hypercholesterolemia Impair the Cytological Structure of the Anterior Pituitary Gland. Biosci Biotech Res Asia 2019;16(3). Available from: https://bit.ly/2mewyLA |
Introduction
Diabetes is a public health problem associated with increased blood sugar level and decrease of insulin production from the damaged beta cells of theIslet of Langerhans of pancreas which developed metabolic dysfunction of the body organs (Yoon and Jun, 2005).
Diabetes was found to increase blood sugar level and ketone bodies which involved in the reduction of pituitary hormones may be linked in the development of diabetic ketoacidosis (Barnes et al., 1978).
Previous studies reported ovarian failure of hypercholesterolemic rat associated with altering the anti-oxidative enzymes and decreased reproductive hormonal levels (El-Sayyad et al., 2018 and 2019). Also, diabetes was associated with male infertility through reduction of insulin production in the spermatogenic cells through disrupting of the hypothalamic-pituitary gonadal axis. Streptozocin-induced diabetic rats produced approximately half of the insulin protein in testis (Schoeller et al., 2012) reflecting its importance in spermatogenesis. Diabetic patients were found to exhibit low plasma level of testosterone (Gluud et al., 1982) associated with decreased sperm production and development of infertility (Agbaje et al., 2007).
It is known that the spermatogenesis need the production of lactate from glucose by the Sertoli cell. The transport of glucose from blood to the spermatogenic cell and the recovery of the metabolic intermediates is controlled by the blood-testis-barrier (Riera et al., 2009). The glucose transporter Glut1, Glut2, Glut3,Glut 4 and Glut8 have been demonstrated in the testis ( Carosa et al., 2005; Verma and Haldar, 2016). Diabetes was found to alter the GLUT8 immunoreactivity in the acrosomic system of spermatids, and at low levels in Leydig cells as well as reduced testicular insulin levels which involved in retarding spermatogenesis(Gómez et al., 2009) STZ-induced hyperglycemic rats was found to upregulate the pro-apoptotic factors Bad, Bax and c-Jun N-terminal kinases parallel with an increase in germ cell death (Koh, 2007).
This work was carried out to exhibit the cytological structure of hormonal secreting cells of the anterior pituitary gland in relation to hypercholesterolemia and/ or diabetes.
Materials and Methods
Biochemical Investigations
The serum levels of high density lipoproteins (HDL) (Grove, 1979) and total cholesterol (TC) (Deeg and Ziegenhorn, 1982) were investigated. The level of low density lipoproteins (LDL) was calculated from the total concentration of triglycerides, total cholesterol (TC) and HDL-cholesterol (Friedewald et al , 1972). The blood glucose levels were measured by blood glucometers one touch ultra (Life Scan Milipitas, CA, USA).
Application of Hypercholesterolemia
Hypercholesterolemia was induced by feeding the experimental group on diet containing 3% cholesterol beside 7% fat, 1% cholic acid and 0.3% thiouracil for 12 weeks according to Enkhmaa et al.(2005). The control group was fed on a standard diet free from hypercholesterolemic components
Induction of Diabetes
It was occurred by intraperitoneal injection of a single dose of streptozotocin (60 mg/kg) in the citrate buffer (pH 4.5) plus nicotinamide 100mg/kg body weight (Povoski et al., 1993). The control received saline solution as a vehicle. Hyperglycemia was maintained by assaying the blood glucose with approximately 250-280 mg/dL.
Experimental Work
The study was carried out on 24 male Wistar albino rats weighing almost 100g body weight, obtained from Farm of Ministry of Health, Giza, Egypt. The animals were housed in aerated room with approximately12-hour light and dark cycle. Free access of standard diet and water was allowed ad-libitum. The rats were arranged into four groups (n=6) such as control, hypercholesterolemic group, diabetic group, hypercholesterolemic and diabetic group. At the end of treatment, the studied groups were anesthetized, sacrificed and blood was collected, and glucose levels were estimated. Sera were separated and subjected for estimating total cholesterol and LDL-c. The heads were removed, and brain dissected, and the pituitary glands were separated and fixed in cacodylate buffered 2% glutaraldehyde (pH 7.4) and post fixed in 1% osmium tetroxide at 4°C. The specimens were dehydrated in ascending ethyl alcohol (70-100%0, cleared in propylene oxide and embedded in epoxy resin. Ultrathin sections were cut with a LKB Ultratome IV, mounted on grids and stained with uranyl acetate and lead citrate, and examined on a Joel 100CXl transmission electron microscope of Mansoura University Lab.
Results
Biochemical Markers
Diabetes increased blood levels of glucose, and serum HDL, LDL and total cholesterol levels in comparison with the control groups (Fig.1).
 |
Figure 1: Chart illustrating the blood glucose level and sera levels of LDL-C, HDL and total cholesterol levels of diabetic mother supplemented watery cinnamon extract. |
Cytological Observations
In control, the gonadotroph cells appeared aligned near to the blood capillaries. It takes large round or polyhedral structure. The nuclei possessed peripheral marginated heterochromatin on the nuclear envelope and euchromatin forming the bulk of the nucleoplasm. The cytoplasm is rich of electron-dense secretory granules of varying sizes. The somatotroph cells possessed irregular-shaped nuclei with abundant euchromatin and thin coat of peripheral heterochromatin. Mitochondria are detected dispersed in the cytoplasm. Rough endoplasmic reticulum and their ribosomes are distributed throughout the cytoplasm. Secretory granules are abundant around the nucleus and take their homogenous size. The corticotroph cells appeared elongated with eccentric nuclei. Rough endoplasmic reticulum and polysomes are detected within the cytoplasm. Mitochondria and rough endoplasmic reticulum are more abundant in the cytoplasm of the sommato-and gonadotroph cells (Fig.2 A-A5).
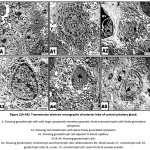 |
Figure 2(A-A5): Transmission electron micrographs of anterior lobe of control pituitary gland. |
In diabetic group, the nuclei of the pituitary cells including gonadotroph, somatotroph, and throtroph cells showed different pattern of karyolysed chromatin materials and reduction of the cytoplasmic granules. Some somatotroph and gonadotroph cells exhibited atrophied nuclei with compacted chromatin materials (Fig. 3A-D).
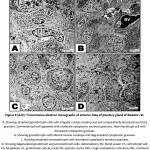 |
Figure 3 (A-D): Transmission electron micrographs of anterior lobe of pituitary gland of diabetic rat. |
In hypercholesterolemic group, both gonadotroph and somatotroph cells exhibited electron-dense nuclear chromatin materials associated with vesiculated cytoplasmic materials and reduction of secretory granules. Many pituitary cells appeared with pyknotic nuclei (fig. 4 A-D).
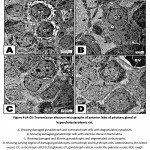 |
Figure 4 (A-D): Transmission electron micrographs of anterior lobe of pituitary gland of hypercholesterolemic rat. |
In diabetic and hypercholesterolemic group, there was a detected massive degeneration of the anterior pituitary cells especially gonadotroph and somatotroph cells. The cells possessed pyknotic nuclei, depletion of secretory granules and increase of lysosomes. Fibrotic pattern of somatotroph cells were detected. The endothelial cells lining the blood vessels were damaged. Increased deposition of fat globules was detected within the pituitary cells illustrating the most pathological observation (Fig. 5 A-D). In some other specimens, there was apparent increase of gonadotroph and somatotroph cell exhibited convoluted nuclear enveloped and possessed compacted nuclear chromatin materials. Gonadotroph cells with pyknotic nuclei with vesiculated cytoplasm and decreased secretory granules and fragmented rough endoplasmic reticulum (Fig. 6 A-D).
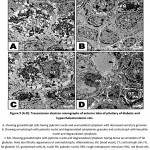 |
Figure 5 (A-D): Transmission electron micrographs of anterior lobe of pituitary of diabetic and hypercholesterolemic rats. |
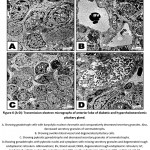 |
Figure 6 (A-D): Transmission electron micrographs of anterior lobe of diabetic and hypercholesterolemic pituitary gland. |
Discussion
The present findings reported that diabetes and or hypercholesterolemia are linked to marked increase of blood levels of glucose, LDL and total cholesterol level. Similar findings of the association of diabetes with altering lipid profiles were reported by El-Sayyad et al., (2010, 2011, 2014). Hypercholesterolemic diet was found to develop type 2 diabetes via increase blood sugar level and glycosylated haemoglobin (HbA1c) after 30 weeks (Morris et al., 2016) as well as increase plasma level of triglycerides and decreased HDL-cholesterol (Ramalho et al., 2017).
The mammalian reproductive cycles are tightly regulated by the hypothalamus (gonadotrophin-releasing hormone)-pituitary (follicle-stimulating hormone) and ovarian axis (17β- estradiol) (Nett et al., 2002; Kermath and Gore, 2012). FSH represents an important indicator of both normal and abnormal testis function. It is known that follicle stimulating hormones promoted de novo biosynthesis of the steroid hormone from the circulating lipoprotein and cholesterol in the theca and granulosa cells (Miller and Auchus, 2011).
Also, the gonadotroph cells are important for secreting two kinds of hormones; follicle stimulating hormone and luteinizing hormone. The follicle stimulating hormone is involved for managing spermatogenesis, meanwhile luteinizing hormone activated the Leydig cells for testosterone secretion (Nicholson and Ricks, 2011).
The observed hypofunction of gonadotroph secretion which was assessed by the observed reduction of the gonadotroph secretory granules in diabetic and or hypercholesterolemic group impaired testosterone section which disrupt testicular structure and function. Also, depletion of testosterone secretion impair the prostatic function and damaging the prostate (Gründker and Emons, 2017). Rat and rabbit fed on a hypercholesterolemic diet was found to damage Leydig cells associated depletion of serum free testosterone level (Dupont et al., 2014) and consequently depleted semen volume, sperm cell count, and percentage of sperm motility (Saez Lancellotti et al., 2013; Raad et al., 2017) and developed infertility (McPherson and Lane, 2015).
It is known that under oxidative stress of increased blood sugar level from diabetes (El-Sayyad et al., 2010, 2011, 2014), the damaged cell organelles released reactive oxygen species (Dumollard et al., 2007) which enhanced further dysfunction of mitochondrial and endoplasmic reticulum (Luzzo et al., 2012). These was involved in depletion of the ATP associated decrease of the oxidative phosphorylation , liberation of oxygen free radical and activation of caspases leading to DNA fragmentation (Wu et al., 2011; Yang et al., 2012).
GH is one of the glucose counter-regulatory hormones. Low IGF-I and growth hormone levels linked directly to deficient insulin levels or lack to circulating binding protein. In type 2 diabetes, growth hormone is decreased due to opposite alterations in hypothalamic somatostatin (Giustina and Wehrenberg, 1994) or to type 2 diabetes as in 17-year-old non-obese female due to disrupting glucose and lipid metabolism (Henry and Menon , 2018).
The observed damage of corticotroph cells supported the findings of Rhyu et al., (1994) whom reported mpairing of cortisol and/or growth hormone secretion due to severe hypoglycemia.
Type 2 diabetes and alterations of thyroid hormone secretion is of great importance. Gene polymorphism, abnormal gene expression and regulation, enhanced absorption of dietary glucose from intestine, decreased its utilization by tissues and aberrations in hepatic tissues similar to T2DM. Hypo-and hyperthyroidism have been associated with impaired glucose metabolism in T2DM (Wang, 2013; Jayanthi and Srinivasan et al., 2019).
Finally the author concluded that diabetes is associated with degranulation of the secretory function of gonadotrophs, sommatotroph, thyrotroph and corticotroph cells, disrupting hormonal secretion and development of diseases.
References
- Agbaje, I.M., Rogers, D.A., McVicar, C.M., McClure, N., Atkinson, A.B., Mallidis, C., Lewis, S.E. Insulin dependant diabetes mellitus: implications for male reproductive function. Acta Endocrinol (Copenh). 1982; 100(3):406-9.
- Barnes, A.J., Kohner, E.M., Bloom, S.R., Johnston, D.G., Alberti, K.G., Smythe, F. Importance of pituitary hormones in aetiology of diabetic ketoacidosis. Lancet. 1978;1(8075):1171-4.
- Carosa, E., Radico, C., Giansante, N., Rossi, S., D’Adamo, F., Di Stasi, S.M., Lenzi, A., Jannini, E.A. Ontogenetic profile and thyroid hormone regulation of type-1 and type-8 glucose transporters in rat Sertoli cells. Int J Androl. 2005;28(2):99-106.
- Deeg, R., Ziegenhorn, J. Kinetic enzymic method for automated determination of total cholesterol in serum. ClinChem. 1983; 29: 1798–1802.
- Dumollard, R., Duchen, M., Carrol,l J. The role of mitochondrial function in the oocyte and embryo. Curr Top Dev Biol. 2007; 77:21-49.
- Dupont, C., Ralliard-Rousseau, D., Tarrade, A., Faure, C., Dahirel, M., Sion, B., Brugnon, F., Levy, R., Chavatte-Palmer, P. Impact of maternal hyperlipidic hypercholesterolaemic diet on male reproductive organs and testosterone concentration in rabbits.J Dev Orig Health Dis. 2014;5(3):183-8.
- El-Sayyad, H.I.H., Ahmed, A. El-mansi, Samia, M. Efekrin. Dietary supplements of barley and date-palm fruit improved the growth defects of ovaries of rat offspring maternally fed on hypercholesterolemic diet. Biosci., Biotech. Res. Asia. 2019;16(2), 359-376.
- El-Sayyad, H.I.H., Al-Haggar, M.M.S., El-Ghawet, H.A., Bakr, I.H.M. Effect of maternal diabetes and hypercholesterolemia on fetal liver of albino Wistar rats. Nutrition 2014;( 30)326–336.
- El-Sayyad, H.I.H., Amoura, M.A., Gadallah, A.A., Bakr, I.H., Protective effects of Allium sativum against defects of hypercholesterolemia on pregnant rats and their offspring. Int J Clin Exp Med 2010;3(2):152-163.
- El-Sayyad, H.I.H., El-Sherbiny, M., Sobh, M.A., Abou-El-Naga, A.M., Ibrahim, M.A.N. and Mousa, S.A. Protective effects of Morus alba leaves extract on ocular functions of pups from diabetic and hypercholesterolemic mother rats. Int.J.Biol.Sci. S.A 2011; 7: 715-728.
- El-Sayyad, H.I.H., El-Shershaby, E.M.F., El-Mansi, A.A. , El-Ashry, N.E. Anti-hypercholesterolemic impacts of barley and date palm fruits on the ovary of Wistar albino rats and their offspring. Reproductive Biology. 2018; 18(3): 236-251
- Enkhmaa, B., Shiwaku, K., Anuurad, E., Nogi, A., Kitajima, K., Yamasaki, M. Prevalence of the metabolic syndrome using the third report of the national cholesterol educational program expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (ATP III) and the modified ATP III definitions for Japanese and Mongolians. Clin Chim Acta 2005; 352:105e13.
- Friedewald, W.T., Levy, R.I., Fredrickson, D.S. Estimation of low-density lipoprotein cholesterol in plasma without use preparative ultracentri-fuge. ClinChem. 1972; 18: 499–502.
- Giustina, A.1., Wehrenberg, W.B. Growth Hormone neuroregulation in diabetes mellitus. Trends Endocrinol Metab.1994;5(2):73-8.
- Gluud, C., Madsbad, S., Krarup, T., Bennett, P. Plasma testosterone and androstenedione in insulin dependent patients at time of diagnosis and during the first year of insulin treatment. Hum Reprod. 2007; 22(7):1871-7
- Gómez, O., Ballester, B., Romero, A., Arnal, E., Almansa, I., Miranda, M., Mesonero, J.E., Terrado, J. Expression and regulation of insulin and the glucose transporter GLUT8 in the testes of diabetic rats. Horm Metab Res. 2009;41(5):343-9.
- Grove, T.H. Effect of reagent PH on determination of the high-density lipoprotein cholesterol by precipitation with sodium phototungestate-magnesium. ClinChem. 1979; 25: 560–564.
- Gründker, C., Emons, G. The Role of gonadotropin-releasing hormone in cancer cell proliferation and metastasis. Front Endocrinol (Lausanne). 2017; 8:187.
- Henry, R.K., Menon, R.K. Type 2 Diabetes Mellitus, a Sequel of Untreated Childhood Onset Growth Hormone Deficiency Developing in a 17-Year-Old Patient. Case Rep Endocrinol. 2018; 24:4748750.
- Jayanthi, R., Srinivasan, A.R. Sex hormone independent associations between insulin resistance and thyroid status -a gender based biochemical study on clinically euthyroid non-obese, overweight and obese type 2 diabetics. Diabetes Metab Syndr. 2019;13(3):2286-2291.
- Kermath, B.A., Gore, A.C. Neuroendocrine control of the transition to reproductive senescence: lessons learned from the female rodent model. Neuroendocrinology 2012; 96 (1) , pp. 1-12
- Koh, P.O. Streptozotocin-induced diabetes increases apoptosis through JNK phosphorylation and Bax activation in rat testes. J Vet Med Sci. 2007;69(9):969-71.
- Luzzo, K.M., Wang, Q., Purcell, S.H,, Chi, M., Jimenez, P.T., Grindler, N., Schedl, T., Moley, K. H. High fat diet induced developmental defects in the mouse: oocyte meiotic aneuploidy and fetal growth retardation/brain defects. PLoS ONE 2012; 7, e49217.
- McPherson, N.O., Lane, M. (). Male obesity and subfertility, is it really about increased adiposity? Asian J Androl. 2015;17(3):450-8.
- Miller W.L., Auchus, R.J. . The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr. Rev. 2011; 32, pp. 81-151
- Morris, Molly R., Ludwar, Bjoern Ch. , Swingle, Evan., Mamo, Mahelet, N., Shubrook, Jay H. A New Method to Assess Asymmetry in Fingerprints Could Be Used as an Early Indicator of Type 2 Diabetes Mellitus. J Diabetes Sci Technol. 2016; 10(4): 864–871.
- Nett, T.M., Turzillo, A.M., Rispoli, L.A. Pituitary effects of steroid hormones on secretion of follicle-stimulating hormone and luteinizing hormone. Domestic Animal Endocrinology 2002; (23)Issues 1–2, Pages 33-42
- Nicholson, T.M., Ricke, W.A. Androgens and estrogens in benign prostatic hyperplasia: past, present and future. Differentiation. 2011; 82(4-5): 184–199.
- Povoski, S.P., McCullough, P.J., Zhou, W., Bell, J.r. R.H. Induction of diabetes mellitus in Syrian golden hamsters using stored equilibrium solutions of streptozotocin. Lab Anim Sci 1993; 43:310e4.
- Raad, G., Hazzouri, M., Bottini, S., Trabucchi, M., Azoury, J., Grandjean, V. Paternal obesity: how bad is it for sperm quality and progeny health? Basic Clin Androl. 2017; 27:20.
- Ramalho, L., da Jornada, M. N. , Antunes, L. C., Hidalgo, M. P. Metabolic disturbances due to a high-fat diet in a non-insulin-resistant animal model. Nutrition & Diabetes 2017; (7) 245.
- Rhyu, M.S., Jan, L.Y., Jan, Y.N. Asymmetric distribution of numb protein during division of the sensory organ precursor cell confers distinct fates to daughter cells. Cell 1994; 76(3): 477-491.
- Riera, M.F., Galardo, M.N., Pellizzari, E.H., Meroni, S.B., Cigorraga, S.B. Molecular mechanisms involved in Sertoli cell adaptation to glucose deprivation. Am J Physiol Endocrinol Metab. 2009;297(4):E907-14.
- Saez Lancellotti, T.E., Boarelli, P.V., Romero, A.A., Funes, A.K., Cid-Barria, M., Cabrillana, M.E., Monclus, M.A., Simón, L., Vicenti, A.E., Fornés, M.W. Semen quality and sperm function loss by hypercholesterolemic diet was recovered by addition of olive oil to diet in rabbit. PLoS One. 2013;8(1):e52386.
- Schoeller, E.L., Schon, S., Moley, K.H. The effects of type 1 diabetes on the hypothalamic, pituitary and testes axis. Cell Tissue Res. 2012;349(3):839-47.
- Verma, R., Haldar, C. Photoperiodic modulation of thyroid hormone receptor (TR-α), deiodinase-2 (Dio-2) and glucose transporters (GLUT 1 and GLUT 4) expression in testis of adult golden hamster, Mesocricetus auratus. J Photochem Photobiol B. 2016; 165:351-358.
- Wang, C. The Relationship between Type 2 Diabetes Mellitus and Related Thyroid Diseases. J Diabetes Res. 2013; 390534.
- Wu, L.L., Norman, R.J. and Robker, R.L. The impact of obesity on oocytes: evidence for lipotoxicity mechanisms. Repro Fertil Dev. 2011; 24(1):29–34.
- Yang, X., Wu, L.L., Chura, L.R., Liang, X., Lane, M., Norman, R.J .and Robker, R. Exposure to lipid-rich follicular fluid is associated with endoplasmic reticulum stress and impaired oocyte maturation in cumulus-oocyte complexes. Fertil Steril. 2012; 97(6):1438–1443.
- Yoon, J.W., Jun, H.S. Autoimmune destruction of pancreatic beta cells. Am J Ther. 2005; 12:580–591.

This work is licensed under a Creative Commons Attribution 4.0 International License.





