Manuscript accepted on : 12-June-2019
Published online on: 28-06-2019
Plagiarism Check: Yes
Reviewed by: Monica Butnariu
Second Review by: Dr. Farzana Khan Perveen
Madhavi M*, Babu Rao G and Srinivas V
Department of Zoology, Nizam College, Osmania University, Hyderabad, Telengana State, India.
Corresponding Author E-mail: prsmadhavi@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2756
ABSTRACT: Betulinic acid is a compound isolated from bark of Ziziphus jujuba. Betulinic acid,a triterpenoid. Betulinic acid exhibits a broad range of biological activities. It is an insect growth regulator, we observed the effect of different concentrations (10, 8, 6, 4and 2 μg / μl doses) Betulinic acid on Callosobruchus chinensis growth and development, in our observations we observed various morphological abnormalities like degeneration, deformation in larval, pupal stages. Untreated Callosobruchus chinensis showed normal in the developmental stages with the larval instar stages and henceforth developimg into pupa without any deformities. However Betulinic acid affected larval instars showed disrupted structures of the cuticle like tanning of cuticle and abnormal larvae over-aged larva with either complete or partial damage of pupa. The results demonstrated that Betulinic acid causes rapid cessation of growth due to disruption of larval structure and inhibition of growth following topical treatment on 4th 5th instar and pupae of Callosobruchus chinensis.
KEYWORDS: Betulinic Acid; Callosobruchus Chinensis; Larvae; Pupa
Download this article as:| Copy the following to cite this article: Madhavi M, Babu R. G, Srinivas V. Morphological Abnormalities of Betulinic Acid from Ziziphus Jujuba Against the Callasobruchus Chinensis (Coleoptera: Bruchidae). Biosci Biotech Res Asia 2019;16(2). |
| Copy the following to cite this URL: Madhavi M, Babu R. G, Srinivas V. Morphological Abnormalities of Betulinic Acid from Ziziphus Jujuba Against the Callasobruchus Chinensis (Coleoptera: Bruchidae). Biosci Biotech Res Asia 2019;16(2). Available from: https://bit.ly/2Xz7yR9 |
Introduction
The modern insecticide researches started 65 years before with the chlorinated hydrocarbons, organophosphates, methyl carbamates and botanicals, these organic insecticides are used to control insect pests.9 In the past few years, for pest management use of synthetic pesticides has become controversial. The pesticides are known to cause environmental threat as the pesticides accumulate in different concentrations in various levels of ecosystem. Another reason of controversy is to pest developing resistance to pest. Even though insects are exposed to insecticide for a prolonged period of time they manifest gradually, insect will not only develop resistance against particular insecticides to which they are exposed to, but also a group of insecticides will also develop resistance by a method of cross-resistance. To control these problems, the use of cultural practices, biological control, use of antifeedants, hormonal insecticides (IGRs) plant extracts are the other methods developed for pest control.2,31
The search for safe insecticide approaches, interest on products of plant acting like an insecticides has grown more, such that pesticides are removed from use because of environmental and food safety problems14 Diverse biological effects on the insects are provided by the plant kingdom which is the store house of chemicals. Several plants with insecticidal properties have been recognized in current years. Environmental safe pesticides have been particularly toxic, and are not bio-accumulate, and show short persistence in the environment. More particular ways of work and decreased risks for non-target organisms and the environment they are more advisable in modern integrated pest management programs, in previous two decades with expansion of natural and synthetic compounds efficient of interfering with process of growth, development, metamorphosis of selected insects. Plant products (insecticides) seem to be eco-friendly and mixtures of biologically active substances, because they have been found to be selective24 and cause low negative effects to the ecosystems apart from conventional insecticides.27
Use of plant products to insect pest control that untimely affect insect growth and development without causing environmental hazards. These substances were categorized as “insect growth regulators” (IGRs) or “Insect hormone mimics”. They were considered for having less risk as reported by the29 to being soft to beneficial insects and target specific for juvenile stages. The insect growth regulators are – hormonal, enzymatic and chitin synthesis inhibitors. Both juvenile hormone analogues and ecdysone inhibitors disrupt the ratio of hormones in the young insect. The correct ratio of juvenile hormone and ecdysone must be present for an insect to be moult in the next stage. Primary moulting hormone that is Ecdysone is necessary for insects to change from the larval to pupal stage. If the ratio of one hormone to other is not correct, the insect fail to become adult, decreasing reproduction and subsequently population increase. With some IGRs, adults even fail to produce usable eggs.
Plants are potential producer of novel chemical compounds which cannot yet be synthesized. Estimations suggest that over 2000 plant species have the potential to identify and develop new chemistries to reduce bacteria, fungal, and insect/arthropod pests.13 The complexity in chemical compounds in biorational products can also make development of resistance by insect pests is more difficult.21 These environmental issues have driven agricultural researchers to search for better ecofriendly based pesticides.30 Callosobruchus chinensis was believed to be of eastern origin but became a cosmopolitan species spreading throughout the world with the transport of food stuffs. Its absence from apparently suitable habitats ex. Flour mills in Iraq, Iran and areas of Pakistan was believed to be due to low moisture contents of stored food in such places.Callosobruchus chinensis attacked stored products like Bengal grams, cotton, wheat and wheat bran, Sorghum, rice and rice bran, maize, groundnut seed and cakes, cereals, pulses, oil cakes, nuts, dried fruit and various processed foods, redgram, greengram, cowpea, and gingelly cakes in descending order based on the severity of infestation and damage.
Callosobruchus chinensis is a pest of stored grain. The quality of the grain will deteriorate unless protected from pests. The larvae of Callosobruchus chinensis damage the grains of rice and maize by feeding under silken webs. In case, of whole grain Kernels are bound into lumps. When infestation increases entire stock of grains have a possibility to be connected into a webbed mass. Eventually a typical bad smell develops and the grain is rendered unfit for human consumption. Larvae are serious economic pests that cause quantative and qualitative losses in tropical and sub-tropical regions. In the present study Betulinic acid which was isolated from Ziziphus jujuba. We investigated the developmental activity effects of Betulinic acid on the fourth and fifth instars and pupae of Callosobruchus chinensis, in laboratory assays.
Materials and Method
Betulinic Acid Isolated from Z. jujube
At room temperature the pulverized dried roots of Z. jujuba were macerated with MeOH (2×60 L) for each one week. To give a crude extract the MeOH extract was concentrated in vacuo. The extract which was concentrated is suspended in H2O and acidified with 1N HCl to pH 3. To yield EtOAc fraction the acidic solution was extracted with EtOAc. The aqueous residue with NaOH was basified to pH 9 and extracted with CHCl3 for an alkaloid fraction which was not applied in the present study. The fraction of EtOAc was subjected to a normal silica gel CC with mixture of CHCl3 and MeOH (100:1 to 1:1) and 10 subfractions (EA-1-10) are given. The EA-3 fraction was fractioned by normal silica gel CC with a mixture of MeOH (100:1 to 5:1) and CHCl3, giving seven fractions. By re-crystallization with 100% MeOH, Fraction 3 and 4 yielded Betulinic acid.
Test Product: Betulinic Acid
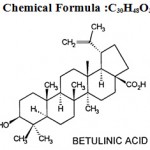
Betulinic Acid (C30H48O3) Betulinic acid is a compound isolated from bark of Ziziphus jujuba. Its medicinal benefits are indicated in Ayurveda and Chinese Medicine. Betulinic acid, a triterpenoid present in many plant species, has captivated the attention due to its important pharmacological properties, such as anti-cancer and anti-HIV activities, anthelmintic activity, antifeedant activity. It also exhibits antibacterial, anti- inflammatory and anti-malarial properties.
 |
Scheme 1
|
Preparation of test Solution
By dissolving a known amount of Betulinic acid in 1 μl of acetone the different concentrations of Betulinic acid doses were prepared 10, 8, 6, 4 and 2 μg / μl doses.
Test Insect
The larvae of Callosobruchus chinensis caused damage to stored products firstly by feeding and secondly by leaving silken threads wherever they move, thus forming a dense webbing leading to the formation of silken galleries. In case of heavy infestation, food materials become tightly matted together with webbing, larval galleries, cocoons and excreta. Severe pest attack resulted in entire destruction of the stored productsand leads to damage of the foodstuffs. Various insecticides provide effective and quick control of pest. With the aspect of their adverse effects on environment and other different non-targeted organisms now there is an attempt made to review biology, biological management methods, historical distribution which are most prominently suited to the programme of integrated pest management.
Collection of Larvae , their Maintenance
Larvae
The larvae start feeding immediately after hatching. They grow in 25-35 days. There are five instars. The larvae alone cause damage to the grains. Pre-pupal stage: It is the non feeding stage when the larvae make silken cocoons among the grains for pupation. The duration of this stage is 4-5 days. Pupal Stage: This stage spreads over 7-8 days and the insect is in a quiescent state. This arrangement has helped the larvae and adults to evolve in different directions, the larvae specializing in food gathering and the adults developing advanced means of reproduction and dispersal .The adult moths of Callosobruchus chinensissurvive for 8-10 days and are of great economic importance. The adults have a siphoning type of mouth parts, so adults do not feed on the grains. The larva has a well-developed chewing type of mouth parts. It is the larva that feeds on the grains and forms infesting stage .Based on the body weight and head capsule size the larval stages were classified for the current study. IV Instar, chosen for the present study was 45- 55 mg on an average in weight. The head capsule size was 0.72-0.78 mm.
V Instar larvae weighed 76-85 mg and the head capsule size was 103-108mm .The completely grown fourth instar and fifth instar larvae were classified and kept in a separate glass dish at room temperature for the experiment For each experiment 3-5 replicates were done and each experiment was repeated at least 5 times.
Treatment with Betulinic Acid
With the help of Hamilton micro syringe freshly thirty moulted fourth instar, fifth instar larvae and thirty zero-hour pupae on the abdominal region were treated topically with 2, 4, 6, 8 and 10 μg/ μl of Betulinic acid by acetone as carrier solvent .Each time by Betulinic acid thirty larvae and pupae were treated and in triplicate the experiments were performed. The acetone controls were treated each time with an equivalent volume of carrier solvent. After total absorption of Betulinic acid.
Into the diet the larvae and pupae were transferred. The treated resultant females were observed for morphological deformities and compared with controls for results.
Results and Discussion
A plant product which causes the morphogenetic and physiologic abnormalities may result in insects reproductive failure can be applied to control the Callosobruchus chinensis. It is one of the relatively new method to control the Callosobruchus chinensis.11 During this stage, to initiate the development ecdysone is released and reproductive organs differentiation, leading for metamorphosis, the internal reproductive organs morphological abnormality and testis and ovaries histological disruption were similar to findings reported by other researchers.4,19 On fifth instar, fourth instar larvae and pupae the plant product Betulinic acid of different concentrations is applied. the inhibition rate is increasing as per concentration increased because of the phytochemical exhibiting the ecdysis inhibition. Abnormalities in larvae, pupae, adults were developed in treated resultants (Figure-1). As per the increase of concentration the survival rate of adult is decreased. With treatment by Betulinic acid some of the treated larvae pupated abnormally and henceforth drastically affected.
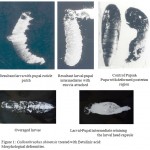 |
Figure 1: Callosobruchus chinensis treated with Betulinic acid: Morphological deformities.
|
The incompletely development of larvae abnormalities such as deteriorated and defective pupae and partially developed adults and overaged larvae are formed in the presence of Betulinic acid. With results of this study and other reports, application by Betulinic acid on the Callosobruchus chinensis will not only produces morphological deformities, but will also cause the sterility in adults. We found the Betulinic acid application it had a direct role on fecundity, fertility of treated insects, so it can be concluded that this compound had an effect on growth of larvae, pupae. Decrease in fecundity similar observations had been reported by22 on Aphis glycines and 4 on Rhyzoperthadominica.
Conclusion
On the basis of overall findings, it can be concluded that Betulinic acid is toxic to Callosobruchus chinensis, as it mimics the action of JH and maintains insect in an immature state. Betulinic acid caused mortality in larvae and produced abnormal adults and it also affected sterility in adults. This can be because of the destructive effects of these compounds on reproductive organ development. Growth and development and fecundity of the Callosobruchus chinensis are inhibited by the plant extract. Thus Betulinic acid may be considered as a leading target compound having the potential to control Callosobruchus chinensis and can therefore form an important component of various Integrated Pest Management (IPM) programs.
References
- Basu, K. and Lamsal, P.P. (1947). Investigations on Indian medicinal plants-II. Hydrocotyle asistica. Quart. J. Pharm. Pharmacol. 20:135-136.
- Bell, A., L.E. Fellows and S.J.Simmonds, (1990). Natural products from plants for the control of insect pests. In: Safer insecticide development and use. Hodgson, E and R. J. Kuhr (eds.), Marcel. Dekke, USA.
- Balasubrmanian R, Selvaraj P, Sahayaraj K. Partial Purification and characterization of phytoecdysone from Chrystella Parasitic (L) and screening its pesticidal properties on lepidopteran pest. J. Biopesticides. 2008; 1(2): 201-205.
- Chanbang Y, Arthur FH, Wilde GE , Throne JE, Subramanyam BH. Susceptibility of eggs and adult fecundity of the lesser grain borer, Rhyzopertha dominica, exposed to J. Insect Sci. 2008; 8: 1-5.
- Fathpour H, Mir T. Effects of juvenile hormone, pyriproxyfen, on German cockroach (Dictyoptera: Epilampridae; Blattella germanica). Research Bulletin of Isfahan 2003; 17(1): 87-102.
- Gaikwad SM, Muniv YS, Chavan JA, Bhawane GP. (2011), Population Density and Natural Enemies of Papilio polytes polytes , (Lepidoptera: Papilionidae), Biological Forum-An International Journal. 2011; 3(1): 41-43.
- Hami M, Taibi F, Soltani-Mazouni N. Effects of flucycloxuron, chitin synthesis inhibitor on reproductive events and thickness of chorion in mealworms. Commun. Agric. Biol. Sci. 2004; 69 (3): 249–255.
- Hermawan W, Kajiyama S, Tsukuda R, Fujisaki K, Kobayashi A, Nakasuji Antifeedant and antioviposition activities of the fractions of extract from a tropical plant, Andrographis paniculata (Acanthaceae), against the diamondback moth, Plutella xylostella (Lepidoptera: Yponomeutidae). Appl.Entomol. Zool. 1994; 29:533–538.
- Hoffmann KH, Lorenz MW. The role of ecdysteroids and juvenile hormones in insect Trends in Comparative Biochemistry and Physiology.1997; 3: 1-8.
- Indrasith LS, Sasaki T, Yaginuma T, Yamashita The occurrence of premature form of egg-spesific protein in vitellogenic follicles of Bombyx mori. J. Comp. Physiol. 1988; 158 (1): 1–7.
- Karhu RR, Anderson SH. Effects of pyriproxyfen spray, powder, and oral bait treatments on the relative abundance of nontarget arthropods of black- tailed prairie dog (Rodentia: Sciuridae) towns. Journal ofMedical Entomology. 2000;37(4): 612-8.
- Klocke JA. Plant compounds as source and models of insect control agents. In: Hosttettmann K (ed.) Economic and medicinal plant research. Academic, London. 1989; 103-144.
- Koul O, Isman, MB, Ketkar CM. Properties and uses of neem, Azadirachta indica Juss. Can. J. Bot. 1990; 68: 1-11.
- Kumar RA, Sridevi K, Vijaya Kumar N, Nanduri S, Rajagopal S. Anticancer and immunostimulatory compounds from Andrographis paniculata. Journal of 2004; 92: 291–295.
- Studies on seasonal occurrence, biology and control of citrus butterfly, P. demoleus Linnaeus (Papilionidae : Lepidoptera) M.Sc (Ag) thesis submitted to Acharya NG Ranga Agricultural University, Hyderabad. 2000; 141.
- Lingampally V, Solanki VR and Sabita Raja S. Andrographolide: An effective anti- fertility agent for the control of Triboliumconfusum. Asian Journal of Plant Science and 2012b; 2(3): 313-317.
- Lu WJ, Lee JJ, Chou DS. A novel role of andrographolide, an NF-kappa B inhibitor, on inhibition of platelet activation: the pivotal mechanisms of endothelial nitric oxide synthase/cyclicGMP. Journal of Molecular Medicine. 2011; 89(12): 1263–1271.
- Masner P, Hangartner W, Suchy, M. Reduced titers of ecdysone following juvenile hormone treatment in the German cockroach. Blattella germanica. Journal of Insect Physiology. 1975; 21: 1755-1762.
- Reddy VLN, Reddy SM, Ravikanth V. A new bisandrographolide ether from Andrographis paniculata nees and evaluation of anti-HIV activity. Natural Product 2005; 19(3): 223–230.
- Regnault-Roger C, Philogene BJR, Vincent C. Biopesticides origine vegetale, Techiques and documents, Paris. 2002.
- Richardson ML, Lagos DM. Effects of a juvenile hormone analogue, pyriproxyfen, on the apterus form of soybean aphid (Aphis glycines). J. Appl. Entomol. 2007; 131: 297–302.
- Rembold H, Seiber KP. Inhibition of oogenesis and acdysteroid synthesis by 1981.
- Saxena RC, Justo HD, JrEpino PB. Evaluation and utilization of neem cake against the brown planthopper, Nilaparvata lugens (Homoptera: Delphacidae). J. Entomol. 1984; 77: 502-507.
- Saxena S, Jain DC, Gupta MM, Bhakuni RS, Mishra HO, Sharma RP. High performance thin layer chromatographic analysis of hepatoprotective diterpenoids from Andrographis Phytochem Anal. 2000; 11: 34-36.
- Shamsuzzoha M, Rahman MS, Ahmed MM.. Antifertility activity of a medicinal plant of the genus Andrographis Wall (family Acanthaceae). Part II. Bangladesh Med Res Counc Bull 1979; 5: 14-18.
- Stark JD, Walter JF. Neem oil and neem oil components affect the efficacy of commercial neem insecticides. J. Agric. Food Chem. 1995; 43: 507-512.
- Telfer WH, Rubenstein E, Pan ML. Regulation of Insect Development and Behaviour. Technical University Press, Wroclaw. 1981.
- US EPA. Fenoxycarb, in pesticide fact hand book, Noyes Data Corp, Park Ridge, NJ, USA.1988 373-377.
- Wood HA, Granados RR. Genetically engineered baculoviruses as agents for pest Annu. Rev. Microbiol. 1991; 45: 69-87.
- Yankanchi SR, Patil SR. Field efficacy of plant extract on larval populations of Plutella xylostella L. and Helicovepa armigera Hub. and their impact on cabbage infestation. J. Biopesticide. 2009; 2: 32-36.
- Kellouche A, Soltani N. Impact of hexaflumuron, a chitin synthesis inhibitor, on growth, development and reproductive performance of the progeny in Callosobruchus maculates after adult treatments. Afr. J. Agric. Res. 2006; 1(3): 57–64.

This work is licensed under a Creative Commons Attribution 4.0 International License.





