Manuscript accepted on : 28 May 2018
Published online on: 13-06-2018
Ramanjeet Kaur, Lubna Aslam, Nisha Kapoor and Ritu Mahajan
School of Biotechnology, University of Jammu, Jammu.
Corresponding Author E-mail: ritufeb@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2637
ABSTRACT: Wild pomegranate is an ancient fruit with known medicinal and curative properties, attributing to overall positive health. The present study focuses on phytochemical analysis and the antioxidant potential of the fruits (red and green), leaves and flowers of wild pomegranate. High amount of carbohydrates and proteins were observed in red fruits, followed by green fruit, while they were low in flowers and leaves. However, leaves possessed higher amounts of phenolics and tannins as compared to other tissues and fruit extracts. Highest radical scavenging effect was observed in red fruit with EC50 value 70.33µg/ml as compared to other tissues. The ferric reducing potential was significantly higher in red fruit (310.99 ± 0.98 µmol Fe2+/ g dry matter) in comparison to green fruit extracts of wild pomegranate. The results indicated that methanolic extract of red fruits are pharmacologically more active and can be exploited for studying the pharmacokinetics of various bioactive compounds present in wild pomegranate.
KEYWORDS: Antioxidants; Antioxidants; Phytochemicals; Secondary metabolites; Wild pomegranate
Download this article as:| Copy the following to cite this article: Kaur R, Aslam L, Kapoor N, Mahajan R. Phytochemical Analysis and Antioxidant Activity of Wild Pomegranate Collected from Patnitop, Jammu and Kashmir. Biosci Biotech Res Asia 2018;15(2). |
| Copy the following to cite this URL: Kaur R, Aslam L, Kapoor N, Mahajan R. Phytochemical Analysis and Antioxidant Activity of Wild Pomegranate Collected from Patnitop, Jammu and Kashmir. Biosci Biotech Res Asia 2018;15(2). Available from: https://www.biotech-asia.org/?p=29966 |
Introduction
Plants produce a wide range of bioactive molecules referred as secondary metabolites which are used as a source of medicines for treatment of various diseases. Secondary metabolites apart from being safe for human consumption are also environment friendly. Most of the medicinal plants species present in India are used as potent therapeutic agents. Epidemiological studies reveal that consumption of fruits and vegetable rich in phytochemicals, reduces the risk of cancer and chronic degenerative disorders.1,2
Wild pomegranate is an ancient plant which may have been originated from Iran. In India, wild pomegranate grows only in the three regions of north western Himalayas (Jammu and Kashmir, Himachal Pradesh and Uttrakand).3 The commercial use of wild pomegranate is in the form of anardana which is used as acidulent in culinary preparation like curies and chutneys. Most of the parts of the plant are rich in phytochemicals that further contributes to health promoting benefits4. Rind and arils of cultivated and wild pomegranate are rich in ellagic acids, flavonoids, anthocynanins and hydrozyable tannins.5,6
In context to above apprehensions, the present study was undertaken to study different parts of wild pomegranate plant for its phytochemical constituents and antioxidant properties.
Materials and Methods
Collection of Plant Materials
Fruits (green and red), leaves and flowers of wild pomegranate were collected from the Patnitop, Jammu and Kashmir (2,024m above sea level). They were washed with deionised water and disinfected with 0.1% HgCl2 solution for 5 min and then dried in shade. The plant parts were separately grounded to fine powder using an electrical blender, sieved and then stored in vials at 4⁰C.
Preparation of Extracts
Ten grams of powdered material of four samples (flowers, leaves, green fruits and red fruits) were extracted with 20 ml of methanol. After extracting thrice with methanol, the supernatant obtained was air dried using vacuum, in a rotary-evaporator at 40⁰C. The extracts were then stored at 4⁰C for further phytochemical analysis.
Phytochemical Screening
Phytochemical tests were conducted in four samples (tissues and fruits) with the methanolic extracts using the standard methods.7,8
Test for Carbohydrates
One ml of the each extracts was added to 2 ml of molish reagent (α-napthol in 5% alcohol), followed by the addition of 1 ml concentrated H2SO4. Formation of red or dull violent colour at interphase indicated presence of carbohydrates.
Test for Proteins
One ml of methanolic extract of each sample was added to 0.5 ml of 40% sodium hydroxide in tube followed by addition of few drops of 1% copper sulphate. Formation of violet color indicated the presence of proteins.
Test for Flavonoids
One ml each of the extracts was added to 1 ml of 2N sodium hydroxide in a tube. Formation of yellow colour indicated the presence of flavonoids.
Test for Phenols
One ml of each of the methanolic extract was added to 2 ml of 10% ferric chloride in test tube. Formation of blue/green colour indicated the presence of phenols.
Test for Tannins
One ml of the each of methanolic extract was taken in tube to which 1 ml of 5% ferric chloride was added. Formation of dark blue /greenish black colour indicated the presence of tannins.
Test for Terpenoids
One ml of methanolic extract of each sample was added to 2 ml chloroform in test tube followed by addition of concentrated sulphuric acid. Formation of red brown colour at the interface indicated the presence of terpenoids.
Test for Saponins
One ml of each of the extracts was added to 1 ml distilled water in a tube and the mixture was further shaken for 15 min. Formation of 1 cm layer of foam indicated the presence of saponins.
Test for Steroids
One ml of the methanolic extract of each sample was added to 2 ml chloroform followed by the addition of 1 ml sulphuric acid. Formation of reddish brown ring at interface indicated the presence of steroids.
Quantitative Determination
Determination of carbohydrates
Total carbohydrate content in methanolic extracts of four samples was calculated using DNS method.9 DNSA reagents and 40% potassium sodium tartarate were freshly prepared. 1 ml DNSA reagent was added to each tube containing different extracts and incubated for 5 min at 90⁰C, followed by addition of 1 ml potassium sodium tartarate. Absorbance was recorded at 540 nm. Dextrose was used as standard.
Determination of Total Protein Content
Total protein content was estimated using Lowry’s method.10 The reagents prepared were solution A (2% sodium carbonate in 0.1N NaOH) and solution B (0.5% copper sulphate solution in 1% sodium potassium tartarate). Alkaline copper sulphate solution was prepared by mixing solution A and B in the ratio of 50:1. Folin–Ciocalteau (FC) reagent was diluted with equal volume of water just before use. 5ml of copper sulphate solution was added to each extract and incubated at room temperature for 10 min, followed by addition of 0.5 ml FC reagent. Absorbance was recorded at 660nm. BSA was taken as standard.
Determination of Total Phenolic Content
Total phenolics were estimated using Folin-ciocalteau assay.11 1N folin –ciocalteau reagent was freshly prepared and added to 100 µl of each methanolic extract. The reaction was stopped by adding 1ml of 7.5% sodium carbonate and the mixture was further incubated at room temp for 2 hours. Absorbance was recorded at 760nm. Gallic acid was used as standard. Concentration of phenolics of each sample was determined by the following equation:
Absorbance = 0.0364 gallic acid (µg) + 0.009
Determination of Total Tannins
Total tannin content was estimated using method described by Rebaya et al.,12 100 µl methanolic extract of each sample was mixed with 3 ml vannilin solution followed by the addition of 1.5 ml concentrated HCl. The mixture was incubated for 5 min and absorbance was taken at 500nm. Tannic acid was used as standard.
Determination of Antioxidant Activity
The antioxidant activity of all four samples was studied using DPPH assay and FRAP assay.
Diphenyl-1-picrylhydrazyl (DPPH) assay
The DPPH radical scavenging activity was determined by protocol as described by Ebrahimzadeh et al.,13 The DPPH (2,2-diphenyl-1-picrylhydrazyl) free radical is very stable which reacts with compounds having weak A-H bond. Reduction of DPPH by the antioxidants present in extracts results in decolourization of DPPH methanol solution. DPPH reagents were prepared by mixing 18 mg of DPPH in 100 ml of methanol. The 3 µl extracts were mixed with 100 µl of DPPH followed by addition of 2 ml of acetate buffer. The tubes were incubated at room temperature in the dark for 30 min and absorbance was recorded at 517nm. BHT was used as a reference. The results were expressed as percentage reduction of the initial DPPH absorption in relation to the control and IC50 value was determined.
Ferric Reducing Anti-oxidant Power (FRAP assay)
Antioxidant activity of four methanolic extracts was assessed by FRAP assay.14 It is based on the principle of reducing ferric to ferrous ions at low pH that results in the formation of a coloured ferrous –tripyridyltriazine complex. FRAP reagents includes 300 mmol/l acetate buffer (pH 3.6), 10mmol/l TPTZ in 40 mmol/l HCl and 20mmol/l FeCl3. 3 ml of the FRAP reagent was added to 100 µl of each extract and incubated at 37⁰C for 5 min. Absorbance was taken at 593nm using FeSO4 as the standard solution.
Statistical Analysis
All Experiments were conducted in triplicate and results are presented as the mean ± standard Error (SE).
Results and Discussion
Secondary metabolites have pharmaceutical properties that contribute towards human health.15 Many plants rich in flavonoids, alkaloid and terpenoids have anti-cancerous properties.16
Qualitative Phytochemical Analysis
Table 1: Qualitative analysis of phytochemicals in wild pomegranate extracts
| S.No. | Phytochemical tests | Green fruit | Red fruit | Flower | Leaf |
| 1. | Terpenoids | ++ | ++ | ++ | + |
| 2. | Saponins | ++ | + | + | + |
| 3. | Steroids | ++ | ++ | ++ | + |
| 4. | Flavonoids | ++ | +++ | ++ | ++ |
| 5. | Coumerins | ++ | ++ | ++ | + |
| 6. | Tannins | ++ | + | ++ | +++ |
| 7. | Proteins | ++ | +++ | ++ | ++ |
| 8. | Carbohydrates | + | +++ | + | + |
| 9. | Phenolics | ++ | + | ++ | +++ |
(+++) = present in very high amount (++) = High amount (+) = Traces (-) = Absent
Quantitative Phytochemical Analysis
Total Carbohydrate Content
A significant amount of carbohydrate content in different parts of wild pomegranate was observed. Maximum carbohydrate content was observed in the red fruit extract (270.58 mg DE/g) followed by green fruit (149.25 mg DE /g), flower (138.58 mg DE/g) and was least in the leaves (137.41 mg DE/g of extract) (Figure 1). This is due to the process of fruit ripening which causes hydrolysis of starch that leads to accumulation of simple sugars in ripe fruit.17 These results are in agreement with Zarie et al.,18 where increase in total sugar content from 7.40 mg/100g (20 days) to 17.88 mg/100 g (140 days) was observed during ripening in cultivated pomegranate.
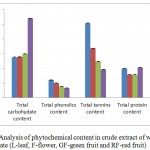 |
Figure 1: Analysis of phytochemical content in crude extract of wild pomegranate (L-leaf, F-flower, GF-green fruit and RF-red fruit)
|
Determination of Total Phenolic Compounds
The total phenolics were estimated using the Folin-Ciocalteau colorimetric reagent. Total phenolic content was highest in leaves (59.67 mg gallic acid equivalent /g of extract), followed by flowers (49.55 mg GAE /g), green fruits (37.90 mg GAE /g) while low phenolics were observed in red fruits (32.86 mg gallic acid equivalents/g) (Figure 1). This decline in the total phenolic level in fruits as compared to tissues is due to the oxidation of phenolic content by polyphenol oxidase that characterizes the stages of maturity.19
Determination of Tannins
The concentration of tannins was recorded highest in leaves (253.98 mg TAE equivalent/g of extract) as compared to flowers (167.84 mg TAE/ g of extracts) (Figure 1). In fruits, green fruits had more tannins (123.55 mg TAE/g of extracts) as compared to red fruits (95.67 mg TAE / g) as the content of total tannins decreases considerably during ripening and low tannins content also reduces the astringency of red fruits. The decrease in condensed tannin content during ripening process is due to increase in activity of enzymes such as anthocyanin synthase and 3-glycosyl transferase which are involved in formation of anthocyanins.20
Determination of Proteins
Highest protein content was observed in red fruit (102.83 mg BSA equivalent /g of extract) while it was only 78.05 mg/g in green fruit. Flower and leaves contained 78.03 mg/g and 99.06 mg BSA equivalent / g of extract of proteins respectively (Figure 1). This is due to synthesis of proteins during ripening which was also observed in ripe fruits of cultivated pomegranate.21
Determination of Antioxidant activity of wild pomegranate extracts
Plants contain many phytonutrients which helps in preventing the damage caused by reactive oxygen species. The antioxidant activity of wild pomegranate extracts was determined by DPPH (2, 2-diphenyl-1-picrylhydrazyl) assay and FRAP (Ferric reducing antioxidant activity) assay.
Antioxidant activity by DPPH assay
Antioxidant activity of different extracts of wild pomegranate was determined by DPPH assay and EC50 value was calculated. Highest antioxidant activity was recorded in red fruits (EC50 =70.33µg/ml) followed by green fruit (EC50 =111.51µg/ml), flower (EC50 = 123.51µg/ml) while it was lowest in leaves (EC50 = 120.78µg/ml).The EC50 value for BHT taken as reference was 23.5 ± 0.551µg/ml (Fig 5). Singh et al.,22 reported the highest antioxidant activity in methanolic extracts of peels and seeds of cultivated pomegranate using various in vitro models while Shiban et al.23 observed the highest DPPH scavenging activity in methanol extracts of fruit peels.
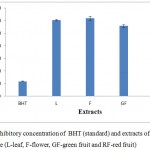 |
Figure 2: Inhibitory concentration of BHT (standard) and extracts of wild pomegranate (L-leaf, F-flower, GF-green fruit and RF-red fruit)
|
FRAP Assay
The antioxidant potential of four extracts of wild pomegranate was determined from their ability to reduce 2,4,6 – tripyridyl-s- triazine (TPTZ) –Fe (III) complex to ferrous form TPTZ –Fe (II) which has an intense blue colour which can be observed by measuring the change in aborption at 593nm. More the blue colour, more is the reducing power. Maximum antioxidant activity was observed in red fruits (310.99 ± 0.98 µmol Fe2+/g of extract) while it was 253.99 ± 0.67 µmol Fe2+/g of extract in green fruit. However, the antioxidant potential in flower was 95.99 ± 0.31µmol Fe2+/g of extract while in leaves, it was 69.99 ± 0.45 µmol Fe2+/ g dry matter (Figure 3). Wang & Lin24 also observed a linear correlation between fruit ripeness and increase in antioxidant potential of fruit. The increase in antioxidant potential is attributed to increase in content of flavonoids and anthocynanins during ripening process. Hajimahmoodi et al.25 observed the higher FRAP values in peel extracts of cultivated pomegranate which contributed to its higher antioxidant activity.
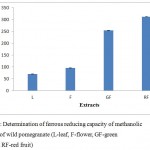 |
Figure 3: Determination of ferrous reducing capacity of methanolic extracts of wild pomegranate (L-leaf, F-flower, GF-green fruit and RF-red fruit)
|
Conclusions and future prospects
The present study proves the use of wild pomegranate plant parts as potential sources of natural antioxidants with efficient antioxidant potential. The high antioxidant activity of the methanolic extracts is due to the presence of phenolics and tannins. So, the plant parts can be used as potent source of secondary metabolites in pharmaceutical industries.
Acknowledgements
The authors are thankful to INSPIRE fellowship and DST, New Delhi for providing the financial support as major research project on wild pomegranate. The authors are also thankful to School of Biotechnology, University of Jammu for providing the basic facilities to carry out this research work.
Conflict of Interest
Authors would hereby like to declare that there is no conflict of interests that could possibly arise.
References
- Dujaili E.A.S. Natural Polyphenols: Potential for Disease Prevention. EC Nutr. 2012;2(2):337-345.
- Wang T.Y, Li Q, Bi K.S. Bioactive flavonoids in medicinal plants: Structure, activity and biological fate. J. Pharm. Sci. 2018;13:12-23.
CrossRef - Mahajan R, Javed A, Kapoor N. Characterization of genetic diversity of wild pomegranate collected from Himachal Pradesh. India. Pl. Sci. 2018;7.2:2042-2046.
CrossRef - Noda Y, Kaneyuki T, Mori A, Packer Antioxidant activities of pomegranate fruit extract and its anthocyanidins: delphinidin, cyanidin, and pelargonidin. J. Agr. Food. Chem. 2002;50:166-171.
CrossRef - Sudhakar S, Venugopal D, Vignesh D, Karthikeyan S. Anticancer Activity of the Pomegranate and Their Role in Cancer Prevention and Therapy. J. Life. Sci. Res. 2015;3.3:77-84.
- Wu S, Tian L. Diverse Phytochemicals and Bioactivities in the Ancient Fruit and Modern Functional Food Pomegranate (Punica granatum). 2017;22:1606; doi:10.3390/molecules22101606.
CrossRef - Yu Q, Qi J, Yu H.X, Chen L.L, Kou J.P, Liu S.J, Yu B.Y. Qualitative and quantitative analysis of phenolic compounds in the leaves of Aquilaria sinensis using liquid chromatography-mass spectrometry. Anal. 2013;24:349-56.
- Gul R, Jan S.U, Faridullah S, Sherani S, Jahan N. Preliminary Phytochemical Screening, Quantitative Analysis of Alkaloids, and Antioxidant Activity of Crude Plant Extracts from Ephedra Intermedia Indigenous to Balochistan. World. J. 2017;5873648.
- Miller G. L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Chem. 1959;31:426–428.
CrossRef - Lowry O. H, Rosebrough N. J, Farr A. L, Randall R. J. Protein measurement with the Folin phenol reagent. Biol. Chem. 1951;193:265-275.
- Ainsworth E.A, Gillespie K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin- Ciocalteu reagent. Protoc. 2007;2: 875–877.
CrossRef - Rebaya A, Belghith S.I, Baghdikian B, Leddet V. M, Mabrouki F, Olivier E, Cherif J.K, Ayadi M.T. Total Phenolic, Total Flavonoid, Tannin Content, and Antioxidant Capacity of Halimium halimifolium (Cistaceae). Appl. Pharma. Sci. 2014;5:052-057.
- Ebrahimzadeh M.A, Hosseinimehr S.J, Hamidinia A, Jafari M. Antioxidant and free radical scavenging activity of Feijoa sallowiana fruits peel and leaves. Ol. 2008;1:7-14.
- Chai T.T, Wong F.C. Antioxidant properties of aqueous extracts of Selaginella willdenowii. Med. Plants Res. 2012;6:1289-1296.
- Zhang J, Wu Y, Zhao X. Chemopreventive effect of flavonoids from Ougan (Citrus reticulata cv. Suavissima) fruit against cancer cell proliferation and migration. Funct. Foods., 2014;10:511–519.
CrossRef - Patel K, Kumar V, Rahman M, Verma A, Patel D.K. New insights into the medicinal importance, physiological functions and bioanalytical aspects of an important bioactive compound of foods Hyperin: Health benefits of the past, the present, the future. Beni-Suef. Univ. J. Appl. Sci. 2018;7:31–42.
CrossRef - Villanueva M.J, Tenorio M.D, Esteban M.A, Mendoza M.C. Compositional changes during ripening of two cultivars of muskmelon fruits. Chem. 2004;87:179-185.
CrossRef - Zarie M, Azizi M, Bashir S.Z. Evaluation of physicochemical characteristics of pomegranate (Punica granatum) fruit during ripening. Fruits. 2011;66:121–129.
CrossRef - Maiman S.A.A, Ahmed D. Changes in physical and chemical properties during pomegranate (Punica granatum) fruit maturation. Food. Chem. 2002;76:437-441.
CrossRef - Robbins M.P, Bavudage D, Strudwicke C, Morris P. Genetic manipulation of condensed tannins in higher plant. Plant. Physiol. 1998;116:1133–1144.
CrossRef - Anand P, Aradhya S. M. Chemical changes and antioxidant activity in pomegranate arils during fruit development. Food. Chem. 2005;93:319-324.
CrossRef - Singh R.P, Chidambara K.N, Jayaprakasha G.K. Studies on the antioxidant activity of pomegranate (Punica granatum) peel and seed extracts using in vitro J. Agric. Food. Chem. 2002;50:81-86.
CrossRef - Shiban M.S, Mutlag M.A, Najeeb S, Zoreky A. Antioxidant Activity of Pomegranate (Punica granatum) Fruit Peels. Food. Nutr. Sci. 2012;3:991-996.
CrossRef - Wang S.Y, Lin H.S. Antioxidant Activity in Fruits and Leaves of Blackberry, Raspberry, and Strawberry Varies with Cultivar and Developmental Stage. Agric. Food. Chem. 2000;48:140–146.
CrossRef - Hajimahmoodi M, Oveisi M. R, Sadeghi N, Jannat B, Hadjibabaie M, Farahani E, Akrami M. R, Namdar R. Antioxidant properties of peel and pulp hydro extract in ten Persian pomegranate cultivars. Pak. J. Biol. Sci. 2008;11:1600-4.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





