Manuscript accepted on : 12-June-2019
Published online on: 28-06-2019
Plagiarism Check: Yes
Reviewed by: Revathi Shenoy
Second Review by: Muzaffar Hassan
Agro-Industrial Waste: A Potential Feedstock for Pullulan Production
Daniel Joe Dailin*1,2, Luo Zaini Mohd Izwan Low1,2, Kugan Kumar1,2, Roslinda Abd Malek1, Khairun Hani Natasya1, Ho Chin Keat1, Dalia Sukmawati3 and Hesham El-Enshasy1,2,4
1Institute of Bioproduct Development, Universiti Teknologi Malaysia, 81310, Skudai, Johor, Malaysia.
2School of Chemical and Energy Engineering, Universiti Teknologi Malaysia, 81310, Skudai, Johor, Malaysia.
3Department of Biology, Faculty of Mathematics and Natural Sciences, Universitas Negeri Jakarta, Kampus B, Pemuda Street No. 10 Rawamangun, Indonesia.
4Bioprocess Development Department, City for Scientific Research and Technology Applications (CSAT), New Burg Al Arab, Alexandria, Egypt.
Corresponding Author E-mail: jddaniel@utm.my
DOI : http://dx.doi.org/10.13005/bbra/2740
ABSTRACT: Nowadays, the growing interest of using of biopolymer to replace petroleum based material as are increasing tremendously. Microbial biopolymers are usually water-soluble gum which have innovative and unique physical characteristics. Pullulan is a biodegradable and water soluble exopolysaccharide synthesized by the yeast-like fungus Aureobasidium pullulans. This polymorphic fungus is well known as producer of the polysaccharide, pullulan and other by-products such as oil, organic acids, pigment, and others. Pullulan has extensive applications in pharmaceutical, cosmetic, biomedical, and food industries because of its advantageous chemical and physical properties. Pullulan’s structure is co-existence of α-(1, 4) and α-(1, 6) linkages which is nontoxic, tasteless and non-mutagenic. Some of its excellent properties are low viscosity, non-toxicity, slow digestibility, high plasticity, and excellent film-forming capabilities. Although pullulan shows great potential in several industries, its high production cost is a major drawback. Therefore, cheaper and accessible substrate which can minimize the production cost is needed. This review highlights the potential use of agro-industrial waste as an alternative source feedstock for pullulan production and its biosynthesis, chemical structure, production process and applications.
KEYWORDS: Agriculture Waste; Aureobasidium Pullulans; Biopolymer; Bioprocess; Pullulan
Download this article as:| Copy the following to cite this article: Dailin D. J, Low L. Z. M. I, Kumar K, Malek R. A, Natasya K. H, Keat H. C, Sukmawati D, El-Enshasy H. Agro-Industrial Waste: A Potential Feedstock for Pullulan Production. Biosci Biotech Res Asia 2019;16(2). |
| Copy the following to cite this URL: Dailin D. J, Low L. Z. M. I, Kumar K, Malek R. A, Natasya K. H, Keat H. C, Sukmawati D, El-Enshasy H. Agro-Industrial Waste: A Potential Feedstock for Pullulan Production. Biosci Biotech Res Asia 2019;16(2). Available from: https://bit.ly/32frFlM |
Introduction
Since the beginning of the twentieth century, the function of microbe and microbial biotechnology in enhancing the quality of human life in each perspective has been perceived around the world. Biotechnology knowledge related to microbial bioprocessing for the production of bioproducts like biopolymers, enzymes, antibiotics and high cell mass have matured tremendously. Biopolymers are the most abundant molecules in living matter. Microorganism is proficient of producing a wide variety of biopolymers, including carbohydrates, polynucleotides, polyamides, polyesters, glycoproteins, polysaccharides and many others. Pullulan is an alternative type of valuable biopolymer as they can substitute the ordinary polysaccharides produced by plants. It has been reported that the retail price of pullulan is around $25 per kilogram (Ma et al., 2012). Pullulan has been chosen as the biotechnology product manufactured since 1976 by the Hayashibara Company Ltd 9 (Okayama, Japan), which remains the leader supplier (Singh et al., 2008). Pullulan produced by non-pathogenic polymorphic and oligotrophic yeast-like fungus, namely Aureobasidium pullulans. A. pullulans is a species of the class Ascomycetous yeast, which belongs to the family Dothideaceae of the order Dothideales. A. pullulans is a black yeast, which can be found mainly on leaves and other environment surface like concrete, limestone wood, soil, forest barks, fresh and sea water, plant and animal tissues (Shingel, 2004). It was first isolated and observed by Bernier (1958) and found to play as a valuable product in biotechnology business. Bender at the year 1959 discovered the unique glucan and proposed it “pullulan”. At the year of 1960, the basic empirical structure of pullulan was established. Pullulan is broadly used as biomaterial applied in the food and medical sector because of its characteristics such as structure flexibility, low viscosity, nontoxicity, slow digestibility, high plasticity, and biofilm. Furthermore, pullulan is also edible and biodegradable in the environment. This review will explore the potential of feedstock from agro-industrial waste. Several low-cost feedstock that potentially can be used for supporting pullulan production includes potato starch waste, olive oil wastes, carob pod, corn steep liquor, coconut by-products, jaggery and rice hull hydrolysate. Bioprocess including basic medium optimization, cultivation in shake flask level up to batch bioreactor were discussed. The metabolic pathways of pullulan synthesis and applications of pullulan are discussed extensively.
Pullulan Specifications: Chemical Structure, Molecular, and Physical Properties
The chemical formula of the natural biopolymer secreted from A. pullulans has been investigated by many authors and well established (Sugumaran and Ponnusami, 2017; Kumar et al., 2012; Singh et al., 2008; Rekha and Sharma, 2007; Shingel, 2004; Jakovljevic et al., 2001). Pullulan is a neutral polymer consist of repeating glucose units with α-1,6 and α-1,4 glyosidic bonds and no branching (Figure 1). The linear pullulan chemical structure may also contain maltotetrose subunits. Basic linkages in extracellular polymer and its enzymatic hydrolysis sites is shown in Figure 2. Pullulan contain both hydrophobic and hydrophilic features which are appropriate for its unique structure. The chemical formula derived from IR spectroscopic of pullulan is (C6H10O5) with molecular weight reaching 45-600 kDa and optical rotation of +192 in a 1 g/dL solution (Shingel, 2004) (Table 1). The final purified of polysaccharide has a molecular weight of ca. 250 kDa. The structure consists of hydroxyl groups and the well-ordered alternation of α (1-4) and α (1-6) bonds on pullulan chains provides the polymer with characteristic physiological activity, structural flexibility and increasing solubility established (Sugumaran and Ponnusami, 2017; Ma et al., 2012; Kumar et al., 2012; Singh et al., 2008; Rekha nad Sharma, 2007; Shingel, 2004; Jakovljevic et al., 2001).
Pullulanase (EC 3.2.1.41, pullulan 6-glucanohydrolase), is well known as debranching enzyme is able to hydrolysis the α-1,6- glucosidic bond in pullulan structures and convert it to amylaceous polysaccharides. The pullulan also undergoes enzymatic hydrolysis by both α-1,6 and α-1,4 glycosidic bonds D pullulanases. The pullulanase enzyme, acting to cleave the (1-6) α-D-glucopyranoside linkages. From this actions, it can contribute perfect hydrolysis process of pullulan using (1-6)-α-D pullulanase yields maltotriose as an utmost outcome along with traces of maltotetraose. Furthermore, the (1-4)-α-D-pullulanases act on (1-4)-α-D-glucosidic linkages at their reducing ends adjacent to (1-6)- α-D linkages. Complete hydrolysis of pullulan with (1-4)-α-D-pullulanase present with isopanose as the primary product. Products of enzymatic pullulan degradation are usefulness in food and pharmaceutical industry (Oğuzhan and Yangılar 2013).
Pullulan is white colored, odorless and tasteless powder currently exploited in the food industry due to its variety of unique properties. Its natures are nontoxic, nonimmunogenic, non-mutagenic, non-hygroscopic in nature and non–carcinogenic. Pullulan is also highly soluble in water and insoluble in organic solvents. For the molecular weight, pullulan molar mass was stated in the range of 58-9000 kDa. Pullulan can be converted to other components or derivatives or chemically modified by using several steps such as esterification, carboxymethylation and sulfation (Prasongsuk et al., 2018; Sugumaran and Ponnusami, 2017; Kumar et al., 2012; Ma et al., 2012; Singh et al., 2008; Rekha nad Sharma, 2007; Shingel, 2004; Jakovljevic et al., 2001). The great potential of pullulan ion vast variety of areas and applications ensures its bright future in microbial biotechnology. The aqueous solutions of pullulan are stable and its viscosity is relatively low compared to other polysaccharides. Pullulan can withstand and decompose at 250-280°C (Singh et al., 2008). The main quality parameters of pullulan are shown in Table 1.
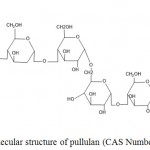 |
Figure 1: Molecular structure of pullulan (CAS Number, 9057-02-7).
|
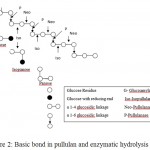 |
Figure 2: Basic bond in pullulan and enzymatic hydrolysis site.
|
Table 1: Pullulan molecular information.
| Name | IUPAC name | Pullulan |
| Other names | E1204 | |
| Identifiers | CAS Number | 9057-02-7 |
| EINECS Number | 232-945-1 | |
| ChemSpider | none | |
| E number | E1204 | |
| ECHA InfoCard
UNII |
100.029.938
8ZQ0AYU1TT |
|
| Properties | Molecular formula | (C6H10O5)n |
Table 2: Quality parameters of pullulan (Ma et al., 2013; Singh et al., 2008).
| Parameter | Specification |
| Appearance (external) | A white or yellowish-white powder |
| The degree of water of solubility (25C) | Soluble very well |
| Specific optical activity [α] D2O (1% in water) | Min. +160 ̊ |
| Polypeptidies (%) | Max. 0.5 |
| pH (solution) | Within 5-7 scale |
| Mineral residue-ash (sulfated, %) | Max. 3 |
| Moisture level (loss of drying, %) | Max. 6 |
| Molecular weight (kDa) | Range between 100-250 |
Table 3: Characteristics of microbial Pullulan (Prasongsuk et al., 2018; Sugumaran and Ponnusami, 2017; Kumar et al., 2012; Shingel, 2004; Rekha and Sharma, 2007; Jakovljevic et al., 2001; Singh et al., 2008; Ma et al., 2013).
| Characteristics of microbial pullulan | |
| Non-toxic | Non -carcinogenic |
| Non-mutagenic | Odourless |
| Tasteless | Edible |
| Biodegradable | Transparent/ Impermeable to oxygen |
| Low viscosity | Non-hygroscopic |
| Insoluble in organic solvents | Oil resistant |
| Water soluble | Non-reducing |
| High adhesion and film forming abilities | Film; thermostable, anti-static, elastic |
| Non-ionic polysaccharide | Blood compatible |
| Non-immunogenic | Dilute alkali |
| Insoluble in alcohol | Edible |
| Low viscosity | White to off-white Colour |
Mechanism of Pullulan Biosynthesis
Exopolysaccharides produced serves as an outer protection for the producer containing high water content, to ensure greater resistance against desiccation and predation (Kumar et al., 2017). A. pullulans is known as the major producer of pullulan and aubasidan-like components (Sheng et al., 2015). This fungus disperses due to the production of yeast-like propagules and found globally but reported in the intense cold environment, as investigations on fungal diversity are limited to frozen Antarctic soils and Siberian permafrost where basidiomycetous yeasts were found (Gaur et al., 2010). It has unique metabolic features and the cellular morphologies characteristics of A. pullulan are more luxuriant.
Pullulan biosynthesis is accomplished through mediation of sugar-nucleotide-lipid carrier intermediates associated with the cell membrane fraction. It is synthesized extracellular at the cell of the membrane wall and secreted out to the cell surface to form amorphous solid which consists of maltotriose and maltotetraose with bond α-(136) and α-(134) linkages. For instance, the regular alternation of α-1,4 and α-1,6 bonds results in two distinctive properties, structural flexibility and enhanced solubility (Moubasher et al., 2014).
There are 3 main stages of the precursor of the pullulan molecule. The first stage is formation of Lph-Glu, through the intermediary uridine-diphosphate-glucose (UPDG) which is catalysed by ATP. Next stage is transfer an additional D-glucose produced by UPDG to form isomaltose molecule (Lph-Glu-(1-6)-Glu). Lastly, in the final stages, isomaltose will interact with the glycosyl lipid precursor from stage one to produce molecule of isopanosyl (Lph-Glu-(1-6)-Glu-(1-4)-Glu). The isopanosyl molecules will polymerised into a pullulan chain (Donot et al., 2012). The biosynthesis of pullulan is mainly performed by key enzymes such as uridine diphosphate glucose pyrophosphorylase (UDPG-pyrophosphorylase), α-phosphoglucose mutase and glucosyltransferase.
A. pullulans is able to consume various carbon sources such as mannose, sucrose, maltose, fructose, galactose, xylose and even the agro-industrial waste. The presence of isomerase and hexokinase are necessary for carbon source to be converted to UDPG which is an important precursor to synthesis pullulan (Sugumaran et al., 2017). UDPG is important in medium for pullulan production where A. pullulans incorporates 14C-labeled glucose into lipid-linked glucose, isomaltose, panose and isopanose that participate in reaction with lipid-linked glucose (Leathers et al., 2003). In addition, they proposed a reaction mechanism in which pullulan is formed by the polymerization of isopanosyl into the pullulan chain using glucosyl tranferase enzyme. However, there are limited studies about the exact mechanism of pullulan synthesis by A. pullulans which has not been understood due to the complex physiological and cytological characteristics of the microorganism (Cheng et al., 2011). The proposed pathway of pullulan synthesis is summarized in Figure 3.
 |
Figure 3: Biosynthesis of pullulan (1,α-phosphoglucose mutase; 2, UDPG-pyrophosphorylase; 3, glucosyltransferase) Cheng et al. (2011).
|
Pullulan can be synthesized from sucrose with enzymes from A. pullulans when both ATP and UPDG participate in reaction mixture. ADPG cannot be replaced by the UDPG because the pullulan precursor is originated from UPDG. Unfortunately, the formation pathway still remains unclear. It is only known that, maltose containing media, the carbohydrate metabolites needed for the polymer formation, which are panose [α-Glc-(1fi6)-α-Glc-(1fi4)-α-Glc] and or isomaltose [α-Glc-(1fi6)-α-Glc] can be synthesized via a glucosyl transfer reaction in A. pullulans.
Cheng et al. (2011) explained that A. pullulans does not convert glucose directly into polysaccharide instead it involved in polymerization of carbohydrate precursors stored inside the cells. The cells will accumulate sugars and consume the carbohydrate for their last stage of life cycle in pullulan production. The hypothesis was proved that an inverse correlation between the concentration of pullulan and the content of intracellular glycogen.
Besides A. pullulans, the newly isolated pullulan producing fungus Eurotium chevalieri has also been reported to produce non-melanin pullulan which demonstrate the higher yield of pullulan production (Gaur et al., 2010). Hydrolytic products of the polysaccharide produced by E. chevalieri using pullulanase specifically hydrolyses the α-1,6 linkage of the linear α-d-glucan, releasing maltotriose with the reducing end from pullulan to determine the purity of the polysaccharide produced. Forabosco et al. (2006) stated that Cryphonectria parasitica which is fungal virulence of chesnut cranker also produced pullulan where all cases pullulan was much richer in a-(1→6) maltotetraose subunits than the pullulan(s). The maximum amount of a-(1→6) maltotetraose subunits was believed to be 7%.
As studied by Olivia et al. (1986), they mentioned that pullulan is also produced by Cyttaria darwinii which is a fungal species that form a tumour that infect the tree. The pullulan structure of Cyttaria darwinii was confirmed by the pulullanase treatment. Chi and Zhao (2003) have found new pullulan-producing yeast strain Rhodotorula baracum that was collected from Chinese plant leaves from South China which produced large amount of pullulan and did not produce melanin pigment. Pullulanase hydrolyzes β-1,3-glucan was carried out. The result was confirmed that polysaccharide produced by R. bacarum is pullulan. Apart from A. pullulans, some other microbial strains are also reported as pullulan producer such as Eurotium chevalieri (Gaur et al., 2010), Cryphonectria parasitica (Forabosco et al., 2006) Rhodotorula baracum (Chi and Zhao, 2003) and Cytaria darwinii (Olivia et al., 1986).
Production Process
In production process, there are many factors that affect the production of pullulan. Some of the factors that contributed towards the efficiency of pullulan production in the industry are the medium components, processing parameters and other factors such as labour skills, bioreactor type and design used. Some of the factors that may influencing pullulan production are summarize in Figure 4.
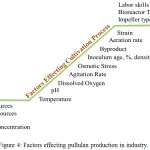 |
Figure 4: Factors effecting pullulan production in industry.
|
Carbon Source
Carbon sources is an important nutrient in living cells under the category as macronutrient as they are needed mostly for energy. Quite numbers of previous studies reported that sucrose was the best carbon source supporting for high pullulan production. Jiang et al. (2018) reported that the production of pullulan obtained by Aureobasidium melanogenum TN1-2 strain isolated from natural honey was 97 g/L when sucrose was used as carbon source in the cultivation medium. Earlier, Özcanaa et al. (2014) reported that maximal pullulan production of 38.77 g/L achieved using 95.2 g/L of sucrose concentration. Another study conducted by Sheng et al. (2016) using various types of carbon sources for pullulan production such as sucrose, maltose, glucose, fructose, mannose, galactose xylose and soluble starch. They found that highest pullulan yield obtained when cells were cultivated in medium containing sucrose. Ma et al., (2014) reported that maximal pullulan production of 65.3 g/L obtained when 120 g/L of sucrose was used in the cultivation medium. Nevertheless, there are also other studies reported that other carbon sources besides sucrose is best when used for pullulan production. Chen et al., (2017) reported that 19.8 g/L of pullulan obtained and highest compared to other carbon sources tested such as sucrose, fructose and maltose using mutant A. pullulans. By using fructose as carbon source, 50.1 g/L of pullulan obtained for A. pullulans NCPS2016 and was the highest among other carbon sources tested that were glucose, sucrose, maltose, xylose and soluble starch. (Yang et al., 2018). This is probably due to the capability of strain used for pullulan production were different as well as the cultivation conditions.
pH of Cultivation
The pH of cultivation medium is highly influencing not only to the pullulan production but also to the morphology and cultivation time of A. pullulans. It was reported that the pullulan production increasing as the pH increase from 2.5 to 5.5 and decreased after that (Ponnusami et al., 2014). This result was similar with the result obtained by Singh et al. (2012) in which they found that pH 5.5 was optimal for pullulan production. Chen et al., (2017) reported that the optimal pH for pullulan production was pH 4 using mutant A. pullulans. Study conducted by Sheoran et al. (2012) showed that optimal pH is pH 5.9 and that pH ranging from 5.3 to 6.2 did not gave significant effect on pullulan production. Singh et al. (2018) reported that pH 5 was best in producing pullulan. Optimal pH values for pullulan production are varied. This is probably due to the variety of strains and cultivation condition used.
Temperature
Cultivation temperature is one of the most crucial factors influencing the production of pullulan. It was reported by Singh et al. (2018) that the optimal temperature for high pullulan production was 37°C. Another interesting study conducted by Singh et al. (2012) where they have isolated a pullulan producer strain that is thermotolerant and non-melanin producer that can be grown and produced pullulan at temperature up to 42°C. Nevertheless, it was reported that the pullulan production was favoured in lower temperature and as low as 25°C (Hilares et al., 2019) and 26°C (Xia et al., 2011). This variation could be mostly due to the capability and origin of the strain being isolated.
Fermentation Time
Pullulan production is directly related to the fermentation time. It was reported by previous researches that the fermentation time takes to produce the maximum yield of pullulan is different to one another. This is probably due to different cultivation conditions used for each experiment reported for pullulan production. Ponnusami and Sugumaran (2014) reported that the maximum yield of pullulan obtained was maximum on day 4. Another study by Göksungur et al. (2014) reported that the pullulan production was maximum when cultivated for 5.36 days in an air lift bioreactor. Lin and Thibault (2013) reported that highest pullulan concentration of 23.3 g/L produced at 78 hours of cultivation time. In other study, it was reported that the maximal production of pullulan can be achieved within 48 hours of cultivation time (Singh et al., 2012). Therefore, in order to get high pullulan production, the fermentation time can be in the range of 48 to 120 hours depending muchly on the strains and cultivation conditions.
Agro-Industrial Waste as Feedstock
In a recent review, it is estimated the cost of the raw materials for pullulan production is three times higher than other polysaccharides and 30% of the total production cost comes from the raw material (Mishra et al., 2017). There are many approaches have been taken to reduce the cost for pullulan production which include using genetically modified strains, engineering innovations but the best solution so far is by identifying cheaper and effective carbon source. Agro-industrial waste which is nutritionally rich enough to support the growth of the microorganisms as well as the production of pullulan can be used as an alternative approach in reducing the production cost as they are abundant available to be used.
Pullulan can be produced using different type of substrates incorporated into either the defined (synthetic) or non-synthetic media. Using agro-industrial waste as substrate, it can be sound advantages for both ecological and economical. This is because it can lower down the negative costs when synthetic chemicals are being used as the sole substrate. Since the usage of agro-industrial waste as feedstock can reduce the environmental pollution, it is desired to find the suitable material that can be used as substrate. The pollution problem which is associated with the accumulation of agro-waste and by-products increased the usage of bioconversion of the plant biomass to value-added compounds economically.
Starch Waste
Potato is a cheap and easily available agriculture product. Potato mainly constitute of starch and little amount of sugar. Normally the wastes of potato starch from the manufactures of the potato crisp or other potato processing industries are in the form of homogenous substrate which normally free from extraneous materials. Starch from potato has been used as an alternative carbon source for various industrial fermentations. Certain A. pullulans strains possess the starch degrading enzymes but this activity was greater against linear α-1,4-glucans, but very little if against any polysaccharides with α-1,6-linkages. Therefore, in order for the starch waste to be considered as a good substrate for the production of pullulan, it must be hydrolysed partially. Normally, the starch should be hydrolysed to become sugar right before the fermentation process starts. But, there is no need of adding the expensive β-amylase when the potato is being used as a carbon source. This is mainly because potato contains considerable amount of highly active β-amylase. Besides that, the production of pullulan is largely dependent on the degree of the hydrolysis of the starch or dextrose equivalent. This indicates that the total amount of reducing sugar as a percentage of glucose. Some of the unhydrolyzed starch has the dextrose equivalent of 0 and glucose has 100 of dextrose equivalent. By using the hydrolysed starch waste as the substrate, the pullulan content of the agglutinating substances increased during the course of the fermentation process and reached more than 90% (w/w) on day six.
Olive oil Waste
Olive oil fruit contains large amount of bioactive compounds and substances which is highly interest. This olive oil is known for its health properties which help to contribute to form a protective effect towards human body. During the olive oil processing, most of them remain as olive oil wastes. Although the olive oil processing generated by two phase extraction process, it represents the major disposal and potentially can be the best solution for most of the pollution problem. The waste from the olive oil is basically the effluent caused by the mills that produce by the olive oil. It is considered to be one of the major pollutants and can cause huge problems in olive tree cultivation areas mainly in Mediterranean countries. The fresh waste from this olive oil can be phytotoxic due to the presence of phenolic compounds and for the time being there is no ecological or economic solution to overcome this problem. Although there are many microorganisms which cannot grown with the presence of the olive oil waste which is mainly due to the presence of toxic phenol compounds, A. pullulans has the ability to grow and produce pullulan. According to the experiment conducted by Israilides et al., 1993, the A. pullulans grown well regardless of the presence of the olive oil waste but most importantly it reduced the phenolic compound content up to 41% during the sixth day of observation. The concentration of the pullulan that agglutinate ethanol substance increased when the phenols content was completely removed which indicates the absence of the phenols plays pivotal inhibitory role during the pullulan production (Israillides et al., 1999).
Carob pod
The carob pod is a type of fruit from the carob tree (Ceratonia siliqua). This tree mainly can be found at the Mediterranean regions and at some semiarid regions of North America. The carob pods contain special polyphenolics compounds, carbohydrates and also contain low level of insoluble dietary fibres, minerals and lipids and proteins. It consists of high amount of soluble sugars which is around 40% to 60% that enables it to be used as good substrates. When it comes to the ripen seeded carob cod, it contains high level of tannins which makes it to be used partially for the production of health confections. It is also mainly being used for animal feeding besides the fact that the presence of the tannin decreases the nutritional value of the pod. Somehow due to the difficulties and the high cost for the harvesting purposes, most of the carob pods are left unutilised. Due to the compositions of the carob pods, it is highly used as an animal feed (Mishra et al., 2018). According to Rouks and Billaderis (1994), the yield of pullulan increased up to 89% when carob cod was used as the substrate during the fermentation process.
Corn Steep Liquor
Corn steep liquor (CSL) is the by-product from the corn wet milling process and also from other commercial corn processes. This waste which comes from the corn milling processing plant considered being non-synthetic and it has been used as non-synthetic input for most of the liquid fertilizer formulations for any organic crop production. It normally comprises of 10 g fiber/kg dry matter, 130-220 g carbohydrate/kg dry matter, 205 g crude protein /kg dry matter, 525 g/kg dry matter, 88 g ash/kg dry matter and a small amount of sulfurous acid (<0.01 g/kg DM) (Chiani et al., 2010). CSL also contains 42% of protein (Mishra et al., 2018).
According to the experiment done by Sharma et al. (2013), when five different types of agricultural wastes which are rice bran oil cake, soya bean oil, cotton seed oil cake, mustard seed oil cake and corn steep liquor used as substrate for pullulan production, the corn steep liquor gave the highest pullulan production up to 77.92 g/L. This fermentation procedure also validated in 7-L fermenter where the economics of the process was analysed and it was found that, CSL can reduce the cost of raw material up to three times compared to the conventional process. This finding can be used for the development of cost-effective pullulan production.
Coconut by-Product
Coconut water contains naturally occurring lipid which can be found inside the coconut. The coconut milk which tastes sweet can be derived from the meat of the mature coconut. The coconut water particularly considered as a waste product especially from the factories that produce copra desiccated coconut and other meat coconut product. Moreover, the coconut water can be an active pollutant due to its high biological oxygen demand. Increasing pollution problem also increased the interest on coconut water and motivated for its utilization for industrially important biopolymer production. Coconut water contains easily digestible carbohydrate which normally can form simple sugars and electrolytes. Due to the high demand for biological oxygen demand of the by-product from coconut, this agro waste has been used as a substrate for efficient pullulan production (Mishra et al., 2018). Several researches has been done on utilizing this by-product as a substrate in attempt to reduce its waste in the environment. One of it is, according to the study done by Thirumavalavan et al., (2009), both the coconut water and coconut milk can be used as a good substrate for the production of pullulan. However, the coconut milk tends to be more efficient when it comes to pullulan production comparing with coconut water. According to Thirumavalavan et al., (2009), this mainly due to the higher amount of carbon and nitrogen ratio in coconut milk than in coconut water.
According to the results obtained by Thirumavalavan et al., (2009), coconut water contains around 40 g/L of reducing sugar while the tender coconut water contains 22 g/L of reducing sugar. Coconut milk contains around 48 g/L of reducing sugar. The highest production of pullulan which is around 54 g/L was obtained from coconut milk during the fermentation period of 144 hours. This is mainly because the coconut milk and coconut water are rich with mineral source and amino acids. Besides that, both of the by-product does not require any additional pre-treatment methods like other substrates to enhance the pullulan production.
Jaggery
Jaggery can be defined as natural and traditional sweetener which can be made from concentrated sugarcane juice and it is known in different local names according to the country all over the world. It is the traditional unrefined and non-centrifugal sugar that being consumed in regions likes Asia, Latin America and Africa. It contains all the basic minerals and vitamins which also present in sugarcane juice which make it to be one of the healthiest sugar in the world (Singh et al., 2018). Jaggery is largely produced in India which is around 70% of total. Basically, jaggery is prepared by concentrating the sugarcane juice and there are three types of jaggery which are solid jaggery (cube shape), liquid jaggery and granular or powder. Moreover, the sap that being collected from some of the palm trees like coconut palm, wild date palm and sago palm is being used for the preparation of jaggery (Nath et al., 2015).
Since jaggery contains different sugar and minerals like sucrose, glucose, sucrose which is about 75% to 85%, potassium and calcium, it can be used as important components for the growth media for A. pullulans. According to the experiment done by Ganduri et al., 2016, it was revealed that jaggery can be a good carbon source due to high composition of sucrose which can be utilized by A. pullulans for pullulan production. This can be a good strategy to deliver cost effective pullulan production. Likewise, it was reported that the pullulan yield was highly dependent on the concentration of the jaggery used (Vijayendra et al., 2001). Therefore, the pullulan yield was examined by adding different concentration of jaggery which were at 50%, 75% and 100% respectively and 50% concentration showed the highest pullulan production which is up to 6 g/L.
Rice hull hydrolysate
Rice hull is one of the most widely used and available agricultural by product in many rice producing countries like Thailand and China. 1000 kg of paddy grain can produce about 200 kg (20%) of rice hulls (Hossain et al., 2018). Only some small part of the rice hull used for variety of purposes like building materials, fertilizers, fuel, insulation material and more importantly most of them are being thrown away as wastes (Ma et al., 2011). Owing the presence of lignocellulosic material as the main component, the rice hull is highly used for the fermentable sugar production in recent time where the rice hull also been categorised as desirable candidate to be used as carbon source for production of bioethanol and other bio-based materials. Although the rice hull hydrolysate contains little amount of silicon and ashes, it consists significant amount of glycans that can be decomposed into fermentable sugars by acids (Rahman et al., 2011).
For the best conversion of rice hull into the fermentable sugars, the important part is the physiochemical pre-treatment of the biomass. Normally, the diluted sulphuric acid hydrolysis method was used to recover the sugars from the lignocellulosic material under high efficiency. But during this process, most of the inhibitory compounds for example furfurals and acetic acid will be generated along with the released of fermentable sugars (Hickert et al., 2013). According to research done by Wang et al. (2014), the presence of acetic acid during hydrolysis process exert some negative effect on pullulan production. This indicates that acetic acid might have function as an inhibitor for the pullulan production. This is somehow lowers down the yield of pullulan due to the high level of acetic acid in the rice hull hydrolysate. To overcome the negative effects of the acetic acid, two of these methods which is detoxification of the hydrolysate or adaptive evolution of the microorganisms can be applied. Table 4 shows the list of agro-industrial by-products for potential used as feedstock in pullulan production.
Table 4: List of agro-industrial by-products for potential used as feedstock in pullulan production.
| Agro-industry by-products | Strain | Pullulan yield (g/L) | References |
| Sugarcane bagasse
Starch waste |
A. pullulans LB83
A. pullulans P56 |
25.19
79.40 |
Hilares et al., 2019
Barnett et al., 1998 |
| Olive oil wastes | A. pullulans RBF 4A3 | 73.98 | Israillides et al., 1999 |
| Carob pod | A. pullulans SU No. M18 | 68.20 | Mishra et al., 2018 |
| Corn Steep Liquor | A. pullulans RBF 4A3 | 77.92 | Mishra et al., 2018 |
| Coconut byproduct | A. pullulans MTCC2195 | 38.30 (Coconut water); 58.00 (Coconut Milk) | Thirumavalavan et al., 2009 |
| Jaggery | A. pullulans CFR-77 | 50.00 | Vijayendra et al., 2001 |
| Rice Hull Hydrolysate | A. pullulans CCTCC M2012259 | 22.20 | Wang et al., 2014 |
Pullulan Applications
Polysaccharides are ubiquitous in nature. Apart from cellulose, which is the plentiful biomass material on earth, there are other natural polysaccharides such as pullulan. The biologically important natural polysaccharides can be used for developing functional bio-based polymer (Danjo et al., 2017). Currently, pullulan is used in film and food industry extensively (Rekha and Sharma, 2007). It is a molecule which is tasteless and digested slowly in human. Hence, resulted in rising of blood glucose level slowly (Wolf et al., 2003). Nevertheless, recently pullulan is being focused in pharmaceutical applications such as targeted drug or gene delivery, nanoparticles for drug or gene delivery, cancer therapy, medical imaging, molecular chaperone plasma expander and tissue engineering (Rekha and Sharma, 2007).
Food Industry
Over the past decades, pullulan films and coatings have received gigantic attention in the food industry (Farris et al., 2014). Extending shelf life, minimizing foodborne illness, improving postharvest quality is very pivotal in industry (Trinetta and Cutter, 2016). Hence, the development and utilization of pullulan film and coating are used to enhance the quality and strengthen the shelf life of agricultural products (Shao et al., 2018).
Edible films or coatings can be defined as the thin layers that applied and isolate the food products. Thus, the fruits or vegetable can be protected from chemical, physical and microbiological activity (Falguera et al., 2011). Wu et al. (2016) stated that Laminaria japonica-derived oligosaccharides (LJOs) incorporated pullulan coatings were found to diminish respiratory intensity, weight and vitamin C loss. In another study, an edible coating based on chitosan and pullulan were found to increase the quality and strengthen the shelf lifetime of fresh pineapple (Treviño-Garza et al., 2017). A similar finding was found in Kraśniewska et al (2017), pullulan was found to delay deterioration and prevent the drying and wilting of the fruits especially in high temperature conditions. In addition, it was found to inhibit microbes. Pullulan coating containing oregano essential oil were found to inhibit the yeast and mold and populations of Aspergillus niger (Kraśniewska et al., 2016).
Pullulan has been applied in protection systems for omega 3 oils and development of inulin-based encapsulation technology. In order to prolong the oxidative stability and shelf life of the microencapsulated fish oils, whey protein isolate and pullulan were used as emulsifier and stabilizers respectively to prepare tuna oil microcapsules (Bakry et al., 2016). Besides, pullulan was a thickener that can use to form semipermeable films. Pullulan coating based incorporated with antibacterial agents which consists of 1% pullulan, 0.8% glutathione + 1% chitooligosaccharides, and 0.8% glutathione + 1% chitooligosaccharides + 1% pullulan on apple was used to examine during cold storage. It was found to be effective to prolong the shelf life of apple as delaying the browning, inhibiting the microbial growth and maintaining the firmness (Wu and Chen, 2013). It can be utilized in various ways as thickeners in beverage or sauces. It also stabilizes emulsions. This property created smooth and viscous texture. Moreover, the consistency of pullulan to high salt and pH are utilized to impart viscosity to foods such as barbecue and soy sauces (Chaen, 2009).
In addition, pullulan can be used as low calories food ingredient as it only slightly depolymerized by digestive enzymes. It has been demonstrated that replacing flour with pullulan to make biscuit or doughnut in the baking industry (Tsujisaka and Mitsuhashi, 1993). Furthermore, pullulan acts as a humectant and binder by retaining moisture. It has been applied by adding of pullulan to have a fluffy sponge cake. Additionally, it can used as a binding agent to bind food pastes or glazing agents due to its strong adhesive property (Chaen, 2009).
Pharmaceutical industry
Over the past decades, intense research has been carried out in order to understand bioactive polysaccharides utilize its medical properties in naturally produced pharmaceuticals (Giavasis, 2014). Pullulan is non-toxic, water soluble, non-mutagenic, odorless, low oxygen, and moisture permeability (Aguilar-Vázquez et al., 2018). These mechanical properties resulting pullulan can be used as another alternative for gelatin in the production of the capsule coating for dietary supplements and medical products (Park and Khan, 2009). In addition, it can be used as a denture adhesive. Adhesive can be prepared by dissolving pullulan ester in a mixture of water and acetone. Sugar-coated pharmaceutical compositions contain pullulan in sugar layer of tablet can prolong the shelf life (Singh, Saini and Kennedy, 2008). Pullulan films can be applied in oral care product and have been commercialized (Leathers, 2003).
Tissues Engineering
Bone is a dynamic tissue that capable of altering its structure and mass throughout the lifetime (Weatherholt, Fuchs and Warden, 2012). Osteogenesis with appropriate scaffolds for bone regeneration can be enhanced by applying tissue engineering technique to bone defects (Moreau et al., 2007). Cholesteryl group- and acryloyl group-bearing pullulan (CHPOA) nanogels is used to deliver two distinct growth factors FGF18 and Bone Morphogenetic Protein 2 (BMP-2) to a critical size skull bone defect for bone repair using CHPOA/hydrogel systems. Studied indicate that synergistic effect between FGF18 and BMP-2 increase the thickness of the bone. This hydrogel is having potential as a drug delivery systems containing multiple growth factors to regulate and induce osteogenesis. Thus, aided in developing of an efficient delivery system of osteogenic factors that contribute a very stable bone regeneration (Fujioka-Kobayashi et al., 2012). Pullulan provides good solubility and its hydrogels demonstrate great mechanical stability with high water retention capacity (Li et al., 2011). Hence, it is used as a composite based of photocrosslinkable polysaccharide hydrogel for human co-culture model of human osteoblast and endothelial cells. In this study, pullulan-amylose hydrogel composites are demonstrated to have great potential as carrier systems, especially concerning endothelial enhancement by addition of SDF-1. After incubation of hydrogels with the growth factor BMP-2 and SDF-1 respectively, the cell growth occurred and this highlighted the retained function of growth factors after entrapment and release from the hydrogel matrix (Ritz et al., 2016). Li et al. (2016) reported HLC/pullulan hydrogel may enhance the fibroblasts attachment and inhibit the cell death.
Besides, the great mechanical strength with reduced inflammation delayed hydrogel degradation may posse advantages in vivo applications. A scaffold composed of pullulan and dextran with hydroxyapatite particles (nHA) was developed to examine bone healing process. This study revealed that the composite based-polysaccharide scaffold (Matrix + nHA) retained subcutaneously local growth factors like BMP-2, induced the formation of dense mineralized tissue in mice. After that, implanted this scaffold in different size of animal models. High mineralized tissue was observed in all the animal models which including rat and goat. Therefore, proposing this composite matrix able to stimulate bone cell differentiation and bone formation (Fricain et al., 2013). In another similar study, pullulan has been supplemented with nHA in a rat model. The result showed an increasing of newly formed tissue and osteoid tissue around the scaffold. This study suggests pullulan based scaffold favored bone mineralization and formation. Besides, it also enhances vessel ingrowth into the defect site. Therefore, this suggests the scaffold possible meet the clinical trial as it capable of repairing small size defect (Schlaubitz et al., 2014). An enzymatically crosslinked biocompatible hydrogels were established using pullulan and silk fibroin under condition presence of horseradish peroxidase (HRP) and hydrogen peroxide (H2O2) as an oxidant. Besides, the rabbit bone marrow-derived mesenchymal stem cell was encapsulated in silk fibroin/ pullulan hydrogels for 7 days. The result showed that about 90% live cell was present in this hydrogel. This indicates silk fibroin/ pullulan hydrogel had good cytocompatibility and this can be proposed as a cell carrier candidate to have application in musculoskeletal tissue engineering (Li et al., 2018).
Film Industry
Pullulan coating prolonged the shelf life of kiwifruits and strawberries. Accumulation of ethylene in pullulan coated fruits prevent ethylene translocation from the internal to external fruit atmosphere and maintain the firmness during the storage (Diab et al., 2001). Pullulan based films are clear, low toxicity, highly oxygen-impermeable with excellent mechanical properties and good biodegradability (Farris et al., 2012). As a result, it is known as “edible” packing. Normally, they function as protecting food from lengthening the shelf life of food products against moisture and gases (Rinaudo, 2008). In this study, glutaraldehyde and glycerol were used to enhance physical properties and water resistance of pullulan films. The result possess that film will have stronger tensile strength when 2% (w/w) of glutaraldehyde added. Furthermore, glycerol act as plasticizer assists to ameliorate flexibility of films though with reduced water resistance (Chen et al., 2016). In addition, pullulan can be used as wound healing film. In this study, hyaluronic acid grafted pullulan (HA-g-Pu) polymers with hyaluronic acid were synthesized to examine the rate of wound healing process. Results showed applying of HA-g-Pu film will speed up the healing process compared to the natural wound healing process. Due to the HA composition with porous microstructure, high swelling ratio, prevent accumulation of exudates and fast hemostasis ability, hence, HA-g-Pu film is used as wound healing materials (Li et al., 2018). Oral thin films (OTF) is a thin film that composed of the drug molecule and other excipients. It can be produced through extrusion method or solvent casting that capable dissolves rapidly on patient’s tongue (Chowdary et al., 2012). Pullulan as hydrophilic polymers is used as film formers for OTF. In this study, pullulan based oral thin film (OTF) of zolmitriptan was made with PEG 400 as a plasticizer and sucralose as a sweetener in lab scale. Result showed PEG400 and sucralose are compatible and have a good quality overall. In addition, PEG 400 showed having no negative effect on drug release rate and having excellent stability in aluminium sachet stored at 40˚C (Prajapati et al., 2017). In another similar study, protein loaded orodispersible films (ODFs) were prepared based on blends of trehalose/pullulan by air- and freeze-drying. Based on the excellent protein stabilizing capacity of trehalose and film-forming ability of pullulan, these two carbohydrates were selected. Trehalose has a very weak performance on film former, therefore, pullulan is being selected as the main material for ODFs. Combination of these 2 materials are believed can have strong protein stability and exhibit great film-forming properties (Tian et al., 2018). Another pullulan-based nanocomposite films composed of lysozyme nanofiber (LNFs) for functional food packaging were developed. LNFs able to maintain with good mechanical properties and several functionalities such as withstand temperature up to 225 ˚C, antioxidant activity and especially antibacterial activity towards Staphylococcus aureus food pathogenic bacteria increase with the content of nanofibers. These properties not only showed pullulan based nanocomposite is an edible film for food packaging and serve as multifunctional purposes to protect and prolonged the shelf life (Silva et al., 2018).
Nanoparticles Drug Delivery
Emerging in biotechnology have led to research and development of the protein-based drug. However, the bioavailability of protein drugs is low as it always being filtered out from the body via proteolysis and renal filtration. Thus, developed a delivery of therapeutic proteins is attractive in the biopharmaceutical industry and biotechnology research community (Nuttall and Walsh, 2008). In this study, Hybrid hyaluronan (HA) hydrogel modified with 2-aminoethyl methacrylate with the presence of cholesteryl group-bearing pullulan (CHP) nanogel. Just immersing hybrid hydrogels into the drug solutions will allow therapeutic proteins to be trapped in the CHP nanogel in the HA gel. In vitro and in vivo, CHP/protein complex nanogel will be released from the hybrid hydrogel in a sustained manner. Hence, this hybrid hydrogel system possess biocompatible sustained for releasing the protein without denaturation of protein (Hirakura et al., 2010). Finding appropriate strategies to deliver proteins has become a crucial issue and transmucosal administration is the first-line option for their systemic delivery. Recently, nanoparticles have been suggested as protein carriers due to its structural flexibility and biodegradability and biocompatibility (Antosova et al., 2009). In this study, pullulan based nanoparticles were produced with sulfated and aminated derivatives of the polymer. These derivatives were then complexed with carrageenan and chitosan to synthesize nanocarrier. In this work, pullulan based nanoparticles capable to release 30% of protein up to 24 hours. In addition, there is evidence indicating an absence of toxicity of the pullulan based nano-delivery systems on a respiratory cell line (Dionísio et al., 2013). Generally, infectious pathogens invade their hosts through mucosal surfaces of respiratory and gastrointestinal tracts. Nasal and oral vaccines are developed to target various infectious disease (Bahamondez-Canas and Cui, 2018). In this study, cationic pullulan nanogel is being proposed as a mucosal drug delivery system. The nanogel composed of cholesteryl-group-bearing pullulan (CHP) which make protein easier incorporated within the internal space of CHP nanogel. These unique properties make it function as a molecular chaperone. Cationic cholesteryl-group-bearing pullulan recognized as safety and its efficacy in generating antigen-specific protective immunity (Nakahashi-Ouchida, Yuki and Kiyono, 2018). Nanosystem has been focused for drug delivery system to the tumor cell. In this study, pullulan serves as a multifunctional function such as vehicle and design which further assisted by folic acid and disulfide crosslinking, a PTX-loaded redox-responsive nanoplatform was designed for dual targeted liver cancer treatment. In vivo, therapeutic efficacy studies indicated enhancing of antitumor effect and reducing systemic toxicity compared taxol were achieved using FA-Pull-LAPTX CLNPs. In addition, a reversibly disulfide-crosslinked pullulan nanoparticle with folic acid (FA-Pull-LA CLNPs) was reported could target ASGPR and FR-positive human hepatic tumor xenografts. In conclusion, combining dual-targeting and reversible crosslinking can serve as drug delivery systems for the transport of lipophilic drugs (Huang et al., 2017).
Medical Imaging
Nanotechnology has been applied for earlier detection of cancerous cell grow in the body. Quantum dots are a nano-size semiconductor which gaining a lot of interest in biological field. The main purpose of quantum dots is used for cell tracking as fluorescent probes. Cholesterol pullulan and amino group modified cholesterol pullulan nanogel is developed for the delivery of quantum dots into cells in comparison to a conventional cationic liposome which having the difficulty forming aggregates ones gets into the cells. They compared the intensity of fluorescence per cell with conventional cationic liposome. They concluded that cellular uptake of cholesterol pullulan was improved by introducing cationic groups and simultaneously the quantum dot better than the conventional cationic liposomes and these nanoparticles could be a promising fluorescent probe for medical imaging (Prajapati, Jani and Khanda, 2013).
Molecular Chaperons
A molecule having chaperon-like activity are capable to catch or release proteins. It will bind to the denatured protein to prevent irreversible aggregation. Then the chaperons release the protein in its refolded form. Water-soluble polymers such as polyethylene oxide (PEO) will try to increase the recovery yield of native protein during refolding (Cleland et al., 1992). This polymer will block the exposed hydrophobic surface to prohibit aggregation of proteins. Nomural et al. (2003) developed hydrophobized pullulan nanogel possessing properties of molecular chaperons. In the presence of cyclodextrins, complexed proteins will release rapidly from nanogels in their refolded forms. They concluded that this denatured protein and cyclodextrin will be trapped by these amphiphilic nanogels and it acts as an effector to control the binding ability of chaperon molecule to proteins.
Plasma Expander
Pullulan explored as a potential blood plasma substitute. Polymer which is highly water-soluble can be used as plasma expanders. Due to its unique structure, pullulan is water soluble in nature. It has been reported that pullulan should have molecular weight about 60 kDa then only can be used as plasma expanders (Rekha and Sharma, 2007). It was stated that pullulan with high molecular weight will raise the venous pressure whereas pullulans with low molecular weight will exclude from the organism leaving the stage of secondary hemorrhagic shock. Therefore, pullulan should be in the therapeutic range of molecular weight in order to be used as a plasma expander. An anionically modified pullulan is being developed through gamma irradiation which was used as a base for blood plasma substitute (Shingel and Petrov, 2002). Table 5 summarize the potential applications of pullulan mainly in the food and pharmaceutical industries.
Table 5: List of applications of pullulan in food and pharmaceutical industries.
| Industry | Pullulans | Application | Reference |
| Food industry | Chitosan-PU | Preservation of fresh-cut pineapple | (Treviño-Garza et al., 2017) |
| CS-BOPP | Food packaging | (Cozzolino et al., 2016) | |
| Pullulan-CMC-TP | Food preservation | (Shao et al., 2018) | |
| Pullulan Coating | Delaying deterioration and controlling microbial growth on blueberry | (Kraśniewska et al., 2017) | |
| Pullulan coating containing oregano essential oil | Preservation of Brussels Sprouts | (Kraśniewska et al., 2016) | |
| Pu-OSA | Edible coating on fruits | (Shah et al., 2016) | |
| Laminaria japonica-incorporated pullulan coatings | Preservation of cherry tomatoes | (Wu, Lu and Wang, 2016) | |
| WPI-pullulan | Better oxidative stability of tuna oil inside microcapsules | (Bakry et al., 2016) | |
| WPI/PUL/NS nanocomposite | Increasing the shelf life of food products | (Hassannia-Kolaee et al., 2016) | |
| Pharmaceutical | CAPL/PBAE/PLGA nanoparticles | Excellent hepatoma-targeting capability and increased synergistic effects of PTX and CA4 on tumor growth and tumor angiogenesis | (Zhang et al., 2016) |
| CHAP | Drug carrier for tumor treatment | (Tao et al., 2016) | |
| CMC-pullulan hydrogel | Injectable in situ anti-adhesive agent | (Bang et al., 2017) | |
| Crosslinked carboxymethylated pullulan/chondroitin sulfate hydrogel | Facilitate chondrogenesis | (Chen et al., 2016) | |
| Crosslinked pullulan/cellulose acetate fibrous scaffolds | Bone tissue engineering | (Atila, Keskin and Tezcaner, 2016) | |
| CS-ADH/oxPL hydrogel | Cell delivery carrier scaffold in cartilage tissue engineering | (Li et al., 2018) | |
| Curcumin pullulan acetate | Effective hepato-protective agent against diethyl nitrosamine induced liver damage | (Ganeshkumar et al., 2016) | |
| FA-Pull-LAPTX CLNPs | Anti-tumor Liver Drug Delivery | (Huang, Tu and Sun, 2017) | |
| FA-CP/SeNPs | Anticancer drug template in drug delivery systems | (Chen et al., 2018) | |
| FPA NPs | Drug carriers for targeted therapy of the folate-receptor overexpressed cancers | (Chen et al., 2016) | |
| FPDP | Co-delivery of DOX and shBeclin1 for cancer therapy | (Nonsuwan et al., 2018) | |
| HA-g-Pu | Wound healing materials | (Li et al., 2018) | |
| OGG3P | Genetic photodynamic therapy | (Zhou et al., 2018) | |
| PABA-QP | Human cancer treatment | (Laksee et al., 2018) | |
| PAMAM-pullulan | Delivering gene into liver cells | (Askarian et al., 2017) | |
| PDP | Co-delivery of drug and gene for potential cancer therapy | (Chen et al., 2017) | |
| PPSS | As gene delivery vector and efflux inhibitor | (S. and R., 2016) | |
| PSCFO | Therapeutic applications in targeting tumors | (Eslaminejad, Nematollahi-Mahani and Ansari, 2016) | |
| Pullulan | Pharmaceutical oral films | (Vuddanda et al., 2017) | |
| Pullulan-CMCS | Wound dressing | (Wang et al., 2016) | |
| Pull-LA-CLNPs | Delivery of paclitaxel into ASGPR over-expressed cancer cells | (Huang et al., 2017) | |
| PuPGEA) | Codeliver lncRNA and pDNA to treat Hepatocellular carcinoma | (Ren et al., 2016) | |
| Pullulan-g-poly | Controlled delivery of indomethacin | (Constantin et al., 2017) | |
| Pullulan-poly(vinyl alcohol) | As controlled release drug delivery system | (Soni and Ghosh, 2017) | |
| ssPBAE-oxPL-DOX | Exhibited in vitro hepatoma-targeting property and condensing genes including plasmid DNA and fluorescein-labeled oligoDNA | (Wang et al., 2016) |
Conclusion
The advancement and needs to design for an efficient bioprocess is the most important step in any biotechnology industry be it food, pharmaceutical or any other. There are needs to have an economical and robust process that can be reproducible effortlessly. Agro-industrial wastes are produced in huge amounts every year and they are rich in nutrients comprising variability of sugars and minerals. This nutrient offers good supports for cell growth and pullulan production. In addition to that, it can help to contribute in minimizing waste and creates environmental eco-friendly.
Acknowledgements
The financial support from the Institute of Bioproduct Development (IBD), is gratefully acknowledged. We would like also to acknowledge the support of Ministry of Higher Education Malaysia (MOHE) and Universiti Teknologi Malaysia-Research Management Centre (UTM-RMC) through HICOE grant no. R.J130000.7851.4J386 and PAS grant no. Q.J130000.2746.03K29.
References
- Aguilar-Vázquez, G., Loarca-Piña, G., Figueroa-Cárdenas, J.D., Mendoza, S., Electrospun fibers from blends of pea (Pisum sativum) protein and pullulan. Food Hydrocolloids. 2018; 83: 173-181. doi:10.1016/j.foodhyd.2018.04.051.
CrossRef - Antosova, Z., Mackova, M., Kral, V., Macek, T., Therapeutic application of peptides and proteins: parenteral forever?. Trends in Biotechnology. 2009; 27: 628-635. doi: 1016/j.tibtech.2009.07.009.
CrossRef - Askarian, S., Abnous, K., Ayatollahi, S., Farzad, S.A., Oskuee, R.K., Ramezani, M., PAMAM-pullulan conjugates as targeted gene carriers for liver cell. Carbohydrate polymers. 2017; 157: 929-937. doi: 10.1016/j.carbpol.2016.10.030.
CrossRef - Atila, D., Keskin, D., Tezcaner, A., Crosslinked pullulan/cellulose acetate fibrous scaffolds for bone tissue engineering. Materials Science and Engineering: C. 2016; 69: 1103-1115. doi: 10.1016/j.msec.2016.08.015.
CrossRef - Bahamondez-Canas, T.F., Cui, Z., Intranasal immunization with dry powder vaccines. European Journal of Pharmaceutics and Biopharmaceutics. 2018; 122: 167-175. doi: 1016/j.ejpb.2017.11.001.
CrossRef - Bakry, A.M., Fang, Z., Ni, Y., Cheng, H., Chen, Y.Q., Liang, L., Stability of tuna oil and tuna oil/peppermint oil blend microencapsulated using whey protein isolate in combination with carboxymethyl cellulose or pullulan. Food Hydrocolloids. 2016; 60: 559-571. doi: 10.1016/j.foodhyd.2016.04.026.
CrossRef - Bang, S., Ko, Y.G., Kim, W.I., Cho, D., Park, W.H., Kwon, O.H., Preventing postoperative tissue adhesion using injectable carboxymethyl cellulose-pullulan hydrogels. International Journal of Biological Macromolecules. 2017; 105: 886-893. doi: 10.1016/j.ijbiomac.2017.07.103.
CrossRef - Barnett, C., Smith, A., Scanlon, B., Israilides, C., Pullulan production by Aureobasidium pullulans growing on hydrolysed starch. Carbohydrate Polymers. 1998; 38: 203-209. doi: 10.1016/S0144-8617(98)00092-7.
CrossRef - Chaen, H., Pullulan. Food stabilisers, thickeners and gelling agents. Blackwell. 2009; 266-274.
CrossRef - Chen, C.T., Chen, K.I., Chiang, H.H., Chen, Y.K., Cheng, K.C., Improvement on physical properties of pullulan films by novel cross‐linking strategy. Journal of Food Science. 2017; 82: 108-117. doi: 10.1111/1750-3841.13577.
CrossRef - Chen, F., Yu, S., Liu, B., Ni, Y., Yu, C., Su, Y., Zhu, X., Yu, X., Zhou, Y., Yan, D., An injectable enzymatically crosslinked carboxymethylated pullulan/chondroitin sulfate hydrogel for cartilage tissue engineering. Scientific Reports. 2016; 6: 20014.
CrossRef - Chen, G., Wang, J., Su, Y., Zhu, Y., Zhang, G., Zhao, H., Liu, H., Yang, Y., Nian, R., Zhang, H., Wei, Y., Xian, M., Pullulan production from synthetic medium by a new mutant of Aureobasidium pullulans. Preparative Biochemistry and Biotechnology. 2017; 47: 963-969. doi: 10.1080/10826068.2017.1350979.
CrossRef - Chen, H., Jia, Y., Nan, W., Tang, H., Zhou, Z., Wei, X., Qin, J., Lin, J., Feng, H., Yuan, Z., Cytotoxicity and cellular internalization of epirubicin-loaded folate-conjugated pullulan acetate nanoparticles in cancer cells. Science of Advanced Materials. 2016; 8: 2029-2036. doi: 10.1166/sam.2016.2989.
CrossRef - Chen, L., Ji, F., Bao, Y., Xia, J., Guo, L., Wang, J., Li, Y., Biocompatible cationic pullulan-g-desoxycholic acid-g-PEI micelles used to co-deliver drug and gene for cancer therapy. Materials Science and Engineering: C. 2017; 70: 418-429. doi: 10.1016/j.msec.2016.09.019.
CrossRef - Chen, L., Qian, M., Zhang, L., Xia, J., Bao, Y., Wang, J., Guo, L., Li, Y., Co-delivery of doxorubicin and shRNA of Beclin1 by folate receptor targeted pullulan-based multifunctional nanomicelles for combinational cancer therapy. RSC Advances. 2018; 8: 17710-17722. doi: 10.1039/C8RA01679H.
CrossRef - Cheng, K., Demirci, A., Catchmark, J.M., Pulullan: biosynthesis, production, and application. Appllied Microbiology Biotechnology. 2011; 92: 29-44. doi: 10.1007/s00253-011-3477-y.
CrossRef - Chi, Z., Zhao, S., Optimization of medium and cultivation conditions for pullulan production by a new pullulan-producing yeast strain. Enzyme and Microbial Technology. 2003; 33: 206-211. doi:10.1016/s0141-0229(03)00119-4.
CrossRef - Chiani, M., Akbarzadeh, A., Farhangi, A., Mehrabi, M.R., Production of desferrioxamine B (Desferal) using corn steep liquor in Streptomyces pilosus.Pakistan Journal of Biological Sciences. 2010; 13: 1151-1155. doi: 10.3923/pjbs.2010.1151.1155.
CrossRef - Chowdary, Y.A., Soumya, M., Madhu Babu, M., Aparna, K., Himabindu, P., A review of fast dissolving drug delivery systems- a pioneering drug delivery technology. Bulletin of Environmental Pharmacology and Life Sciences. 2012; 1: 08-20.
- Cleland, J.L., Hedgepeth, C., Wang, D.I., Polyethylene glycol enhanced refolding of bovine carbonic anhydrase B. Reaction stoichiometry and refolding model. Journal of Biological Chemistry. 1992; 267: 13327-13334.
- Constantin, M., Bucătariu, S., Stoica, I., Fundueanu, G., Smart nanoparticles based on pullulan-g-poly (N-isopropylacrylamide) for controlled delivery of indomethacin. International Journal of Biological Macromolecules. 2017; 94: 698-708. doi: 10.1016/j.ijbiomac.2016.10.064.
CrossRef - Cozzolino, C.A., Castelli, G., Trabattoni, S., Farris, S., Influence of colloidal silica nanoparticles on pullulan-coated BOPP film. Food Packaging and Shelf Life. 2016; 8: 50-55. doi: 10.1016/j.fpsl.2016.03.003.
CrossRef - Danjo, T., Enomoto, Y., Shimada, H., Nobukawa, S., Yamaguchi, M., Iwata, T., Zero birefringence films of pullulan ester derivatives. Scientific Reports. 2017; 7: 46342.
CrossRef - Diab, T., Biliaderis, C.G., Gerasopoulos, D., Sfakiotakis, E., Physicochemical properties and application of pullulan edible films and coatings in fruit preservation. Journal of the Science of Food and Agriculture. 2001; 81: 988-1000. doi: 10.1002/jsfa.883.
CrossRef - Dionísio, M., Cordeiro, C., Remuñán-López, C., Seijo, B., da Costa, A.M.R., Grenha, A., Pullulan-based nanoparticles as carriers for transmucosal protein delivery. European Journal of Pharmaceutical Sciences. 2013; 50: 102-113. doi: 10.1016/j.ejps.2013.04.018.
CrossRef - Donot, F., Fontana, A., Baccou, J., Schorr-Galindo, S., Microbial exopolysaccharides: Main examples of synthesis, excretion, genetics and extraction. Carbohydrate Polymers. 2012; 87: 951-962. doi: 10.1016/j.carbpol.2011.08.083.
CrossRef - Eslaminejad, T., Nematollahi-Mahani, S.N., Ansari, M., Synthesis, characterization, and cytotoxicity of the plasmid EGFP-p53 loaded on pullulan–spermine magnetic nanoparticles. Journal of Magnetism and Magnetic Materials. 2016; 402: 34-43. doi: 10.1016/j.jmmm.2015.11.037.
CrossRef - Falguera, V., Quintero, J.P., Jiménez, A., Muñoz, J.A., Ibarz, A., Edible films and coatings: structures, active functions and trends in their use. Trends in Food Science & Technology. 2011; 22: 292-303. doi: 10.1016/j.tifs.2011.02.004.
CrossRef - Farris, S., Introzzi, L., Fuentes-Alventosa, J.M., Santo, N., Rocca, R., Piergiovanni, L., Self-assembled pullulan–silica oxygen barrier hybrid coatings for food packaging applications. Journal of Agricultural and Food Chemistry. 2012; 60: 782-790. doi: 10.1021/jf204033d.
CrossRef - Farris, S., Unalan, I.U., Introzzi, L., Fuentes‐Alventosa, J.M., Cozzolino, C.A., Pullulan‐based films and coatings for food packaging: present applications, emerging opportunities, and future challenges. Journal of Applied Polymer Science. 2014; 131: 40539. doi: 10.1002/app.40539.
CrossRef - Forabosco, A., Bruno, G., Sparapano, L., Liut, G., Marino, D., Delben, F., Pullulans produced by strains of Cryphonectria parasitica-I. Production and characterisation of the exopolysaccharides. Carbohydrate Polymers. 2006; 63: 535-544. doi: 10.1016/j.carbpol.2005.10.005.
CrossRef - Fricain, J.C., Schlaubitz, S., Le Visage, C., Arnault, I., Derkaoui, S.M., Siadous, R., Catros, S., Lalande, C., Bareille, R., Renard, M., Fabre, T., A nano-hydroxyapatite–pullulan/dextran polysaccharide composite macroporous material for bone tissue engineering. Biomaterials. 2013; 34: 2947-2959. doi: 10.1016/j.biomaterials.2013.01.049.
CrossRef - Fujioka-Kobayashi, M., Ota, M.S., Shimoda, A., Nakahama, K.I., Akiyoshi, K., Miyamoto, Y., Iseki, S., Cholesteryl group-and acryloyl group-bearing pullulan nanogel to deliver BMP2 and FGF18 for bone tissue engineering. Biomaterials. 2012; 33: 7613-7620. doi: 10.1016/j.biomaterials.2012.06.075.
CrossRef - Ganduri, V.S.R.K., Rao, S., Kiranmayi, U., Vijaya, L., Production of pullulan using jaggery as substrate by Aureobasidium pullulans MTCC 2195. Current Trends in Biotechnology and Pharmaceutical. 2016; 10: 153-160.
- Ganeshkumar, M., Ponrasu, T., Subamekala, M.K., Janani, M., Suguna, L., Curcumin loaded on pullulan acetate nanoparticles protects the liver from damage induced by DEN. RSC Advances. 2016; 6: 5599-5610. doi: 10.1039/C5RA18989F.
CrossRef - Gaur, R., Singh, R., Gupta, M., Gaur, M. K., Aureobasidium pullulans, an economically important polymorphic yeast with special reference to pullulan. African Journal of Biotechnology. 2010; 9: 7989-7997. doi: 10.5897/AJB10.948.
CrossRef - Gaur, R., Singh, R., Tiwari, S., Yadav, S., Daramwal, N., Optimization of physico-chemical and nutritional parameters for a novel pullulan-producing fungus, Eurotium chevalieri. Journal of Applied Microbiology. 2010; 109: 1035-1043. doi:10.1111/j.1365-2672.2010.04731.x.
CrossRef - Giavasis, I., Bioactive fungal polysaccharides as potential functional ingredients in food and nutraceuticals. Current Opinion in Biotechnology. 2014; 26: 162-173. doi: 10.1016/j.copbio.2014.01.010.
CrossRef - Hassannia-Kolaee, M., Khodaiyan, F., Pourahmad, R., Shahabi-Ghahfarrokhi, I., Development of ecofriendly bionanocomposite: whey protein isolate/pullulan films with nano-SiO2. International Journal of Biological Macromolecules. 2016; 86: 139-144. doi: 10.1016/j.ijbiomac.2016.01.032.
CrossRef - Hickert, L.R., da Cunha-Pereira, F., de Souza-Cruz, P.B., Rosa, C.A., Ayub, M.A.Z., Ethanogenic fermentation of co-cultures of Candida Shehatae HM 52.2 and Saccharomyces Cerevisiae ICV D254 in synthetic medium and rice hull hydrolysate. Bioresource Technology. 2013; 131: 508-514. doi: 10.1016/j.biortech.2012.12.135.
CrossRef - Hilares, R. T., Resende, J., Orsi, C. A., Ahmed, M. A., Lacerda, T. M., da Silva, S.S., Santos, J. C., Exopolysaccharide (pullulan) production from sugarcane bagasse hydrolysate aiming to favor the development of biorefineries. International Journal of Biological Macromolecules. 2019; 127: 169-177. doi: 10.1016/j.ijbiomac.2019.01.038.
CrossRef - Hirakura, T., Yasugi, K., Nemoto, T., Sato, M., Shimoboji, T., Aso, Y., Morimoto, N., Akiyoshi, K., Hybrid hyaluronan hydrogel encapsulating nanogel as a protein nanocarrier: new system for sustained delivery of protein with a chaperone-like function. Journal of Controlled Release. 2010; 142: 483-489. doi: 10.1016/j.jconrel.2009.11.023.
CrossRef - Hossain, S. S., Mathur, L., Roy, P. K., Rice husk/rice husk ash as an alternative source of silica in ceramics: A review. Journal of Asian Ceramic Societies. 2018; 6: 299-313. doi: 10.1080/21870764.2018.1539210.
CrossRef - Huang, L., Tu, J., Sun, C., Reversibly disulfide-crosslinked pullulan nanoparticles for dual-targeted and bio-triggered anti-tumor drug delivery. Journal of Controlled Release. 2017; doi: 10.1016/j.nano.2018.01.015.
CrossRef - Huang, L., Wang, Y., Ling, X., Chaurasiya, B., Yang, C., Du, Y., Tu, J., Xiong, Y., Sun, C., Efficient delivery of paclitaxel into ASGPR over-expressed cancer cells using reversibly stabilized multifunctional pullulan nanoparticles. Carbohydrate Polymers. 2017; 159: 178-187. doi: 10.1016/j.carbpol.2016.11.094.
CrossRef - Israilides, C., Smith, A., Bambalov, G., Production of pullulan from agro-industrial wastes. Proceedings of the Sixth Eurpoean Congress on Biotechnology. 1993;
- Jakovljevic, D., Vrvic, M.M., Radulovic, M., Hranisavljevic-Jakovljevic, M., Fine structural analysis of the fungal polysaccharide pullulan elaborated by Aureobasidium pullulans, CHL-1 strain. Journal-Serbian Chemical Society. 2001; 66: 377-384.
CrossRef - Jiang, H., Xue, S.J., Li, Y.F., Liu, G.L., Chi, Z.M., Hu, Z., Chi, Z., Efficient transformation of sucrose into high pullulan concentrations by Aureobasidium melanogenum TN1-2 isolated from a natural honey. Food Chemistry. 2018; 257: 29-35. doi: 10.1016/j.foodchem.2018.03.003.
CrossRef - Kraśniewska, K., Gniewosz, M., Kosakowska, O., Cis, A., Preservation of brussels sprouts by pullulan coating containing oregano essential oil. Journal of Food Protection. 2016; 79: 493-500. doi: 10.4315/0362-028X.
CrossRef - Kraśniewska, K., Ścibisz, I., Gniewosz, M., Mitek, M., Pobiega, K., Cendrowski, A., Effect of pullulan coating on postharvest quality and shelf-life of high bush blueberry (Vaccinium corymbosum). Materials. 2017; 8: 965 doi: 10.3390/ma10080965.
CrossRef - Kumar, A.S., Mody, K., Jha, B., Bacterial exopolysaccharides – a perception. Journal of Basic Microbiology. 2007; 47: 103-117. doi:10.1002/jobm.200610203.
CrossRef - Kumar, D., Saini, N., Pandit, V., Ali, S., An insight to pullulan: a biopolymer in pharmaceutical approaches. International Journal of Basic and Applied Sciences. 2017; 1: 202-219. doi: 10.14419/ijbas.v1i3.101.
CrossRef - Laksee, S., Puthong, S., Kongkavitoon, P., Palaga, T., Muangsin, N., Facile and green synthesis of pullulan derivative-stabilized Au nanoparticles as drug carriers for enhancing anticancer activity. Carbohydrate Polymers. 2018; 198: 495-508. doi: 10.1016/j.carbpol.2018.06.119.
CrossRef - Leathers, T.D., Biotechnological production and applications of pullulan. Applied Microbiology and Biotechnology. 2003; 62: 468-473. doi: 10.1007/s00253-003-1386-4.
CrossRef - Li, H., Xue, Y., Jia, B., Bai, Y., Zuo, Y., Wang, S., Zhao, Y., Yang, W., Tang, H., The preparation of hyaluronic acid grafted pullulan polymers and their use in the formation of novel biocompatible wound healing film. Carbohydrate Polymers. 2018; 188: 92-100. doi: 10.1016/j.carbpol.2018.01.102.
CrossRef - Li, H., Yang, J., Hu, X., Liang, J., Fan, Y., Zhang, X., Superabsorbent polysaccharide hydrogels based on pullulan derivate as antibacterial release wound dressing. Journal of Biomedical Materials Research Part A. 2011; 98: 31-39. doi:10.1002/jbm.a.33045.
CrossRef - Li, T., Song, X., Weng, C., Wang, X., Sun, L., Gong, X., Yang, L., Chen, C., Self-crosslinking and injectable chondroitin sulfate/pullulan hydrogel for cartilage tissue engineering. Applied Materials Today. 2018; 10: 173-183. doi: 10.1039/C6BM00863A.
CrossRef - Li, T., Song, X., Weng, C., Wang, X., Wu, J., Sun, L., Gong, X., Zeng, W.N., Yang, L., Chen, C., Enzymatically crosslinked and mechanically tunable silk fibroin/pullulan hydrogels for mesenchymal stem cells delivery. International Journal of Biological Macromolecules. 2018; 115: 300-307. doi: 10.1016/j.ijbiomac.2018.04.046.
CrossRef - Li, X., Xue, W., Liu, Y., Li, W., Fan, D., Zhu, C., Wang, Y., HLC/pullulan and pullulan hydrogels: their microstructure, engineering process and biocompatibility. Materials Science and Engineering. C. 2016; 58: 1046-1057. doi: 10.1016/j.msec.2015.09.039.
CrossRef - Lin, Y., Thibault, J., Pullulan fermentation using a prototype rotational reciprocating plate impeller. Bioprocess and Biosystems Engineering. 2013; 36: 603-611. doi: 1002/jctb.1254.
CrossRef - Ma, , Jiang, G., Yao, S., Jin, H., Wang, C., Studies on the optimal culture conditions of Aureobasidium pullulans to produce exopolysaccharides. Journal of Biomedical Science and Engineering. 2012; 5: 203. doi:10.4236/jbise.2012.54027.
CrossRef - Ma, Y., Zhao, X., Zhang, H., Wang, Z., Comprehensive utilization of the hydrolyzed productions from rice hull. Industrial Crop Production. 2011; 33: 403–408. doi: 10.1021/acssuschemeng.8b01738.
CrossRef - Ma, Z.C., Fu, W.J., Liu, G.L., Wang, Z.P., Chi, Z.M., High-level pullulan production by Aureobasidium pullulans melanogenium P16 isolated from mangrove system. Applied Microbiology and Biotechnology. 2014;98: 4865-4873. doi: 10.1007/s00253-014-5554-5.
CrossRef - Mishra, B., Akula, M., Zamare, D., Selection and utilization of agro-industrial waste for biosynthesis and hyper-production of pullulan: A Review. 2017; 96: 89-103. doi: 10.1007/978-981-10-7434-9_6.
CrossRef - Mishra, B., Zamare, D., Manikanta, A., Selection and utilization of agro-industrial waste for biosynthesis and hyper-Production of pullulan: A Review. InBiosynthetic Technology and Environmental Challenges. 2018; 5: 89-103. doi: 10.1007/978-981-10-7434-9_6.
CrossRef - Moreau, J.L., Caccamese, J.F., Coletti, D.P., Sauk, J.J., Fisher, J.P., Tissue engineering solutions for cleft palates. Journal of Oral and Maxillofacial Surgery. 2017; 65: 2503-2511. doi:10.1016/j.joms.2007.06.648.
CrossRef - Moubasher, H., Wahsh, S.S., Pullulan production from Aureobsidium pullulans by continuous culture. Basic Research Journal of Microbiology. 2014; 1: 11-15.
- Nakahashi-Ouchida, R., Yuki, Y., Kiyono, H., Cationic pullulan nanogel as a safe and effective nasal vaccine delivery system for respiratory infectious diseases. Human vaccines & immunotherapeutics. 2018; 14: 1-27. doi: 10.1080/21645515.2018.1461298.
CrossRef - Nath, A., Dutta, D., Kumar, P., Singh, J., Review on recent advances in value addition of jaggery based products. Journal of Food. 2015; 6: 1-4 doi: 10.4172/2157-7110.1000440.
CrossRef - Nomura, Y., Ikeda, M., Yamaguchi, N., Aoyama, Y., Akiyoshi, K., Protein refolding assisted by self‐assembled nanogels as novel artificial molecular chaperone. FEBS letters. 2003; 553: 271-276.
CrossRef - Nonsuwan, P., Puthong, S., Palaga, T., Muangsin, N., Novel organic/inorganic hybrid flower-like structure of selenium nanoparticles stabilized by pullulan derivatives. Carbohydrate Polymers. 2018; 184: 9-19. doi: 10.1016/j.carbpol.2017.12.029.
CrossRef - Nuttall, S.D., Walsh, R.B., Display scaffolds: protein engineering for novel therapeutics. Current Opinion in Pharmacology. 2008; 8: 609-615. doi: 10.1016/j.coph.2008.06.007.
CrossRef - Oğuzhan, P., Yangılar, F., Pullulan: Production and usage in food ındustry. African Journal of Food Science and Technology. 2013; 4: 57-63. ISSN: 2141-5455.
- Oliva, E.M., Cirelli, A.F., Lederkremer, R.M., Characterization of a pullulan in Cyttaria darwinii. Carbohydrate Research. 1986; 158: 262-267. doi: 10.1016/0008-6215(86)84025-3.
CrossRef - Özcan, E., Sargın, S., Göksungur, Y., Comparison of pullulan production performances of air-lift and bubble column bioreactors and optimization of process parameters in air-lift bioreactor. Biochemical Engineering Journal.2014; 92: 9-15.
CrossRef - Park, J.K., Khan, T., Other microbial polysaccharides: pullulan, scleroglucan, elsinan, levan, alternant, dextran. In Handbook of Hydrocolloids. 2009; 592-614.
CrossRef - Prajapati, V.D., Jani, G.K., Khanda, S.M., Pullulan: an exopolysaccharide and its various applications. Carbohydrate Polymers. 2013; 95: 540-549. doi: 10.1016/j.carbpol.2013.02.082.
CrossRef - Rahman, A., Tashiro, M., Sonomoto, Y., Lactic acid production from lignocellulosic-derived sugars using lactic acid bacteria: Overview and Limits. Journal of Biotechnology. 2011; 156: 286-301. doi: 10.1016/j.jbiotec.2011.06.017.
CrossRef - Rekha, M.R., Sharma, C.P., Pullulan as a promising biomaterial for biomedical applications: a perspective. Trends in Biomaterials and Artificial Organs. 2007; 20: 116-121.
- Ren, Y., Li, R.Q., Cai, Y.R., Xia, T., Yang, M., Xu, F.J., X., Effective codelivery of lncRNA and pDNA by pullulan‐based nanovectors for promising therapy of hepatocellular carcinoma. Advanced Functional Materials. 2016; 26: 7314-7325. org/10.1002/adfm.201603041.
CrossRef - Rinaudo, M., Main properties and current applications of some polysaccharides as biomaterials. Polymer International. 2008; 57: 397-430. doi: 1002/pi.2378.
CrossRef - Ritz, U., Kögler, P., Höfer, I., Frank, P., Klees, S., Gebhard, S., Brendel, C., Kaufmann, K., Hofmann, A., Rommens, P.M., Jonas, U., Photocrosslinkable polysaccharide hydrogel composites based on dextran or pullulan–amylose blends with cytokines for a human co-culture model of human osteoblasts and endothelial cells. Journal of Materials Chemistry B. 2016; 4: 6552-6564. doi:10.1039/C6TB00654J.
CrossRef - Roukas, T., Biliaderis, C. G., Evaluation of carob pod as a substrate for pullulan production by Aureobasidium pullulans. Applied Biochememistry and Biotechnology 1994; 55: 27–44.
CrossRef - Schlaubitz, S., Derkaoui, S.M., Marosa, L., Miraux, S., Renard, M., Catros, S., Le Visage, C., Letourneur, D., Amedee, J., Fricain, J.C., Pullulan/dextran/nHA macroporous composite beads for bone repair in a femoral condyle defect in rats. PloS one. 2014; 9: 110251. doi: 10.1371/journal.pone.0110251.
CrossRef - Shah, N.N., Vishwasrao, C., Singhal, R.S., Ananthanarayan, L., N-Octenyl succinylation of pullulan: effect on its physico-mechanical and thermal properties and application as an edible coating on fruits. Food Hydrocolloids. 2016; 55: 179-188.
CrossRef - Shao, P., Niu, B., Chen, H., Sun, P., Fabrication and characterization of tea polyphenols loaded pullulan-CMC electrospun nanofiber for fruit preservation. International Journal of Biological Macromolecules. 2018; 107: 1908-1914. doi: 10.1016/j.ijbiomac.2017.10.054.
CrossRef - Sharma, N., Prasad, G,S., Choudhury, A,R., Utilization of corn steep liquor for biosynthesis of pullulan, an important exopolysaccharide. Carbohydrate Polymers. 2013; 93: 95–101. doi: 10.1016/j.carbpol.2012.06.059.
CrossRef - Sheng, L., Liu, C., Tong, Q., Ma, M., Central metabolic pathways of Aureobasidium pullulans CGMCC1234 for pullulan production. Carbohydrate Polymers. 2016; 134: 333-336. doi:10.1016/j.carbpol.2015.08.016.
CrossRef - Sheoran, S.K., Dubey, K.K., Tiwari, D.P., Singh, B.P., Directive production of pullulan by altering cheap source of carbons and nitrogen at 5 l bioreactor level. ISRN Chemical Engineering. 2012.
CrossRef - Shingel, K.I., Current knowledge on biosynthesis, biological activity, and chemical modification of the exopolysaccharide, pullulan. Carbohydrate Research. 2004; 339: 447-460. doi:10.1016/j.carres.2003.10.034.
CrossRef - Shingel, K.I., Petrov, P.T., Behavior of γ-ray-irradiated pullulan in aqueous solutions of cationic (cetyltrimethylammonium hydroxide) and anionic (sodium dodecyl sulfate) surfactants. Colloid and Polymer Science. 2002; 280: 176-182. doi: 1007/s00396-001-0599-2.
CrossRef - Silva, N.H., Vilela, C., Almeida, A., Marrucho, I.M., Freire, C.S., Pullulan-based nanocomposite films for functional food packaging: exploiting lysozyme nanofibers as antibacterial and antioxidant reinforcing additives. Food Hydrocolloids. 2018; 77: 921-930. doi: 1016/j.foodhyd.2017.11.039.
CrossRef - Singh, R., Gaur, R., Pandey, P., Jamal, F., Pandey, L., Singh, S., Kewat, H., Tiwari, S., Biswas, P., Gaur, M., A novel media optimized for production of pullulan in flask type fermentation system. 2018; 7: 53-61. doi: 20546/ijcmas.2018.704.007.
CrossRef - Singh, R. S., Kaur, N., Kennedy, J. F., Pullulan and pullulan derivatives as promising biomolecules for drug and gene targeting. Carbohydrate Polymers. 2015; 123: 190-207. doi: 10.1016/j.carbpol.2015.01.032.
CrossRef - Singh, R.S., Saini, G.K., Kennedy, J.F., Pullulan: Microbial sources, production and applications. Carbohydrate Polymers, 2008; 73: 515-531. doi: 10.1016/j.carbpol.2008.01.003.
CrossRef - Singh, R., Gaur, R., Tiwari, S., Gaur, M.K., Production of pullulan by a thermotolerant Aureobasidium pullulans strain in non-stirred fed batch fermentation process. Brazilian Journal of Microbiology.2012; 43: 1042-1050. doi: 10.1590/S1517-838220120003000030.
CrossRef - Soni, S.R., Ghosh, A., Exploring pullulan-poly (vinyl alcohol) interpenetrating network microspheres as controlled release drug delivery device. Carbohydrate Polymers. 2017; 174: 812-822. doi: 10.1016/j.carbpol.2017.07.016.
CrossRef - Sugumaran, K. R., Ponnusami, V., Review on production, downstream processing and characterization of microbial pullulan. Carbohydrate Polymers. 2017; 173: 573-591. doi: 10.1016/j.carbpol.2017.06.022.
CrossRef - Sugumaran, K. R., Jothi, P., Ponnusami, , Bioconversion of industrial solid waste—cassava bagasse for pullulan production in solid state fermentation. Carbohydrate Polymers. 2014; 99: 22-30. doi: 10.1016/j.carbpod.2013.08.039.
CrossRef - Tao, X., Xie, Y., Zhang, Q., Qiu, X., Yuan, L., Wen, Y., Li, M., Yang, X., Tao, T., Xie, M., Lv, Y., Cholesterol-modified amino-pullulan nanoparticles as a drug carrier: comparative study of cholesterol-modified carboxyethyl pullulan and pullulan nanoparticles. 2016; 6: 165. doi: 10.3390/nano6090165.
CrossRef - Thirumavalavan, K., Manikkadan, T,R., Dhanasekar, R., Pullulan production from coconut by-products by Aureobasidium pullulans. African Journal of Biotechnology. 2009; 8: 254–258. ISSN 1684–5315.
- Tian, Y., Visser, J.C., Klever, J.S., Woerdenbag, H.J., Frijlink, H.W., Hinrichs, W.L., Orodispersible films based on blends of trehalose and pullulan for protein delivery. European Journal of Pharmaceutics and Biopharmaceutics. 2018; 133: 104-111. doi: 10.1016/j.ejpb.2018.09.016.
CrossRef - Treviño-Garza, M.Z., García, S., Heredia, N., Alanís-Guzmán, M.G., Arévalo-Niño, K., Layer-by-layer edible coatings based on mucilages, pullulan and chitosan and its effect on quality and preservation of fresh-cut pineapple (Ananas comosus). Postharvest Biology and Technology. 2017; 128: 63-75. doi: 10.1016.j.postharvbio.2017.01.007.
CrossRef - Trinetta, V., Cutter, C.N., Pullulan: a suitable biopolymer for antimicrobial food packaging applications. In Antimicrobial Food Packaging Academic Press. 2016; 1: 385-397. doi: 10.1016/B978-0-12-800723-5.00030-9.
CrossRef - Tsujisaka, Y., Mitsuhashi, M., Pullulan in industrial gums. Academic Press. 1993; 13: 447-460. doi: 1016/j.carbpol.2019.04.050.
CrossRef - Vijayendra, S., Bansal, D., Prasad, M., Nand, K., Jaggery: A novel substrate for pullulan production by Aureobasidium pullulans CFR-77. Process Biochemistry. 2001; 37: 359-364. doi: 10.1016/s0032-9592(01)00214-x.
CrossRef - Vuddanda, P.R., Montenegro-Nicolini, M., Morales, J.O., Velaga, S., Effect of plasticizers on the physico-mechanical properties of pullulan based pharmaceutical oral films. European Journal of Pharmaceutical Sciences. 2017; 96: 290-298. doi: 10.1016/j.ejps.2016.09.011.
CrossRef - Wang, D., Yu, X., Gongyu,an, W., Pullulan production and physiological characteristics of Aureobasidium pullulans under acid stress. Applied Microbiology and Biotechnology 2013; 97: 8069–8077. doi: 10.1007/s00253-013-5094-4.
CrossRef - Wang, D., Ju, X., Zhou, D., Wei, G., Efficient pdroduction of pullulan using rice hull hydrolysate by adaptive laboratory evolution of Aureobasidium pullulans. Bioresource Technology. 2014; 164: 12-19. doi: 10.1016/j.biortech.2014.04.036.
CrossRef - Wang, H., Wan, G., Liu, Y., Chen, B., Chen, H., Zhang, S., Wang, D., Xiong, Q., Zhang, N., Wang, Y., Dual-responsive nanoparticles based on oxidized pullulan and a disulfide-containing poly (β-amino) ester for efficient delivery of genes and chemotherapeutic agents targeting hepatoma. Polymer Chemistry. 2016; 7: 6340-6353. doi: 10.1039/C6PY01664B.
CrossRef - Wang, X., Zhang, D., Wang, J., Tang, R., Wei, B., Jiang, Q., Succinyl pullulan-crosslinked carboxymethyl chitosan sponges for potential wound dressing. International Journal of Polymeric Materials and Polymeric Biomaterials. 2017; 66: 61-70. doi: 1080/00914037.2016.1182912.
CrossRef - Weatherholt, A.M., Fuchs, R.K., Warden, S.J., Specialized connective tissue: bone, the structural framework of the upper extremity. Journal of Hand Therapy. 2012; 25: 123-132. doi: 10.1016/j.jht.2011.08.003.
CrossRef - Wolf, B.W., Garleb, K.A., Choe, Y.S., Humphrey, P.M., Maki, K.C., Pullulan is a slowly digested carbohydrate in humans. The Journal of Nutrition. 2013; 133: 1051-1055. doi: 10.1093/jn/133.4.1051.
CrossRef - Wu, S., Jin, Z., Tong, Q., Chen, H., Sweet potato: a novel substrate for pullulan production by Aureobasidium pullulans. Carbohydrate Polymers. 2009; 76: 645–649. doi :1016/j.carbpol.2008.11.034.
CrossRef - Wu, S., Lu, M., Wang, S., Effect of oligosaccharides derived from Laminaria japonica-incorporated pullulan coatings on preservation of cherry tomatoes. Food Chemistry. 2016; 199: 296-300. doi: 10.1016/j.foodchem.
CrossRef - Xia, Z., Wu, S., Pan, S., Effect of two-stage controlled pH and temperature on pullulan production by Auerobasidium pullulans.Carbohydrate Polymers. 2011; 86: 1814-1816. doi: 5402/2012/140951.
CrossRef - Yang, J., Zhang, Y., Zhao, S., Zhou, Q., Xin, X., Chen, L., Statistical optimization of medium for pullulan production by Aureobasidium pullulans NCPS2016 using fructose and soybean meal hydrolysates. 2018; 23: 1334. doi: 10.3390/molecules23061334.
CrossRef - Zhang, C., An, T., Wang, D., Wan, G., Zhang, M., Wang, H., Zhang, S., Li, R., Yang, X., Wang, Y., Stepwise pH-responsive nanoparticles containing charge-reversible pullulan-based shells and poly (β-amino ester)/poly (lactic-co-glycolic acid) cores as carriers of anticancer drugs for combination therapy on hepatocellular carcinoma. Journal of Controlled Release. 2016; 226: 193-204. doi: 10.1016/j.jconrel.2016.02.030.
CrossRef - Zhou, J., Mohamed Wali, A.R., Ma, S., He, Y., Yue, D., Tang, J.Z., Gu, Z., Tailoring the supramolecular structure of guanidinylated pullulan toward enhanced genetic photodynamic therapy. Biomacromolecules. 2018; 19: 2214-2226. doi: 10.1021/acs.biomac.8b00273.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





