Manuscript accepted on : 04-June-2019
Published online on: 27-06-2019
Plagiarism Check: Yes
Reviewed by: Monica Butnariu
Second Review by: Kalai S.
M. Manimekalai1, Anand Sheshadri2, K. Suresh Kumar1 and Ashish Rawson*1
1Department of Food Safety and Quality Testing, Indian Institute of Food Processing Technology, Thanjavur, 613005, India.
2Agilent Technologies, Bangalore, 560048, India.
Corresponding Author E-mail: ashishrawson@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2745
ABSTRACT: Honey is a highly valuable natural product, which is consumed by people of all age groups unaware of the high load of veterinary drug residues present. Sulfonamides are one such class of veterinary drugs, which are used in apiculture in higher amounts and impose a lot of negative health effects. This paper describes an analytical method developed for simultaneous determination of two Sulfonamides (Sulfamethizole and Trimethoprim) in honey using liquid chromatography- tandem mass spectrometry (LC-MS/MS). Suitable fragmentor voltage and collision energy were optimised for both the analytes. Five different sample preparation techniques based on ultrasonication were evaluated. In which ultrasonication at 80% amplitude, 5 minutes at 45°C gave improved recoveries. On validation, the developed method showed good linearity with r2 values in the range of 0.98-0.99 for both the sulfonamides. The effect of matrix on the method developed was evaluated and was found that the sample matrix does not pose considerable interferences with excellent linearities with r2 values above 0.95 for both the analytes. The method developed was found to be very selective and can find application in routine analysis for the determination of sulfonamides from honey samples.
KEYWORDS: Honey; LC-MS/MS; Sulfonamides; Ultrasound
Download this article as:| Copy the following to cite this article: Manimekalai M, Sheshadri A, Kumar K. S, Rawson A. Ultrasound Assisted Method Development for the Determination of Selected Sulfonamides in Honey Using Liquid Chromatography-Tandem Mass Spectrometry. Biosci Biotech Res Asia 2019;16(2). |
| Copy the following to cite this URL: Manimekalai M, Sheshadri A, Kumar K. S, Rawson A. Ultrasound Assisted Method Development for the Determination of Selected Sulfonamides in Honey Using Liquid Chromatography-Tandem Mass Spectrometry. Biosci Biotech Res Asia 2019;16(2). Available from: https://bit.ly/2JA9izu |
Introduction
Honey bees are easily attacked by microorganisms and are highly susceptible to infections caused due to parasitic mites, fungi, bacteria and numerous other viruses due to their very weak immune system. Continuous attack of these pathogens induces a lot of stress to honey bees which results in the reduction of bee colonies worldwide. So, generally veterinary drugs are administered to honey bees, to prevent it from diseases and to limit the losses in apiculture1.But on the other hand due to the resistance of diseases against certain veterinary drugs, the veterinary treatments are administered to bee colonies at huge amounts for a longer period of time which results in leaving the traces of these drug residues in honey2.
Among all the veterinary drugs, Sulfonamides contribute to major portion as they have superior curative properties and also easily available3. Traces of these veterinary drug residues in food system causes a lot of negative health effects such as antimicrobial drug resistance, disruption of intestinal microflora , carcinogenety etc4,5,6. Due to this, Food safety and standards (contaminants, toxins and residues) regulations, 2011(India) has recommended the maximum limit of quantification when determined by LC-MS/MS (Liquid Chromatography- Tandem Mass Spectrometry) shall not exceed 5µg/kg for sulfonamide and its metabolites7.
LC-MS/MS is one of the most widely used analytical methods for the determination of veterinary drug residues in food systems8. Generally, sample preparation techniques based on solvent extraction has been highly reported in honey samples9,10. Recently papers have been published on increasing the efficiency of sample preparation conditions through ultrasonication. The low frequency sound waves create mechanical energy in the form of shear and cavitation which helps in increasing the recovery rate11-13. The present work reports a sensitive method developed for the determination of Sulfonamides in honey samples. The main objective here is to optimise the sample preparation conditions such that the optimised conditions can provide better extraction efficiency and yield higher recovery.
Material and methods
Chemicals and Materials
Veterinary drug standards (Sulfamethizole, Trimethoprim) and formic acid were purchased from Sigma (Dorset, UK). Methanol was supplied by Merck (Darmstadt, Germany). Bondesil– PSA (primary-secondary amine) and C18 endcapped bulk sorbents were supplied by Agilent, Santa Clara, CA. Water and acetonitrile were supplied by Sigma, Dorset, UK. All solvents used were of HPLC grade. Disodium EDTA (Ethylene diamine tetraacetic acid) solution was prepared by dissolving disodium EDTA salt in double de ionized water. Double de ionised water was made available by Merck water purification system. Anhydrous sodium sulphate (extrapure AR) and Sodium sulphate (extrapure AR) was supplied by Sisco research laboratories, India. Captiva premium syringe filters of glass fibre membrane were supplied by Agilent, Santa Clara, CA.
Stock solutions and Working Standards
Sulfonamides exhibited good solubility in acetonitrile; hence this solvent was used in the preparation of stock solutions and working standards. Individual drug standards of 1mg were dissolved in 1ml to prepare stock standards of concentration of 1000ppm.The individual stock standards were vortexed for 2 minutes to ensure complete solubility. From these stock solutions, further dilutions were made to prepare working standards of 100 mg/kg. Stock standards and working standards were stored in dark at deep freeze conditions.
LC-MS/MS Analysis
The method development was carried out with Agilent 6470 Triple Quad LC-MS/MS system (Santa Clara, CA). The triple Quad Mass Spectrometer used was equipped with Agilent JetStream Technology that creates an ion rich zone by efficient desolvation. A mobile phase gradient with water as mobile phase A and methanol acidified with 0.1% formic acid as mobile phase B was chosen. Suitable gradient conditions were found to be 90% of A and 10% of B in 1st minute, followed by change to 50% of A and 50% of B in the next 3 minutes. A further 6 minute gradient was applied by 95% of B and 5% of A. The method has been developed with extra run time to ensure that the analytes and matrix elute out of the column. The MS source conditions were set at a nozzle voltage of 0V, Sheath gas temperature of 350˚C, Gas flow of 10 l/min and nebulizer pressure of 30psi. The sample injection volume was 3µl.
Sample preparation in Honey
Five different sample preparation conditions were evaluated for recovery and the extraction technique with maximum recovery was chosen.
Sample preparation-1
Sample was extracted with acetonitrile. Further ultrasonication was done at 80% amplitude for 5 minutes at 20°C.
Sample preparation-2
Sample was extracted with acetonitrile acidified with 0.1% acetic acid. Further ultrasonication was done at 80% amplitude for 5 minutes at 20°C.
Sample preparation-3
Sample was extracted with acetonitrile which is acidified with 0.1% oxalic acid. Further ultrasonication was done at 80% amplitude for 10 minutes at 20°C.
Sample preparation-4
Sample was extracted with acetonitrile which is acidified with 0.1% oxalic acid. Further ultrasonication was done at 80% amplitude for 10 minutes at 45°C.
Sample preparation-5
Sample was extracted with acetonitrile which is acidified with 0.1% oxalic acid. Further ultrasonication was done at 100% amplitude at 10 minutes at 45°C.
Results and Discussion
Optimisation of Mass spectra
The veterinary drugs are analysed for precursor ion and product ions using ESI by direct infusion of individual working standards into the mass spectrometer. Initially a MS2 scan was done to determine the abundant precursor ion which is monitored based on the molecular formula of the analyte. Following the selection of suitable precursor ion, Collision induced dissociation was carried out to find the optimal collision energy and fragmentor voltages for the product ions.
Optimal fragmentor voltage is essential as it provides structural information. A very low fragmentor voltage will slow down the movement of desired analytes and a very large fragmentor voltage causes fragmentation of ions before the entrance of collision cell thereby reducing the sensitivity of the method. Collision energy, another important instrument parameter is frequently optimised to increase the fragment ion intensity. The collision energy and fragmentor voltage was varied in the range of 8- 30eV and 80-140 V randomly and the optimised MS/MS conditions for the analysis of veterinary drugs are provided in Table 1. The transition 271.2à156 was selected for monitoring of sulfamethizole and 291.2à230 for monitoring of trimethoprim.
Table 1: Mass spectrometry and chromatographic conditions.
| Compound | Precursor ion | Product ion | Fragmentor voltage | Collision energy | Retention time | Dwell time |
| Sulfamethizole | 271.2 | 156 | 100 | 12 | 6.134 | 25 |
| Trimethoprim | 291.2 | 230 | 120 | 30 | 5.816 | 25 |
Comparison of extraction efficiencies of different sample preparation conditions
The extraction efficiencies from different sample preparation procedures were compared. Initially acetonitrile without any acidification was used for extraction. It was found that recoveries were less than 40%. This signifies that acidification plays an important role in the extraction of sulfonamides from honey. Acidic conditions helps in liberating the sulfonamides which are bound to sugar molecules14. Two different acidifying agents were evaluated for its efficient recovery of analytes from honey. It was found that acidification with oxalic acid proves to be more efficient than acetic acid. Similar results of improved extraction with oxalic acid and acetonitrile was reported15.
Table 2: Validation Studies: Linear range, regression equation and correlation coefficients.
| Analyte | Calibration equation | Correlation coefficient | ||
| Standard solution | Honey sample | Standard solution | Honey sample | |
| Sulfamethizole | y= 599.45*x – 4413.89 | y= 22.85*x + 481.37 | 0.995 | 0.969 |
| Trimethoprim | y= 3083.30*x- 23415.8 | y= 5.012*x + 868.43 | 0.981 | 0.978 |
When ultrasonication was done at 80% amplitude with a time period of 10 minutes, it was observed good recoveries. Two different temperature conditions were tested under these ultrasonication conditions. It was observed that at higher temperature conditions of 45°C, appreciable recoveries were obtained for all the analytes. But at reduced temperature of 20°C recoveries were found to be too high above the maximum acceptable limit.
It was observed that ultrasonication at 100% amplitude for 5 minutes at 45°C gave recoveries that were less than 60% for all the analytes. This may be due to the degradation of analytes under prolonged ultrasonication. Similar degradation of antibiotics in honey sample above the optimal period of ultrasound treatment has been reported9. Hence, it becomes necessary to choose the optimal conditions for sonication to increase the recovery and to prevent the degradation of analytes.
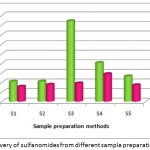 |
Graph 1: Recovery of sulfanomides from different sample preparation techniques.
|
Finalized Sample Preparation Conditions
From the optimised conditions, the sample preparation method-S4 was chosen as the suitable method for simultaneous extraction of all sulfonamides. A brief summary of sample preparation conditions is as follows: 2g of honey sample was taken and spiked with 25ppb concentration of Sulfanomides. 8ml of 0.1M EDTA solution was added to the spiked honey sample. 10ml of acetonitrile acidified with 0.1% oxalic acid was added and kept in a rotary shaker for 30 minutes for complete mixing. 5g of sodium sulphate was added to the mix as the extraction salt and kept for ultrasonication at 80% amplitude for 5 minutes at 45°C. After ultrasonication, the sample was centrifuged at 5000rpm, 5 minutes at 5°C. 6ml of supernatant was collected which was subjected to further cleanup procedures.
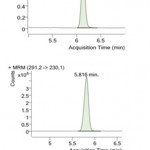 |
Figure 1: MRM Chromatograms of sulfanomide standards: (a) sulfamethizole; (b) trimethoprim.
|
Clean up was done with 50mg of PSA and 100mg of C18. The supernatant was mixed completely with clean up agents by subjecting to vortex for 2 minutes. Then the sample was centrifuged at 5000rpm, 5 minutes at 5°C. The supernatant was collected and filtered using 0.22µ filter. The final clear extract obtained was injected into LC-MS.
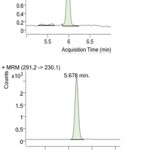 |
Figure 2: MRM Chromatograms of sulfanomides extracted from honey: (a) sulfamethizole; (b) trimethoprim.
|
Method Validation
Validation of the developed method was performed concerning the performance characteristics such as selectivity, linearity and matrix effect. Selectivity of the method was analysed by running different blank matrix samples. Honey samples without spiking veterinary drug standards were subjected to finalized sample preparation conditions and the final extract was injected into LC-MS.As shown in figure 3, it was observed that no interfering peaks were present in the expected retention time of the analytes. Thus, the method proves to be highly selective for the quantification of veterinary drugs eliminating the chances of false positives due to matrix or other co-eluting compounds.
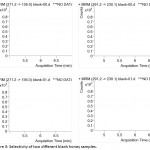 |
Figure 3: Selectivity of two different blank honey samples.
|
Linearity was evaluated for standard solutions in which all the analytes in standard solutions showed excellent linearity with r2 value ranging between 0.98 and 0.99. Matrix effect was evaluated using blank honey samples. Blank honey samples were subjected to finalised sample preparation procedure and the final extracts obtained were fortified with standards at 5 levels from 10ppb to 100ppb. The matrix showed good linearity with r2 values in the range of 0.96-0.97.
Conclusion
A method has been developed for simultaneous determination of sulfonamides in honey. A simple sample preparation and clean up has been proposed in this study. Optimisation of ultrasonic conditions such as amplitude, temperature and time has been performed. It was found that acidification and optimal ultrasonication plays a very important role in extraction of sulfonamides from honey. The method was further validated which proves the robustness of the method.
Acknowledgements
We would like to thank Indian Institute of Food Processing Technology and Agilent Technologies for their continual support.
Conflict of Interest
There is no conflict of interest.
References
- Formato G., Comini A., Giacomelli A., Ermenegildi A., Zilli R., Davis I. Veterinary care of honey bees in the UK. In practice. 2010; 32(9):418-25.
CrossRef - Lozano A., Hernando MD., Uclés S., Hakme E., Fernández-Alba AR. Identification and measurement of veterinary drug residues in beehive products. Food chemistry. 2019;274:61-70.
CrossRef - Dluhošová S., Borkovcová I., Kaniová L., Vorlová L. Residues: Honey Quality in the Czech Market. Journal of Food Quality. 2018.
CrossRef - Beyene T. Veterinary drug residues in food-animal products: its risk factors and potential effects on public health. J Vet Sci Technol. 2016;7(1):1-7.
- Al-Waili N., Salom K., Al-Ghamdi A., Ansari MJ. Antibiotic, pesticide, and microbial contaminants of honey: human health hazards. The Scientific World Journal. 2012.
CrossRef - Rawson, Ashish., Sunil C.K. Veterinary Drug Residue, a Food Contaminant. Ingredients South Asia. 2016.
CrossRef - Food Safety and Standards (Contaminants, Toxins and Residues) Regulations, 2011.
- Masia A., Suarez-Varela MM., Llopis-Gonzalez A., Pico Y. Pesticides and Veterinary Drug Residue Determination in Food by Liquid Chromatography-Mass Spectrometry: A Review. AnalyticaChimicaActa. 2016;10.
CrossRef - El Hawari K., Mokh S., Doumyati S., Al Iskandarani M., Verdon E. Development and validation of a multiclass method for the determination of antibiotic residues in honey using liquid chromatography-tandem mass spectrometry. Food Additives & Contaminants: Part A. 2017;34(4):582-97.
CrossRef - Wang J. Determination of five macrolide antibiotic residues in honey by LC-ESI-MS and LC-ESI-MS/MS. Journal of agricultural and food chemistry. 2004;52(2):171-81.
CrossRef - Chen D., Yu J., Tao Y., Pan Y., Xie S., Huang L., Peng D., Wang X., Wang Y., Liu Z., Yuan Z. Qualitative screening of veterinary anti-microbial agents in tissues, milk, and eggs of food-producing animals using liquid chromatography coupled with tandem mass spectrometry. Journal of Chromatography B. 2016; 1017:82-8.
CrossRef - Manimekalai M., Rawson A., Sengar AS., Kumar KS. Development, Optimization, and Validation of Methods for Quantification of Veterinary Drug Residues in Complex Food Matrices Using Liquid-Chromatography—A Review. Food Analytical Methods.2019; (ARTICLE IN PRESS)
CrossRef - Sunil CK, Chidanand DV, Manoj D, Choudhary P, Rawson A. Effect of ultrasound treatment on dehulling efficiency of blackgram. Journal of food science and technology. 2018; 55(7):2504-13.
CrossRef - Sheridan R., Policastro B., Thomas S., Rice D. Analysis and occurrence of 14 antibacterials and chloramphenicol in honey by solid-phase extraction followed by LC/MS/MS analysis. Journal of agricultural and food chemistry. 2008;56(10):3509-16.
CrossRef - Wang J., Leung D., Chow W., Chang J., Wong JW. Development and validation of a multiclass method for analysis of veterinary drug residues in milk using ultrahigh performance liquid chromatography electrospray ionization quadrupole orbitrap mass spectrometry. Journal of agricultural and food chemistry. 2015;63(41):9175-87.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





