Manuscript accepted on : 24-June-2019
Published online on: 29-06-2019
Plagiarism Check: Yes
Reviewed by: Atul Kadam
Second Review by: Savita Singh
Department of Zoology, Faculty of Science, The Maharaja Sayajirao University of Baroda, Vadodara- 390007, Gujarat, India.
Corresponding Author E-mail: dollymsu@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2761
ABSTRACT: Tribolium casteneum is the most resistant stored grains pest causing more than 40% grains loss per year. Replacement of the conventional fumigants with an eco-friendly alternative seemed an intelligent move to control the pest which has inclined global research towards the efficacy of pesticidal plants. In the race of finding a better insecticidal candidate, we focused on to the chemical composition of the essential oils (EO) derived with polar and non-polar solvents from Artemisia annua and their possible bioactivity against the pest species. GC-MS analyses of Chloroform and n-Hexane derived EOs showed the dominance of Oxygenated Sesquiterpene in the extract. Adults were found more vulnerable to n-Hexane EO (LD50= 0.71 mg adultˉ1) than to chloroform derived EO (LD50= 0.97 mg adultˉ1) in contact toxicity assays. In the fumigant bioassay both the adults and larvae were found susceptible towards n-Hexane EO with LD50 0.66 & 0.53 mg L airˉ1 respectively. Evaluation of the biomolecular profile of adults and larvae at their lethal doses to understand the molecular mechanism underlying oxidative stress has shown significant downfall (p˂0.01) in the activities of protein, AChE, GST, GSH whereas up regulation of LPO was distinctly marked. The basic knowledge of employing potential solvent in eluting EOs of A. annua would prove to be an efficient environmental friendly management tool against T. casteneum.
KEYWORDS: Artemisia Annua; Biomolecules; Contact Toxicity; Essential Oils; Fumigant Toxicity; Tribolium Casteneum
Download this article as:| Copy the following to cite this article: Deb M, Kumar D. Chemical Composition and Bioactivity of the Essential Oils Derived from Artemisia Annua Against the Red Flour Beetle. Biosci Biotech Res Asia 2019;16(2). |
| Copy the following to cite this URL: Deb M, Kumar D. Chemical Composition and Bioactivity of the Essential Oils Derived from Artemisia Annua Against the Red Flour Beetle. Biosci Biotech Res Asia 2019;16(2). Available from: https://bit.ly/2JFtyA2 |
Introduction
Global demand for food is increasing continuously due to overshooting population. This poses great challenge to the sustainable utilization of stored grains which accounts for about more than 70% of their total yield. Unfortunately, stored conditions are a major attraction to different types of infestation mainly by insects due to infinite food resources and favourable abiotic factors (Howe, 1943). Tribolium casteneum (Herbst, 1797) is the most resistant species among the huge list of stored grains pests and known to exploit a wide range of stored products (Hagstrum, 2017). They are testified as the primary pest of wheat flour & other milled products and secondary pest of wheat grains (Good, 1933). Studies reported that more than 40% quantitative and qualitative devastation of wheat flour is caused by the beetle (Ajayi & Rahman, 2006). The flour beetle is also known to secrete toxic quinones which turns the flour greyish and hence reduces its aesthetic and nutritive values (Ladisch et al., 1967). Moreover, carcinogenic attribute of the quinones are affirmed thus poses serious risk to human health (El-Mofty et al., 1989). Hence controlling the pest is of utmost importance.
Fumigation through methyl bromide and phosphine is the most dominant measure practiced in most of the countries to manage stored product pest (Bell, 2000). However, methyl bromide is banned worldwide due to its association in ozone layer depletion (Anbar et al., 1996). Use of phosphine has already triggered much negative impact due to their fast growing resistance (Benhalima et al., 2004). Replacement of these conventional fumigants with an eco-friendly alternative seemed an intelligent move to control the pest which has inclined global research towards the efficacy of pesticidal plants. In the race of finding a better insecticidal candidate, different variants of plants are tested and fortunately emerged as a potent solution towards the challenge (Okwute, 2012; Chaudhary et al., 2017). Plants are known to contain a wide range of essential oils (Sasidharan et al., 2011). The Essential Oils (EO) contain plethora of organic compounds which are relatively non-toxic for the environment and can be used as a potent alternative for the synthetic pesticides (Isman, 2000; Caballero-Gallardo et al., 2011). The views were also supported by previous studies where EOs of Ricinus communis seeds and Datura stramonium extracts were found effective in controlling the red flour beetle (Abbasipour et al., 2011; Babarinde et al., 2011).
Genus Artemisia is the most widely distributed genera of Asteraceae family (Chu et al., 2012) and is extensively used for its medicinal properties in Asian countries (Das, 2012). Artemisia and its EOs are the subject of research interest since decades which is reflected in the wide range of studies conducted across the globe (Bora & Sharma, 2011). The genus was reported to pose toxic effect against pathogens and can be used in human diets and animal fodder (Janssen et al., 1987). It was also testified to possess insecticidal and antifeedant activities (Liu et al., 2006; Gonzalez-Coloma et al., 2012). EOs from the Artemisia sieberi Besser has shown insecticidal properties against three stored grain pest (Negahban et al., 2007). Moreover, EOs from Artemisia princeps are described to be an effective repellent and insecticidal candidate against two major stored grain pests (Liu et al., 2006).
Artemisia annua, the sole producer of Artemisinin, is mainly studied for its efficacy against the malarial parasites, Plasmodium (Dhingra et al., 1999). Artemisinin-based combination therapy is emerged as the most efficient antimalarial drug available in the market against MDR strains (Klayman, 1985; Eastman & Fidock, 2009). However, to the best of our knowledge, studies focusing on the candidature of potent solvents for the extraction of EO from A. annua are still unknown. Taking into account the serious infestation caused by Tribolium which eventually pose negative impact on human health and on country’s economy, the very need for the study was sensed. Hence, authors tried to decipher the chemical composition of the essential oils (EO) eluted with Chloroform (polar) and n-hexane (non-polar) solvents from Artemisia annua and testified their possible bioactivity against Tribolium casteneum. In the present study, fumigant and contact toxicity of A. annua EOs against the pest was evaluated. EO obtained by hydro-distillation was analysed through Gas-chromatography to identify major chemical constituents. Additionally, metabolic interference imposed in the treatment sets of T. casteneum (Herbst, 1797) was evaluated.
Experimental Methods
Insect
Tribolium. casteneum (Herbst, 1797) were collected from a small culture maintained in the division of Entomology of the Department of Zoology, The Maharaja Sayajirao University of Baroda Vadodara, Gujarat, India. Unsexed adults were reared in the defined culture media of wheat flour, wheat grain and yeast in the ratio of 6:3:1. Insects were maintained in the humidity chamber at the suitable temperature and humidity ranges of 27±2˚C, 70±5 RH respectively. Newly emerged adults of 1-10 days old, were used for the toxicity assays. Final larval stages i.e. 14 days old larvae were used in the experiment. All the experiments were conducted in the dark under the same temperature and humidity ranges.
Plant Material
The dried, finely grounded leaves of the A. annua were procured from Professor Neeta Pandya, Department of Botany, The Maharaja Sayajirao University of Baroda, Vadodara, Gujarat, India. Plant powder was then stored in plastic bags at 4˚C until used for the extraction of EOs.
Extraction of Essential Oils
Dried plant powder (25 grams) was subjected to hydro-distillation using a modified Clevenger-type apparatus for the extraction of EOs (Clevenger, 1928). Onset of distillation was marked with the boiling of the selected solvents contained in the round bottom flask. 300 mL of both Chloroform and n-Hexane were used separately for 9 & 2 hours respectively for the extraction of EOs. Distillation process continued till the solvent becomes transparent in the extraction chamber. The oil layer was then separated from the aqueous phase using a separating funnel. EOs were then collected and dried over anhydrous sodium sulphate to remove extra water. Crude extracts were further processed in rotary evaporator to remove extra solvents at their boiling ranges. Oil yield was calculated on a dry weight basis employing the Yield (%) formula.
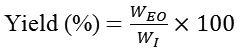
Where, WEO is the weight of dry EO and WI is the weight of fresh plant powder taken for extraction. Extracts were then stored in the airtight containers in a refrigerator at 4°C until it is used.
Chemical analysis of EO- Gas Chromatography-mass spectroscopy (GC- HRMS)
Gas chromatographic analysis was performed on an Agilent 7890N instrument equipped with a flame ionization detector and HP-5MS (30m × 0.25mm × 0.25μm) capillary column, while the EO components were identified on an Agilent Technologies Jeol mass spectrometer. The GC settings were as follows: the initial oven temperature was held at 60°C for 1 min and ramped at 10°C min−1 to 180°C for 1 min, and then ramped at 20°C min−1 to 280°C for 15 min. The injector temperature was maintained at 270°C. The samples (1 μL) were injected neat, with a split ratio of 1:10. The carrier gas was helium at flow rate of 1.0 mL min−1. Spectra were scanned from 20 to 550 m/z at 2 scans s-1. Most constituents were identified using gas chromatography by comparing their retention indices with those of the literature and previous studies. The retention indices were determined in relation to a homologous series of n-alkanes (C8–C24) under the same operating conditions. Further identification was made by comparison of their mass spectra on both columns with those stored in NIST library or with mass spectra from literature. Component relative percentages were calculated based on GC peak areas without using correction factors.
Repellency Test
Repellency in insects was evaluated according to Cosimi et al. (2009) where pests were exposed to different concentrations of chloroform and n-hexane derived EOs dissolved in acetone. Filter papers measuring 7 cm in diameter were cut into two equal halves where one half was treated with the desired concentration of EO and other half with acetone. After drying for two minutes, both the halves of filter paper were attached underside with the cellotape and fixed to the petriplate. 10 unsexed adults were released into the centre of the plate. Five replicates were maintained for each concentration. Readings were taken at the interval of 1, 2, 3, 4, 5, 6, 12 and 24 hours. Insects were then transferred to the plastic vials containing media and checked regularly for 3 days if any mortality is recoded. Raw data was converted to calculate percentage repellency using the following formula:
PR = 2(C – 50)
Where C is the percentage of insects recorded on the untreated half of the disc. Positive values expressed repellency and negative values attractancy. Results of PR were analysed using ANOVA and Tukey’s pairwise comparison test.
Contact Toxicity
To evaluate contact toxicity following the method of Huang et al. (1998), adults and larvae were treated with the desired concentrations of chloroform and n-hexane derived EOs. 10 unsexed adults were taken in plastic vials and kept in the freezer for one minute. This made them unconscious and hence their handling became easy. An aliquot of 5µl of EO was then topically applied on the meso-thoracic region. After 2 minutes they were transferred in the plastic vials containing media. The mortality was recoded till 3 days at the interval of 12 hours.
Fumigant Toxicity
Fumigant toxicity was assessed according to López et al. (2008). Filter papers (What man No.1, 7 cm in diameter) were impregnated with the different concentrations of chloroform and n-hexane derived EOs. Insects were exposed to concentrations ranged from 0.24 to 2.37 mg L airˉ1 of polar solvent and 0.14 to 1.42 mg L airˉ1 of non-polar solvent. Concentrations were decided after standardisation of the process. The filter papers were allowed to air dry for 2 minutes post-treatment to evaporate the solvent. Impregnated paper was then sealed on the screw cap of the plastic vials (25mL). 10 unsexed adults and larvae were tested separately for each concentration. Five replicates were maintained for each concentration. Mortality was determined regularly for 3 days at the interval of 12 hours. Loss of antennal and leg movement was considered as an indication for mortality.
In the toxicity assays, data obtained at the end of the third day was considered as final and processed further for statistical analysis. Probit analysis (Finney, 1971) using Medcalc software was employed in analysing the dosage- mortality response in both the acute toxicity assays.
Biochemical Analysis
Quantitative analyses of biochemical constituents in treated (LC50, LC90) and control sets were assessed. Protein profiling by Biurate kit method (HiMedia Laboratories Pvt. Limited) and enzymatic activities of Acetylcholinesterase (AChE), Glutathione S Transferase (GST), Reduced glutathione (GSH) and Lipid Peroxidases (LPO) were performed following the methods of Ellman et al. (1961), Habig et al. (1974), Jollow et al. (1973) and Buege & Aust (1978) respectively. Analysis of Variance (ANOVA) and Tukey’s Pairwise Comparison Test were employed using Sigma plot 13. 0 statistical software package to compare means.
Results
Chemical Composition of the Essential Oils
The average oil yield from the solvent derived EOs of A. annua was found to be 19.28% and 3.68% w/w for chloroform and n-Hexane respectively. Results of GC-MS showed the qualitative and quantitative composition of EOs of A. annua in Table 1 and 2. Major peaks determined in the solvent extracted EOs of A. annua were marked serially along with their retention indices and % relative composition according to the order of their elution. Twenty four major compounds were identified in the chloroform EO accounted for 97.11% of the total oil (Table 1). Among the major chemical constituents, Bicyclo(2.2.1)heptan-2-one,1,7,7, trimethyl- (15.35%) 3,4-Hexadienal, 2-butyl-2-ethyl-5-methyl- (10.26%), 2H-1-Benzopyran-2-one (8.20%), 2-(4a,8-Dimethyl-1,2,3,4,4a,5,6,7-octahydronaphthalen-2-yl)-prop-2-en-1-ol (6.01%) and 1-Ethyl-3-vinyl-adamantane (5.55%) were enlisted. It is important to mention that Oxalic acid,allyl hexadecyl ester was existed in their stereoisomeric form hence reported with two different retention time in the analysis.
Table 1: Chemical composition of the essential oils of A. annua extracted with chloroform.
| S. No. | Compounds | RIa | RIb | IDc | Relative content (%)d |
| 1 | 2- Pyrrolidinone, 1-methyl- | 920 | 1012 | MS, RI | 4.89 |
| 2 | Bicyclo(2.2.1)heptan-2-one,1,7,7, trimethyl- | 1121 | 1136 | MS, RI | 15.35 |
| 3 | 1- Chloroundecane | 1340 | 1358 | MS, RI | 1.16 |
| 4 | 2H-1-Benzopyran-2-one, 3,4-dihydro | 1392 | 1350 | MS, RI | 0.9 |
| 5 | Bicyclo(7.2.0) undec-4- ene 4,11,11- trimethyl -8- methylene- | 1494 | 1396 | MS, RI | 3.46 |
| 6 | 2H-1-Benzopyran-2-one | 1374 | 1414 | MS, RI | 8.2 |
| 7 | 1,6-Cyclodecadiene, 1-methyl-5-methylene-8-(1-methyethyl)- | 1515 | 1480 | MS, RI | 3.53 |
| 8 | Phenol,2,4-bis(1,1-dimethyethyl)- | 1555 | 1539 | MS, RI | 1.42 |
| 9 | 2-Undecanethiol,2-methyl- | 1433 | 1410 | MS, RI | 3.24 |
| 10 | Caryophyllene oxide | 1507 | 1576 | MS, RI | 3.13 |
| 11 | 2-(4a,8-Dimethyl-1,2,3,4,4a,5,6,7-octahydronaphthalen-2-yl)-prop-2-en-1-ol | 1745 | 1732 | MS, RI | 6.01 |
| 12 | Oxalic acid, allyl hexadecyl ester | 2433 | – | MS | 2.87 |
| 13 | Pulegone | 1212 | – | MS | 2.51 |
| 14 | 1-Ethyl-3-vinyl-adamantane | 1216 | – | MS, RI | 5.55 |
| 15 | Oxalic acid, allyl hexadecyl ester | 2433 | 1514 | MS | 2.16 |
| 16 | 7-Hydroxy, 6- methoxy- 2H-1-benzopyran-2-one | 1784 | 1924 | MS, RI | 2.61 |
| 17 | Deoxyqinghaosu | 1794 | – | MS | 4.27 |
| 18 | 3,4-Hexadienal,2- butyl-2-ethyl-5-methyl | – | – | MS | 10.26 |
| 19 | Phytol | 2045 | 2104 | MS, RI | 4.85 |
| 20 | Oxirane(Tetradecyloxy)methyl- | 1877 | – | MS | 1.45 |
| 21 | 1,4-Methanoazulene-9-methanol,decahydro-4,8,8-trimethyl- | 1635 | 1712 | MS, RI | 2.11 |
| 22 | Didodecyldimethylammonium | – | – | MS | 1.22 |
| 23 | 1,3-Dimethyl-5-3(2-methoxycarbonyl-2 Acetamidoethyl)-1H-indol-2-yl 1-yl uracil | 3566 | – | MS | 3.02 |
| 24 | Squalene | 2914 | 2818 | MS, RI | 2.92 |
| Total | 97.11 | ||||
| Grouped components (%) | |||||
| Oxygenated Sesquiterpene | 20.08 | ||||
| Ketones | 17.86 | ||||
| Esters | 14.13 | ||||
| Alcohols | 12.97 | ||||
| Saturated Hydrocarbons | 9.91 | ||||
| Ethers | 4.58 | ||||
| Phenol | 1.42 | ||||
| Other Metabolites | 16.14 | ||||
aRetention indices were calculated using a homologous series of n-alkanes (C8–C24).
bIdentification of volatile components was carried out by comparing Mass spectrum (MS) and Retention indices (RI) of components with those of the authentic standards in NIST library and previous study.
cResults obtained by peak-area normalization.
On the other hand, chemical composition of the n-Hexane eluted EO was recorded to contain eighteen different compounds accounting for 100% of the total oil (Table 2). The major constituents as depicted in the Figure 2 were identified as 3,4-Hexadienal,2- butyl-2-ethyl-5-methyl-2 (20.98%), Cedran-diol, 8S,13- (8.29%), 4,4-Dimethyladamantan-2-ol (7.37%), Bicyclo(2.2.1)heptan-2-one,1,7,7-trimethyl- (7.20), 2H -1- Benzopyran- 2- one (7.09), Deoxyqinghaosu (6.39%), 1,4-Methanoazulene-9-methanol,decahydro-4,8,8-trimethyl- (6.03).
Table 2: Chemical composition of the essential oils of Artemisia annua extracted with n-hexane.
| Sr. No. | Compounds | RIa | RIb | IDc | Relative content (%)d |
| 1 | Bicyclo(2.2.1)heptan-2-one,7,7-trimethyl- | 1121 | 1146 | MS, RI | 7.2 |
| 2 | 2H -1- Benzopyran- 2- one | 1374 | 1414 | MS, RI | 7.09 |
| 3 | 2-Isopropenyl-4α,8-dimethyl-1,2,3,4,4a,5,6,7-octahydronaphthalene | 1502 | 1473 | MS, RI | 3.22 |
| 4 | Caryophyllene oxide | 1507 | 1576 | MS, RI | 3.64 |
| 5 | Isoaromadendrene epoxide | 1281 | 1590 | MS, RI | 5.59 |
| 6 | Globulol | 1530 | 1578 | MS, RI | 3.05 |
| 7 | 3-Cyclohexane-1-carboxaldehyde,1,3,4-trimethyl | 1204 | 1171 | MS | 3.67 |
| 8 | 4,4-Dimethyladamantan-2-ol | 1203 | – | MS | 7.37 |
| 9 | 2-Propen-1-ol,3-(2,6,6-trimethyl-1-cyclohexane-1-yl)- | 1465 | – | MS | 2.19 |
| 10 | Cedran-diol, 8S,13- | 1786 | – | MS | 8.29 |
| 11 | n-Hexadecanoic acid | 1968 | 1942 | MS, RI | 5.23 |
| 12 | 4,8a-Dimethyl-6-(2-methyl-oxiran-2-yl)-4a,5,6,7,8,8a-hexahydro-1H-naphthalene-2-one | 1742 | – | MS | 1.49 |
| 13 | Deoxyqinghaosu | 1794 | – | MS | 6.39 |
| 14 | 3,4- Hexadienal,2- butyl-2-ethyl-5-methyl-2 | – | – | MS | 20.98 |
| 15 | Z,Z-5,16-Octadecadien-1-ol acetate | 2193 | – | MS | 1.67 |
| 16 | 11,14,15,16- Tetraoxatetracyclo(10.3.1.0 (4,13).0(8,13))hexadecane-10-one,1,5,9-trimethyl | 1903 | – | MS | 1.57 |
| 17 | 1,4-Methanoazulene-9-methanol,decahydro-4,8,8-trimethyl- | 1635 | 1712 | MS, RI | 6.03 |
| 18 | Squalene | 2914 | 2818 | MS, RI | 5.34 |
| Total | 97.11 | ||||
| Grouped components (%) | |||||
| Oxygenated Sesquiterpene | 36.02 | ||||
| Alcohols | 19.56 | ||||
| Esters | 8.76 | ||||
| Saturated Hydrocarbons | 8.56 | ||||
| Ketones | 7.2 | ||||
| Carboxylic acids | 5.23 | ||||
| Aldehydes | 3.67 | ||||
| Ethers | 3.64 | ||||
| Other Metabolites | 7.35 | ||||
aRetention indices were calculated using a homologous series of n-alkanes (C8–C24).
bRetention indices reported in previous studies
cIdentification of volatile components was carried out by comparing MS spectrum and RIs of components with those of the authentic standards in NIST 5 library and previous study.
dResults obtained by peak-area normalization.
In both the EOs, higher % of Oxygenated Sesquiterpene was recorded with 20.08% and 36.02% in case of chloroform and n-Hexane extracts respectively. The second largest group identified was ketones in chloroform EO and alcohol in n-Hexane EO. Chemical groups like aldehydes and carboxylic acids have contributed in the total composition of n- Hexane distillate EO but absent in chloroform distillate (Table 1 & 2). Interestingly, lesser amount of compounds like Caryophyllene oxide and Squalene known for their antimicrobial and anticancer properties were also detected in both the extracts (Falowo et al., 2019; Smith, 2000).
Repellency Test
EOs of A. annua showed strong repellent activity against the adult beetles. In the present study, repellency was more evident in the treatment sets of n-hexane derived EO than the chloroform EO. Highest concentration of the EO (0.90 mg cmˉ2) had demonstrated 93.5% and 88.25% of repellency by n-hexane and chloroform derived EOs respectively (Table 3). However, repellency was recorded to increase insignificantly (p˃0.01) with the increase in concentration.
Table 3: Repellency of solvent derived essential oils of A. annua against T. castaneum adults using Filter paper arena test.
| Solvent used | Concentration (mg cmˉ2) | Duration of exposure (in) hour | Percent Repellency over 24 hours | ||||||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 12 | 24 | ||||||||||
| Mean Repellency (% ± SD) | |||||||||||||||||
| Chloroform | 0.54 | 67±31* | 26±31* | 33±12* | 40±35* | 53±23* | 80±20* | 80±20* | 93±12* | 59 | |||||||
| 0.63 | 40±35* | 80±20* | 73±31* | 13±46* | 53±12* | 80±20* | 93±11* | 93±12* | 65.63 | ||||||||
| 0.72 | 100* | 60±35* | 87±23* | 67±23* | 80±20* | 80±20* | 93±11* | 93±12* | 82.5 | ||||||||
| 0.81 | 80±20* | 100* | 93±12* | 73±23* | 100* | 80±20* | 100* | 100* | 90.75 | ||||||||
| 0.90 | 93±11* | 67±24* | 87±23* | 73±31* | 93±12* | 93±12* | 100* | 100* | 88.25 | ||||||||
| n-hexane | 0.54 | 60* | 67±12* | 60±20* | 87±12* | 80±20* | 60±20* | 80±20* | 87±11* | 72.625 | |||||||
| 0.63 | 80±20* | 87±23* | 80±20* | 87±12* | 87±12* | 93±12* | 93±12* | 80±20* | 85.875 | ||||||||
| 0.72 | 60±20* | 93±12* | 87±23* | 87±23* | 93±12* | 87±12* | 100* | 93±11* | 87.5 | ||||||||
| 0.81 | 87±12* | 93±12* | 100* | 100* | 93±12* | 100* | 100* | 100* | 96.625 | ||||||||
| 0.90 | 87±23* | 87±23* | 100* | 87±23* | 87±23* | 100* | 100* | 100* | 93.5 | ||||||||
Means (±SEM) followed by * indicate no significant difference (p < 0.01) according to the ANOVA.
Contact Toxicity
Topical application was employed to evaluate whether the insecticidal activity of the EO of A. annua against T. casteneum adults and 14 days old larvae was attributable to contact toxicity (Table 4). No mortality was recorded in the control sets. When LD50 and LD90 values were compared, T. castaneum adults were recorded to be more susceptible than its larval stage to chloroform derived EOs (No overlap in 95% confidence interval). Moreover, no significant (p˃0.01) difference in contact toxicity was seen between larvae and adult beetle when treated with n-Hexane EO as overlap in 95% confidence interval is marked. On the basis of LD90 values, adult beetles were found more vulnerable to chloroform derived EO whereas larvae were more responsive towards n-Hexane derived EO.
Table 4: Contact toxicity of essential oils of Artemisia annua applied topically to Tribolium castaneum at 30˚C and 70±80% r.h.
| Plant Extract | Life stage | LD50 (mg adultˉ1) | 95% Confidence interval | LD90 (mg adultˉ1) | 95% Confidence interval | Slope ± SE | χ2 (DF) |
| Chloroform | Adults | 0.97 | 0.48-1.29 | 2.30 | 1.93-3.02 | 0.96±0.2 | 32.174 (1)* |
| 14 days old Larvae | 1.57 | 1.26-1.86 | 2.81 | 2.43-3.48 | 1.04±0.18 | 42.870 (1)* | |
| n-hexane | Adults | 0.71 | 0.57–0.83 | 1.19 | 1.04–1.46 | 2.60±0.44 | 54.373 (1)* |
| 14 days old Larvae | 0.47 | 0.25-0.61 | 1.04 | 0.87-1.35 | 2.24±0.46 | 37.06 (1)* |
LD50= Lethal dose that kills 50% of the test organisms.
LD90= Lethal dose that kills 90% of the test organisms.
χ2= chi square.
DF=degrees of freedom.
*= Significant (P ˂0.01)
Lethal values are expressed as mean of five replicates.
Fumigant Toxicity
Fumigant toxicity was more pronounced in n-hexane eluted EO treated sets than to Chloroform derived EO (Table 8). Zero mortality was found in control set. Results testified the susceptibility of larval stages towards solvents derived EOs when compared with the adults. While comparing the LD90 values, both the stages were found sensitive towards the n-hexane extracted EO treated sets.
Table 5: Fumigant toxicity of essential oils of Artemisia annua to Tribolium castaneum exposed for 24 h at 30°C and 70±80% r.h.
| Extract | Life stage | LD50 (mg L airˉ1) | 95% Confidence interval | LD90 (mg L airˉ1) | 95% Confidence interval | Slope± SE | χ2 (DF) |
| Chloroform | Adult | 1.17 | 0.93-1.38 | 2.13 | 1.83-2.68 | 1.33±0.24 | 37.944 (1)* |
| 14 days old Larvae | 0.98 | 0.71-1.20 | 1.94 | 1. 65-2.46 | 1.34±0.25 | 36.638 (1)* | |
| n-hexane | Adult | 0.66 | 0.52-0.77 | 1.17 | 1.01-1.45 | 2.48±0.43 | 43.757 (1)* |
| 14 days old Larvae | 0.53 | 0.37-0.65 | 1.04 | 0.89-1.31 | 2.50±0.47 | 40.603 (1)* |
LD50= Lethal dose that kills 50% of the test organisms.
LD90= Lethal dose that kills 90% of the test organisms.
χ2= chi square.
DF=degrees of freedom.
*= Significant (P ˂0.01)
Lethal values are expressed as mean of five replicates.
Biochemical Analysis
Quantitative analysis of biomolecules was performed to assess the changes in their normal range on exposure to the plant EOs. The protein concentrations of the whole body homogenate in the adult and 14-days old larvae were found to be in the ranges of 318- 955 µg mlˉ1. However, there was a significant (p˂0.01) downfall in the protein concentration of the treated sets of contact and fumigant bioassays. In chloroform EO treated sets, protein level of adults decreased from 954 µg mlˉ1 (control) to 833 µg mlˉ1 (LD90) and in larvae 407 µg mlˉ1 in control to 326 µg mlˉ1 in LD90 (Fig. 1 i). Results of n-Hexane EO treated sets were similar (Graph 1 ii). However, reduction in protein concentration was more pronounced in fumigant toxicity assays.
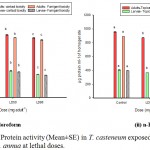 |
Graph 1 (i & ii): Protein activity (Mean±SE) in T. casteneum exposed to solvent derived EOs of A. annua at lethal doses.
|
Columns marked with different letters are significantly different (P˂0.01; ANOVA and Tukey’s honest post-hoc test).
As explained in the Graph 2, level of AChE was reduced significantly (p˂0.01) in the lethal doses when compared with the control. However, insignificant (p˃0.01) reduction is marked between the LD50 & LD90. In contact toxicity bioassays, AChE level in the adults treated with chloroform EO decreased from 0.223 to 0.045 µmoles/min/ml of enzyme and 0.136 to 0.077 µmoles/min/ml of enzyme in larvae. Similar downfall was documented from the n-Hexane EO treated sets (Graph 2 ii). AChE reduction was more pronounced in fumigant toxicity assays from 0.293 to 0.038 µmoles/min/ml of enzyme in adult beetles and 0.14 to 0.069 µmoles/min/ml of enzyme in larvae.
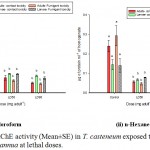 |
Graph 2 (i & ii): AChE activity (Mean±SE) in T. casteneum exposed to solvent derived EOs of A. annua at lethal doses.
|
Columns marked with different letters are significantly different (P˂0.01; ANOVA and Tukey’s honest post-hoc test).
The GST activity was declined significantly (p˂0.01) in the treated sets while comparing their normal range recorded in the control group. But, slight and insignificant (p˃0.01) decrease was noticed in the enzyme activity between the LD50 & LD90 groups. However, as depicted in the Graph 3 i, adults treated topically with the chloroform derived EO has shown significant (p˂0.01) decrease between the sub-lethal (0.115 µmoles/min/ml of enzyme) and lethal group (0.077 µmoles/min/ml of enzyme). Similar results were documented from n-Hexane eluted EO treated sets of adult beetles (Graph 3 ii) where values decline from 0.047 µmoles/min/ml of enzyme in LD50 to 0.038 µmoles/min/ml of enzyme in LD90 groups.
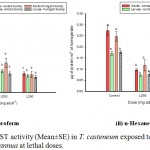 |
Graph 3 (i & ii): GST activity (Mean±SE) in T. casteneum exposed to solvent derived EOs of A. annua at lethal doses.
|
Columns marked with different letters are significantly different (P˂0.01; ANOVA and Tukey’s honest post-hoc test).
A significant (p˂0.01) reduction in GSH activity was well marked in all the treatment sets from their normal range (209- 179 µmoles/mg protein in larvae; 237-181 µmoles/mg protein in adults). Moreover, the enzymatic activity was found to be highly downregulated in n-hexane EO treated sets (Graph 4 i) than the chloroform EO treatment sets (Fig. 4 ii).
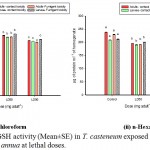 |
Graph 4 (i & ii): GSH activity (Mean±SE) in T. casteneum exposed to solvent derived EOs of A. annua at lethal doses.
|
Columns marked with different letters are significantly different (P˂0.01; ANOVA and Tukey’s honest post-hoc test).
LPO activity was significantly (p˂0.01) upregulated in the treated sets when compared with the control. Moreover, the enzymatic activity was found to be dose- dependent and increased significantly from the LD50 to the LD90 groups (Graph 5). Adults exposed to fumigation of both the EOs, has shown a steep increase in the LPO level (17.983 nmole of MDA/gm of tissue in control to 40.25 nmole of MDA/gm of tissue in larvae). Similar downfall is attained in the fumigant assays as well.
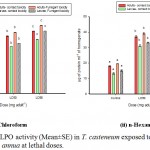 |
Graph 5: (i & ii): LPO activity (Mean±SE) in T. casteneum exposed to solvent derived EO of A. annua at lethal doses.
|
Columns marked with different letters are significantly different (P˂0.01; ANOVA and Tukey’s honest post-hoc test).
Discussion
Our results are quite different from the previous reports. A. annua is well established for possessing Artemisinin which is a potent antimalarial component. However, 1,8-cineole has emerged as a major insecticidal candidate in various studies (Tripathi et al., 2001). Conversely, GC-MS analyses of the present study have clearly depicted the presence of some novel component in the chloroform and n-Hexane derived EOs of A. annua in excessively high amount. Oxygenated sesquiterpene, unique to A. annua is responsible for pharmacological activity is reported to be the major chemical group present in both the EOs (Martínez et al, 2012; Brown, 2010). The group include compounds like 3,4-Hexadienal,2-butyl-2-ethyl-5-methyl and Deoxyqinghaosu in different proportions (Figure 1) which can be attributable to the insecticidal properties of both the EOs. Insecticidal compounds like 2H-1-Benzopyran-2-one (Xiaorong & Taiping, 2008) and Squalene (Chauhan, 2015) were also detected in the EOs which ascertains the insecticidal candidature of plant EOs derived with chloroform and n-hexane.
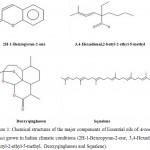 |
Figure 1: Chemical structures of the major components of Essential oils of Artemisia annua grown in Indian climatic conditions (2H-1-Benzopyran-2-one, 3,4-Hexadienal,2-butyl-2-ethyl-5-methyl, Deoxyqinghaosu and Squalene).
|
A. annua showed potent contact, fumigant and repellent activity against T. casteneum with chloroform and n-Hexane derived EOs. However, the insecticidal properties of the EOs varied with solvents and the life stages of the red flour beetle. Presence of Deoxyqinghaosu in the extract was an obvious outcome (Li, 2012; Ni et al., 2012). Correlated to the fact, relatively higher Percent composition of Deoxyqinghaosu in n-hexane EO can be attributable to the better repellent activity and was reported for the first time in the present study from filter paper arena tests.
Results of contact toxicity bioassays have clearly depicted the susceptibility of the adults (LD50= 0.71 mg adultˉ1) and larvae (LD50= 0.47 mg insectˉ1) towards the n-hexane EO. Similarly, Fumigant toxicity has represented n-hexane a better eluent of EO for both the life stages. This could be due to the presence of high percentage of 3,4-Hexadienal,2-butyl-2-ethyl-5-methyl in the crude extract. Moreover, higher Percent composition of Squalene whose insecticidal properties (Chauhan et al., 2015) were already described could have surplused the bioactivity of the plants EOs.
Evaluation of the biomolecular profile of T. casteneum adults and 14- days’ old larvae at their lethal doses were carried out to understand the molecular mechanism underlying oxidative stress following exposure to plant EOs. Decline in protein level with the increase in concentration has been seen in case of both contact and fumigant toxicity bioassays. The results were continuous with a number of earlier investigations where scientists recorded significant downfall in protein level (Smirle et al., 1996; Huang et al., 2004; Macedo et al., 2007). Spectrometric quantification of primary detoxifying enzymes like AChE, GST, GSH and LPO has demonstrated the plants mode of action. Gradual Reduction in the level of AChE, GST and GSH were marked at the lethal doses when compared with the control. In a similar study conducted with the extract of soapnut Sapindus emarginatus against Aedes aegypti has shown significant reduction in larval AChE concentration (Koodalingam et al., 2011). GST is the key cytosolic enzyme for resistance development in insect’s acts by catalysing the conjugation of reduced glutathione to the toxic molecule thus transforms it to less toxic end product (Grant & Matsumura, 1989). Our results on GST level were continuous with a number of previous investigations (Yu, 1982; Vanhaelen et al., 2001). On the contrary, Zibaee & Bandani (2010) has reported a positive correlation between the concentration of plant extract and GST activity after 24 h of exposure. However, reduction in the level of the enzyme was marked with the increase in exposure time. Correlated with GST, GSH level also experienced a significant downfall in the treated sets (Vontas et al., 2001). Upregulation of LPO level was an obvious outcome due to sudden increase in oxidative stress. Similar results were reported by Hasspieler (1990) where an increase in LPO activity was found in mosquito larvae.
Conclusion
The present study has established non-polar solvents as the potent candidate for deriving EOs of A. annua with better insecticidal and repellent activity against T. casteneum. Moreover, newly identified allelochemicals have emerged as an efficient in vivo suppressor of life supporting biomolecules except LPO in the toxicity assays. Hence, these compounds can be used as a potential synergist in pest management by interfering with enzyme mediated detoxification. Moreover, by analysing the gene expression of bioassay survived individuals, future research can focus on modulation of bioassays to channelize the toxicity of Artemisia annua with potent non-polar solvents against other stored grain pests.
Conflict of Interest
There is no conflict of interest.
Funding Source
No organisation has funded the research work.
Compliance with Ethical Standards
All applicable international and national guidelines for the handling of animals were used. All experimentations performed using animals were in accordance with the ethical standards of the institution where the studies were conducted.
References
- Howe, R. W. (1943). An investigation of the changes in a bin of stored wheat infested by insects. Bulletin of Entomological Research, 34(2): 145-158.
- Hagstrum, D. (2017). Infestation records. In K. T. Hagstrum D, Atlas of stored-product insects and mites (pp. 474-483). Minnesota: Elsevier.
- Good, N. E. (1933). Biology of the flour beetles, Tribolium confusum and T. ferrugineum Fab. Journal of Agricultural Research, 46(4): 327-334.
- Ajayi, F. A. and Rahman, S. A. (2006). Susceptibility of some staple processed meals to red flour beetle, Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae). Pakistan Journal of Biological Sciences, 9(9): 1744-1748.
- Ladisch, R. K., Ladisch, S. K. and Howe, P. M. (1967). Quinoid secretions in grain and flour beetles. In Proceedings of the Pennsylvania Academy of Science (pp. 213-219). Pennsylvania Academy of Science.
- El-Mofty, M. M., Sakr, S. A., Osman, S. I. and Toulan, B. A. (1989). Carcinogenic effect of biscuits made of flour infested with Tribolium castaneum in Bufo regularis. Oncology, 46(1): 63-65.
- Bell, C. H. (2000). Fumigation in the 21st century. Crop protection, 19(8-10): 563-569.
- Anbar, A. D., Yung, Y. L. and Chavez, F. P. (1996). Methyl bromide: Ocean sources, ocean sinks, and climate sensitivity. Global biogeochemical cycles, 10(1): 175-190.
- Benhalima, H., Chaudhry, M. Q., Mills, K. A., & Price, N. R. (2004). Phosphine resistance in stored-product insects collected from various grain storage facilities in Morocco. Journal of Stored Products Research, 40(3): 241-249.
- Okwute, S. K. (2012). Plants as potential sources of pesticidal agents: a review. Pesticides-advances in chemical and botanical pesticides. IntechOpen.
- Chaudhary, S., Kanwar, R. K., Sehgal, A., Cahill, D. M., Barrow, C. J., Sehgal, R. and Kanwar, J. R. (2017). Progress on Azadirachta indica based biopesticides in replacing synthetic toxic pesticides. Frontiers in plant science,8: 6-10.
- Sasidharan, S., Chen, Y., Saravanan, D., Sundram, K. M. and Latha, L. Y. (2011). Extraction, isolation and characterization of bioactive compounds from plants’ extracts. African Journal of Traditional, Complementary and Alternative Medicines, 8(1): 1-10.
- Isman, M. B. (2000). Plant essential oils for pest and disease management. Crop protection, 19(8-10): 603-608.
- Caballero-Gallardo, K., Olivero-Verbel, J. and Stashenko, E. E. (2011). Repellent activity of essential oils and some of their individual constituents against Tribolium castaneum Journal of Agricultural and Food chemistry, 59(5): 1690-1696.
- Abbasipour, H., Mahmoudvand, M., Rastegar, F. and Hosseinpour, M. H. (2011). Bioactivities of jimsonweed extract, Datura stramonium(Solanaceae), against Tribolium castaneum (Coleoptera: Tenebrionidae). Turkish Journal of Agriculture and Forestry, 35(6): 623-629.
- Babarinde, S. A., Oyegoke, O. O. and Adekunle, A. E. (2011). Larvicidal and insecticidal properties of Ricinus communis seed extracts obtained by different methods against Tribolium castaneum Herbst (Coleoptera: Tenebrionidae). Archives of phytopathology and plant protection, 44(5): 451-459.
- Chu, S. S., Liu, Z. L., Du, S. S. and Deng, Z. W. (2012). Chemical composition and insecticidal activity against Sitophilus zeamais of the essential oils derived from Artemisia giraldii and Artemisia subdigitata. Molecules,17(6): 7255-7265.
- Das, S. (2012). Artemisia annua (Qinghao): a pharmacological review. Int J Pharm Sci ReS, 3(12): 4573-4577.
- Bora, K. S. and Sharma, A. (2011). The genus Artemisia: a comprehensive review. Pharmaceutical Biology,49(1): 101-109.
- Janssen, A. M., Scheffer, J. J. C. and Svendsen, A. B. (1987). Antimicrobial activities of essential oils. Pharmaceutisch Weekblad,9(4): 193-197.
- Gonzalez-Coloma, A., Bailen, M., Diaz, C. E., Fraga, B. M., Martínez-Díaz, R., Zuñiga, G. E., … and Burillo, J. (2012). Major components of Spanish cultivated Artemisia absinthium populations: Antifeedant, antiparasitic, and antioxidant effects. Industrial Crops and Products,37(1): 401-407.
- Negahban, M. M. (2007). Fumigant toxicity of essential oil from Artemisia sieberi Besser against three stored-product insects. Journal of stored products research, 43(2): 123-128.
- Liu, C. H., Mishra, A. K., Tan, R. X., Tang, C., Yang, H. and Shen, Y. F. (2006). Repellent and insecticidal activities of essential oils from Artemisia princeps and Cinnamomum camphora and their effect on seed germination of wheat and broad bean. Bioresource technology, 97(15): 1969-1973.
- Dhingra, V., Rao, K. V. and Narasu, M. L. (1999). Current status of artemisinin and its derivatives as antimalarial drugs. Life sciences, 66(4): 279-300.
- Klayman, D. L. (1985). Qinghaosu (artemisinin): an antimalarial drug from China. Science, 228(4703): 1049-1055.
- Eastman, R. T. and Fidock, D. A. (2009). Artemisinin-based combination therapies: a vital tool in efforts to eliminate malaria. Nature Reviews Microbiology, 7(12): 864-874.
- Clevenger, J. F. (1928). Apparatus for the determination of volatile oil. The Journal of the American Pharmaceutical Association (1912),17(4): 345-349.
- Cosimi, S., Rossi, E., Cioni, P. L., & Canale, A. (2009). Bioactivity and qualitative analysis of some essential oils from Mediterranean plants against stored-product pests: Evaluation of repellency against Sitophilus zeamais Motschulsky, Cryptolestes ferrugineus (Stephens) and Tenebrio molitor (L.). Journal of Stored Products Research, 45(2): 125-132.
- Huang, Y., & Ho, S. H. (1998). Toxicity and antifeedant activities of cinnamaldehyde against the grain storage insects, Tribolium castaneum (Herbst) and Sitophilus zeamais Motsch. Journal of Stored Products Research, 34(1), 11-17.
- López, M. D., Jordán, M. J., & Pascual-Villalobos, M. J. (2008). Toxic compounds in essential oils of coriander, caraway and basil active against stored rice pests. Journal of Stored Products Research, 44(3): 273-278.
- Ellman, G. L., Courtney, K. D., Andres Jr, V. and Featherstone, R. M. (1961). A new and rapid colorimetric determination of acetylcholinesterase activity. Biochemical pharmacology, 7(2): 88-95.
- Habig, W. H., Pabst, M. J. and Jakoby, W. B. (1974). Glutathione S-transferases the first enzymatic step in mercapturic acid formation. Journal of biological Chemistry, 249(22): 7130-7139.
- Jollow, D. J., Mitchell, J. R., Potter, W. Z., Davis, D. C., Gillette, J. R. and Brodie, B. B. (1973). Acetaminophen-induced hepatic necrosis. II. Role of covalent binding in vivo. Journal of Pharmacology and Experimental Therapeutics, 187(1): 195-202.
- Buege, J. A. & Aust, S. D. (1978). [30] Microsomal lipid peroxidation. In J. Abelson, Methods in Enzymology (Vol. 52, pp. 302-310). Cambridge, Massachusetts, United States: Academic Press, Elsevier.
- Falowo, A. B., Mukumbo, F. E., & Muchenje, V. (2019). Phytochemical Constituents and Antioxidant Activity of Artemisia Afra and Bidens Pilosa Essential Oil in Ground Pork. Journal of Essential Oil Bearing Plants. 1-11.
- Smith, T. J. (2000). Squalene: potential chemopreventive agent. Expert Opinion on Investigational Drugs, 9(8): 1841-1848.
- Tripathi, A. K., Prajapati, V., Aggarwal, K. K. and Kumar, S. (2001). Toxicity, feeding deterrence, and effect of activity of 1, 8-cineole from Artemisia annua on progeny production of Tribolium castanaeum (Coleoptera: Tenebrionidae). Journal of Economic Entomology, 94(4): 979-983.
- Martínez, M. J. A., Del Olmo, L. M. B., Ticona, L. A., & Benito, P. B. (2012). The Artemisia L. genus: a review of bioactive sesquiterpene lactones. In Studies in natural products chemistry(Vol. 37, pp. 43-65). Elsevier.
- Brown, G. D. (2010). The biosynthesis of artemisinin (Qinghaosu) and the phytochemistry of Artemisia annua L.(Qinghao). Molecules, 15(11), 7603-7698.
- Xiaorong, T., & Taiping, H. (2008). Separation and identification of botanical insecticide 7-hydroxycoumarin and its biological activity against Aphis craccivora and Culex pipiens pallens. Natural Product Research, 22(4), 365-370.
- Chauhan, N., Kumar, P., Mishra, S., Verma, S., Malik, A. and Sharma, S. (2015). Insecticidal activity of Jatropha curcas extracts against housefly, Musca domestica. Environmental Science and Pollution Research,22(19): 14793-14800.
- Li, Y. (2012). Qinghaosu (artemisinin): chemistry and pharmacology. Acta Pharmacologica Sinica,33(9): 1141.
- Ni, L., Acharya, K., Hao, X. and Li, S. (2012). Isolation and identification of an anti-algal compound from Artemisia annua and mechanisms of inhibitory effect on algae. Chemosphere,88(9): 1051-1057.
- Smirle, M. J., Lowery, D. T. and Zurowski, C. L. (1996). Influence of neem oil on detoxication enzyme activity in the obliquebanded leafroller, Choristoneura rosaceana. Pesticide Biochemistry and Physiology,56(3): 220-230.
- Huang, Z., Shi, P., Dai, J. and Du, J. (2004). Protein metabolism in Spodoptera litura (F.) is influenced by the botanical insecticide azadirachtin. Pesticide Biochemistry and Physiology,80(2): 85-93.
- Macedo, M. L. R., Freire, M. D. G. M., da Silva, M. B. R. and Coelho, L. C. B. B. (2007). Insecticidal action of Bauhinia monandra leaf lectin (BmoLL) against Anagasta kuehniella (Lepidoptera: Pyralidae), Zabrotes subfasciatus and Callosobruchus maculatus (Coleoptera: Bruchidae). Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology,146(4): 486-498.
- Koodalingam, A., Mullainadhan, P. and Arumugam, M. (2011). Effects of extract of soapnut Sapindus emarginatus on esterases and phosphatases of the vector mosquito, Aedes aegypti (Diptera: Culicidae). Acta tropica, 118(1): 27-36.
- Grant, D. F. and Matsumura, F. (1989). Glutathione S-transferase 1 and 2 in susceptible and insecticide resistant Aedes aegypti. Pesticide Biochemistry and Physiology. 33(2): 132-143.
- Yu, S. J. (1982). Host plant induction of glutathione S-transferase in the fall armyworm. Pesticide Biochemistry and Physiology,18(1): 101-106.
- Vanhaelen, N., Haubruge, E., Lognay, G. and Francis, F. (2001). Hoverfly glutathione S-transferases and effect of Brassicaceae secondary metabolites. Pesticide Biochemistry and Physiology,71(3): 170-177.
- Zibaee, A. and Bandani, A. (2010). A study on the toxicity of a medicinal plant, Artemisia annua(Asteracea) extracts to the sunn pest, Eurygaster integriceps Puton (Hemiptera: Scutelleridae). Journal of Plant Protection Research,50(1): 79-85.
- Vontas, J. G., Graham, J. and Hemingway, J. (2001). Glutathione S-transferases as antioxidant defence agents confer pyrethroid resistance in Nilaparvata lugens. Biochemical Journal,357(1): 65-72.
- Hasspieler, B. M., Arnason, J. T. and Downe, A. E. (1990). Modes of action of the plant-derived phototoxin α-terthienyl in mosquito larvae. Pesticide Biochemistry and Physiology, 38(1): 41-47.

This work is licensed under a Creative Commons Attribution 4.0 International License.






