Manuscript accepted on : 14 February 2017
Published online on: --
Plagiarism Check: Yes
Recent advances and future Perspectives of thermostable xylanase
E. Selvarajan and R. Veena
Department of Genetic Engineering, School of Bioengineering, SRM University, Kattankulathur, Tamil Nadu, India.
Corresponding Author E-mail: selvarajan.e@ktr.srmuniv.ac.in
DOI : http://dx.doi.org/10.13005/bbra/2461
ABSTRACT: The xylan degrading enzyme, xylanase can be used to develop eco-friendly technologies mainly in the paper and pulp industries. By using this enzyme, the lignocelluloses materials can be modified to produce high quality liquid fuel and other products. There is a wide range of applications for the xylanase as an enzyme and more with thermostable xylanase. The Fungal strains are considered most potent for xylanase production, while the yeast and bacteria produce it in low quantities. The production of these enzymes, at low quantity, can be further enhanced by the Genetic engineering techniques like mutation, cloning and expression in various organisms. The genomic studies have helped to come across the basic barriers like low production, enzyme stability etc. The xylanase producing gene is isolated in microorganisms, made modifications and is cloned into a heterologous or a homologous host for the enhanced production, to meet the industrial demand. Thus this review concentrates about the production parameters, immobilization techniques and the applications briefly.
KEYWORDS: Applications Classification; Genetic engineering; Immobilization; Production; Xylanase;
Download this article as:| Copy the following to cite this article: Selvarajan E, Veena R. Recent advances and future Perspectives of thermostable xylanase. Biosci Biotech Res Asia 2017;14(1). |
| Copy the following to cite this URL: Selvarajan E, Veena R. Recent advances and future Perspectives of thermostable xylanase. Biosci Biotech Res Asia 2017;14(1). Available from: https://www.biotech-asia.org/?p=21967 |
Introduction
Thermo stability is the resistance to the thermal inactivation and the thermo stable enzymes like the thermo stable xylanase are most preferred in the industrial applications. In other words, thermo stability can be explained as the requirement of higher optimum temperature for the organism to grow or exceptional stability above 50°C over extended period of time. The thermal stability can also be related to the half life (t1/2). (t1/2, the time required to reduce activity by 50% at a stated temperature provided that the inactivation follows first order kinetics). Higher the thermo stability, higher will be the half life. For example, xylanases produced by thermophilic eubacteria and archaebacteria have considerably longer t1/2 at 80°C or higher temperatures than those from thermophilic fungi (Dahlberg et al, 1993). This thermostability can also be achieved by the introduction of the disulphide bonds in certain microorganisms (Jeffries et al, 1996). The Thermoanaerobacterium xylanase resists the thermal denaturation (SHAO et al, 1994). The thermostable enzymes carry out the enzymatic hydrolysis at high temperature. Thus high temperatures lead to higher mass transfer rates and low viscosity helping in the solubility of the products and reactants. It also reduces the risk of contamination of the mesophilic microbes. But the yields obtained from the thermophils are much lower compared to mesophilic microbes. Thus the yield can be increased by rDNA technology using heterologous host vector system. When considering only temperature, a handful of xylanases that show optimum activity at higher temperatures have been reported from various microorganisms. These include Geobacillus thermoleovorans, Streptomyces sp, Bacillus firmus, Actinomadura sp and Saccharopolyspora sp all of which produce xylanases that show activity between 65 and 90°C. One xylanase, reported from Thermotoga sp has been shown to exhibit a temperature optima between 100 and 105°C.
Xylanases are the class of enzymes, which degrades or hydrolyses the beta 1, 4 Xylan into xylose, a fermentable sugar with high market price. Generally, plant cell wall contains the lignocellulose aromatic compound that has cellulose (45-55%), xylan (25-35%) and lignin (20-30%). Xylan, a renewable hemicelluloses is a second most abundant complex heterogeneous polysaccharide composed of homopolymeric backbone of 1, 4-linked β-D-xylopyranose residues and short chain branches consisting of O-acetyl, α-L-arabinofuranosyl and α-D-glucuronyl residues. It constitutes of 25% hard wood and the rest soft wood and is the major component of the plant cell wall. Hemicelluloses may serve in the matrix as flexible bridges and act as a coat between and around cellulose fibrils (Saha.B, 2003). The chemical and structural composition of xylan and its concentration is different in various plants. Few eukaryotic microorganisms use xylan as the chief carbon source. Endo-xylanase depolymerizes the xylan and also produces several auxiliary enzymes those are necessary for debranching of the substituted xylan (Haltrich et al., 1996).
Xylanases are produced by fungi, bacteria, yeast, marine algae, protozoan, snail, crustaceans, insects, seeds and filamentous fungi but not by mammals. The chief source of xylanase is from filamentous fungi that give higher yield compared to other micro-organisms but the mesophilic fungi does not have the thermo stability. These fungi can also produce cellulase that has to be separated and removed. A few aerobic or anaerobic thermophilic fungi like Thermomyces spp., Humicola spp . (Anand et al), and Aspergillus spp. are capable of producing xylanases only in acidic growth conditions. Xylan and cellulose induces the activity of xylanase, while the readily metabolizable glucose or glycerol inhibits the activity .It can be produced either by solid state fermentation (SSF) or submerged state fermentation (SMF) methods. Enzyme productivity in solid state fermentation (SSF) is higher than that of submerged fermentation (Haltrich et al., 1996) and it is cost effective. The common agricultural residues like the wheat bran and other agricultural wastes are used as substrate in the SSF, while the enzyme is immobilized on the nanoparticles to enhance its activity.
It has many applications in the paper and pulp industry. It basically removes lignin carbohydrate complex (LCC), which is produced during Kraft process and serves as a barrier to the toxic bleaching chemicals like chlorine components. The chemical bleaching process gives out byproducts that are mutagenic, toxic, bio accumulating and may cause disturbance in the bio-system. Due to this reason, the government and the environmentalists ban the use of chlorine products. The best alternate for this can be the usage of cellulase-free xylanase that ensures no damage to the tender pulp fibers and is also used to provide high quality, easily dissolvable pulps. The major commercial applications include chlorine free bleaching of the wood pulp for paper making process. It is also used to increase the digestibility of the silage, poultry, food additives in wheat flour and fermentative composting.
There are studies about the genes that are responsible for xylanase production in a micro-organism. It says that the genes encoding for the xylanase fall either in family 10 (F) or 12 (G), can be cloned into E. coli from the mesophills or thermophills (Shao et al). Thus the gene can be further characterized. The main research now is not only about the hydrolysis of xylan, but the stability of the xylanase enzyme under various conditions like optimum temperature, pH etc. As mentioned earlier, certain types of wild xylanase have desired characteristics like thermostability, pH tolerance etc. But no individual xylanase can provide all the desired characteristics together. This rare type of xylanase that is obtained in a very meager quantity is much needed by the industry at affordable cost, for the viability of the process. The Genetic engineering and Recombination technology plays key role in the large scale production of xylanase. By holding on the future prospects of the xylanase in its application, this review gives a brief about the isolation, production, genetic significance and industrial applications of xylanase. In the article structure of xylan and xylanase are also presented.
Xylan Structure
The term ‘Hemicellulose’ was first coined by Schulze (1891) to represent the isolate obtained from plants that is diluted with alkali .These are the hetero polymers containing the Xylan and consist of D-xylose monomeric units and traces of L-arabinose. The plant cell wall contains lignocelluloses compound that contains 30-35% of Xylan which has homopolymeric backbone of 1, 4-linked β-D-xylopyranose residues and short chain branches consisting of O-acetyl, α-L-arabinofuranosyl and α-D-glucuronyl residues. It occurs mostly in hardwood of angiosperms and less in softwood of gymnosperms. The Xylan from hardwood is 0-acetyl-4–0-methylglucuronoxylan. This polysaccharide consists of at least 70 β-xylopyranose residues. Every tenth xylose residue carries a 4-0-methylglucuronic acid attached to the 2 position of xylose. Thus hardwood Xylan is highly acetylated, degrades on acetyl xylan esterase and thus responsible for partial solubility in water (e.g. Birchwood Xylan). These acetyl groups are removed with addition of alkali. While, Xylan from softwood are composed of arabino-4-0- methylglucuroxylans. These softwoods does not contain acetyl groups instead they have α-L-arabinofuranose units linked by α-1, 3-glycosidic bonds at the C-3 position of the xylose (beg et al, 2001). There are four different types of xylan in general. They are
Arabinoxylans, having only side chains of single terminal units of α-L-arabinofuranosyl substituents. In the particular case of cereals, arabinoxylans vary in the degree of arabinosyl substitution, with either 2-O- and 3-O-mono-substituted or double (2- O-, 3-O-) substituted xylosyl residues.
Glucuronoxylans, in which α-D-glucuronic acid or its 4-O-methyl ether derivative represents the only substituent.
Glucuronoarabinoxylan, in which α-D-glucuronic (and 4-O-methyl-α-D-glucuronic) acid and α-L-arabinose are both present.
Galactoglucuronoarabinoxylans, which are characterized by the presence of terminal β-D-galactopyranosyl residues on complex oligosaccharide side chains of xylan and are typically found in perennial plants.
In plants, these Xylans are present in between the lignin and cellulose layers. These xylan intertwine and form covalent bonds with the sheath of the lignin and produce a layer over the cellulose fibers. This maintains the integrity of the cellulose by non-covalent interaction between the lignin and the Xylan. Thus preventing the cellulose degradation by cellulase and helping with the fiber cohesion (beg et al,2001). These Xylans can also be isolated from esparto grass (Chanda et al., 1950), tobacco stalks (Eda et al., 1976), Marine red algae (Barry et al, 1940), Red seaweed (Tseng et al, 2002).
Enzymatic hydrolysis of xylan
Xylans are well known for their homogeneity and complex chemical nature. Due to this, the complete breakdown requires several complex hydrolytic enzymes with different mode of actions. This proves that the xylan degrading cells are the arsenal of polymer degrading proteins. (Beg et al, 2001). The xylanolytic enzyme system that carries out the xylan hydrolysis is normally composed of a repertoire of hydrolytic enzymes, including endoxylanase (endo-1, 4-β-xylanase, E.C.3.2.1.8), β-xylosidase (xylan-1,4-β-xylosidase, E.C.3.2.1.37), α- glucuronidase (α-glucosiduronase, E.C.3.2.1.139), α-arabinofuranosidase (α-L arabinofuranosidase, E.C.3.2.1.55) and acetyl xylan esterase (E.C.3.1.1.72) (V. Juturu et al, 2012). These convert xylan to simple sugars while the endo xylan cleaves the gycosidic linkage and liberate short xylo-oligosaccharides.
Xylan, being the high molecular weight cannot penetrate into the cell wall while the small fragments like xylose, xylobiose, xylo oligosaccharides, heterodisaccharides of xylose and glucose and their positional isomers, play a chief role in regulating xylanase biosynthesis. Xylanase catalyzes the random hydrolysis of xylan to xylooligosaccharides, while β-xylosidase releases xylose residues from the non reducing ends of xylo-oligosaccharides. The acetyl esterase removes the acetyl group from the beta 1, 4 D-xylose backbone of xylan (D.Verma et al,2012).
Structure of Xylanase
Xylanase comes from the glycosyl hydrolase family, which is further classified into 90 other families based on the amino acid sequences. These xylanases catalyze the hydrolysis of β 1, 4 glycosidic linkage of xylosidase, forming a Hemiacetal sugar and free aglycone non sugars, by replacing it with hydrogen bonds. The xylanase can be classified in three ways: based on molecular weight, isoelectric point and crystal structure and kinetic property. There are 2 major types of xylanase activities: Endoxylanase and exoxylanase. Endoxylanase like endo-1, 4-xylanases, show a preference for internal Xylan bonds. Exoxylanase like exo-1, 4-xylanases, which show a preference for side groups at the terminals of Xylan chains. These Xylanases falls under the 10th and 11th family of glycosyl hydrolase. The 10th family of Xylanases has a molecular weight of approximately 35 kDa and a TIM-Barrel structure that possess the 8 alpha-1-beta chains. The 11th family of Xylanases has the molecular weight of approximately 22 kDa and a jelly-roll structure consisting of two twisted -sheets stacked face to face. The structure is compared with a hand where the palm represents the active centre and the thumb consists of 2 beta strands that covers the active centre and is found in the plant lectins. Not all Xylanases have a high molecular mass (above 30 kDa) and low pI or a low molecular mass (less than 30 kDa) and high pI. Therefore, a more complete system, based on the primary structure and comparison of the catalytic domains, was introduced, analyzing both the structural and mechanistic features (Collins et al, 2005). The characteristics of these enzymes can be found in the carbohydrate database from the NCBI. According to the database, the Xylanases are related to glycoside hydrolase (GH) families 5, 7, 8, 9, 10, 11, 12, 16, 26, 30, 43, 44, 51 and 62. However, the sequences classified in families 16, 51 and 62 appear to be bifunctional enzymes containing two catalytic domains, unlike families 5, 7, 8, 10, 11 and 43, which have a truly distinct catalytic domain with endo-1, 4-β-xylanase activity. Using the same analysis, families 9, 12, 26, 30 and 44 may have residual or secondary xylanase activity. While after the brief study, it is found that the class 5, 7,8,43 are recent and remain very limited. Families 5, 7, 8, 10, 11 and 43 differ in their physicochemical properties, structure, mode of action and substrate specificities (Collins et al, 2005). The anomeric centre of the reducing sugar monomer of the carbohydrate undergoes either retention (families 5, 7, 10 and 11) or inversion (family 8 and 43) (Subramaniyan.S et al, 2002). Family 11 Xylanases have 11 various representative structures in fungi and bacterial species (Table1). Among these the Paecilomyces variotii, Thermomyces lanuginosus and Dictoglomus thermophilum isozymes are thermostable. (Oakley et al, 2003).
Table 1: Xylanase produced from different microrganism
| S.No. | Micro-organism | Source | Isolation media | Substrate | Type of fermentation | Reference: |
| 1. | Trichoderma.sp | Soil sample (uttarpradesh) | Potato-dextrose medium | Birchwood xylan | SSF with vogal’s medium; BOD incubator | [49] |
| 2. | Thermomyces lanuginosus | Soil sample (Durban, South Africa) | Potato-dextrose medium | oatspelts xylan or birchwood xylan | – | [60] |
| 3. | Aspergillus fumigatus | paper industry effluent (Cheranmahadevi, India) | Potato-dextrose medium | Oatspelts xylan ,carboxymethyl cellulose and avicel | – | [4] |
| 4. | Crocus sativus | barley leaves infected with spot blotch symptoms | Potato dextrose agar | Birchwood xylan | SSF | [6] |
| 5. | Trichoderma longibrachiatum | Papersludge sample
(Syracuse, NY) |
potato dextrose agar | Oat spelt xylan, Larch xylan, Poplar xylan | – | [53] |
| 6. | Thermomyces lanuginosus | MIRCEN (Bangkok,
Thailand) |
Basal medium with glucose | Oat spelt xylan | – | [36] |
| 7. | Thermomyces lanuginosus | Purchased from ATCC | yeast
glucose agar plate |
oat spelt xylan or xylose | – | [33] |
| 9. | Chromohalobacter sp. | Soil sample
(tuticorin, tamil nadu) |
Agar medium | Oatspelts xylan | – | [51] |
| 10. | Bacillus pumilus SV-205 | soil samples
(Ambala Cantt., Haryana) |
nutrient agar | Birchwood xylan | SMF | [44] |
| 11. | Streptomyces sp.
RCK-2010 |
soil samples ( DU,Delhi) | xylan agar, Horikoshi medium | Birchwood xylan | SMF with OFAT approach | [37] |
| 12. | Bacillus firmus | Waste water sample from paper industry (Thailand) | Berg’s mineral salts medium | Birchwood xylan | – | [17] |
| 13. | Thermoanaerobacterium sp | Water sample from hot spring pool,
Yellowstone National Park,Wyoming |
Birch xylan medium | oat spelt xylan | 100-liter fermentor under anaerobic conditions | [58] |
| 14. | Streptomyces sp | decaying coconut
fibers (goa, India) |
actinomycete isolation agar | wheat bran
and eucalyptus kraft pulp |
SSF | [10] |
| 15. | Bacillus sp. | Addis Ababa, Ethiopia | High moisture medium | Birchwood xylan | SSF | [27] |
| Bacillus subtilis | hot-spring,
(Azores (Portugal) |
Luria-Bertani (LB) media | Xylose | – | [54] | |
| 16. | Bacillus firmus | Wastewater sample from paper industry
|
Berg’s mineral salts medium | Birchwood xylan | – | [65] |
| 17. | Geobacillus sp. | Compost facility | Agar plates with azo xylan | Birchwood xylan | – | [13] |
| 18. | Bacillus Pumillus | Chandigarh, India | Nutrient Agar | Birchwood xylan | SMF | [38] |
| 19 | R. marinus R-10 (DSM 4252) | submarine alkaline hot
spring in Iceland |
Nutrient agar | Birchwood xylan | – | [22] |
| 20. | Cryptococcus adeliae | Antarctic region, | Potato dextrose agar | Birchwood xylan | – | [28] |
Classification of Xylanase
Family 10 and 11 Glycoside Hydrolase
Generally, the xylanase can be classified either as family 10 or 11. Class 10 xylanase has low molecular weight and the isoelectric point between 8-9.5 while the class 11 has high molecular weight and low isoelectric point. It is said that the beta 1,4 or 1,3 glycosidic bonds tend to hydrolyze aryl β-glycosides of xylobiose and xylotriose at the aglyconic bond. The class 11 Glycoside Hydrolase has purely xylanase. So it is otherwise called as true xylanase. These family 11 xylanase can also hydrolyze Aryl-cellobiosides. In a certain research, the team has isolated a psychrophilic xylanase that belongs to family 8 and which has both a high pI and high molecular mass. This novel xylanase, isolated from the Antarctic bacterium Pseudoalteromonas haloplanktis, is not homologous to family 10 or 11 enzymes but has 20–30% identity with family 8 members. NMR analysis shows that this enzyme hydrolyzes with inversion of anomeric configuration, in contrast to other known xylanases which are retaining. No cellulase, chitosanase or lichenase activity was detected. It appears to be functionally similar to family 11 xylanases. It hydrolyzes xylan principally to xylotriose and xylotetraose and is most active on long chain xylo-oligosaccharides. The class 10 xylanase is more versatile and has low specificity compared to class 11 xylanase. The binding sites for xylose residues in xylanases are termed subsites, and bond cleavage occurring between the sugars residues at the -1 (non-reducing) and the +1 (reducing) ends of the substrate. As reported in assays using arabinoxylan as the substrate, class 10 products have arabinose residues substituted on xylose at the +1 subsite, whereas Class 11 products have arabinose residues substituted at the +2 subsite. These results show that Class 10 enzymes are able to hydrolyze xylose linkages closer to the side (F.L.Motta et al, 2013). Therefore, xylanases from family 11 preferentially cleave the unsubstituted regions of the arabinoxylan backbone, whereas GH10 enzymes cleave the substituted regions along the xylan backbone. The degree of substitution in xylan will influence the hydrolytic products.
Trichoderma reesei produces both these types of Xylanases: xyn1 and xyn2 (anneli et al, 1992). The xyn2 of T. reesei has 5 xylopyranose while xyn1 has 3. The xyn1 has acidic pI of (5.5) pH while xyn2 has a basic pI of (9.0) pH. The xyn1 and xyn2 differ in the lysine and arginine residues by 4 in xyn1 and 10 in xyn2. These react with glucuronic acid side chains of the xylan. This binding reaction occurs only in the acidic pH or they unbound in the basic pH. The catalyst is necessary to maintain a proper pH and has Asparagines amino acid with hydrogen bonding in all the class 11 xylanase. Only Schizophyllum commune xylanase from class 11 is categorized under acidic pI with amino acid arrangements but actually fall under basic category, since the Asp reacts with the acid/basic catalyst. The xyn1 has the Glu 164 linked by Asp 33 and for xyn2 has Glu177 linked by Asp 44. Alkaline-active and extreme-thermophilic enzymes are of current interest of research (Jeffries et al, 1996)
The protein sequence of the xylanase is studied either by pair wise alignment sequences or by the basic local alignment search tool (BLAST). This blast tool is used to identify the sets of mutually exclusive enzymes. The crystallographic studies reports that the major family 1 or A has a protein fold and an active site similar to those of Family 10 xylanase. Members of Family 10 will act on both p-nitro phenyl (PNP)-xylobiose and PNP-cellobiose but however, the overall catalytic efficiency on PNP-xylobiose is -50-fold higher (Jeffries et al, 1996). This suggests that Family 10 enzymes act mainly on xylan residues and also due to the relatively greater solubility of the xylan substrate and the higher reactivity of the xylan glycosidic linkage it increases the hydrolytic rate for xylan compared with cellulose. There are again two major subclasses of thermostable Xylanases namely, xynA and xynB. XynA was reported to have molecular weight of 120kDa and belong to family 10 while the xynB has a molecular weight of 40kDa and belong to family 11 and have optical activity at 92°C and 105°C.
Family 5, 7, 8 and 43 of Glycoside Hydrolase
The class 5 GH is otherwise called as class A, which is one of the biggest families of GH with 7 amino acid residues. Moreover, the xylanase activity is affected by substituents on the xylan backbone, and is unable to cleave linkages adjacent to substituted residues. Hydrolysis studies have shown that the internal xylose residue is substituted by the short fragments called xylotrioses, which is formed from the glucuronoxylan and arabinoxylan. Therefore, the products produced by family 5 are shorter than those produced by family 7 (T.Collins et al, 2005).
The class 8 GH is otherwise called as class D, which contains cellulases, chitosanases, lichenases and endo-1,4, beta Xylanases. T.Collins et al, 2005 reported that these class 8 enzymes, unlike the class 10 and 11, catalyze hydrolysis with the inversion of the anomeric configuration and, under certain conditions, it is found to be inactive on aryl β-glycosides of xylose, xylobiose and xylotriose.
The class 7 GH does the xylanase activity and is yet to research more on it. The class 7 shows similarity with class 10 and 11 GH as they have high molecular weight, low pI value and has 4 subsites with catalytic site at the middle. But class 43 of GH can be compared with class 62 of GH or family F. It is said that this class may display a five-blade β-propeller fold and a glutamate and aspartate in the center of a long V-shaped surface groove formed across the face of the propeller have been reported as the catalytic residues, while its members are believed to catalyze hydrolysis via a single displacement mechanism (Collins et al, 2005).
Genetic Significance
Genetic engineering has enhanced thermostability, but not always with the desired activity at the elevated temperature. The type 1 xylanase (Xyn10A) or type2 (Xyn11A), which is produced by certain micro-organisms, can be cloned by knowing the genetic code. For isolation of the upstream region, in Bacillus Subtilis B230 the genomic DNA was digested with HindIII, self-ligated at 289 K and subjected to PCR with inverse primers for xfor2 (50-GGA ACC GTA TCTGTC AGC GAA C) and xrev1 (50-CCA CTG GGC ACTGAA CGC ACC). A 950 bp PCR fragment was purified, cloned into pGemT and sequenced. The sequence contained 438 bp ORF which showed high homology to the carboxyterminus of the B. subtilis xynB gene, an intergenic region with several potential promoter motifs, a typical B. subtilis ribosome-binding site and the coding region for a 27 amino acid leader peptide. The downstream region was isolated by inverse PCR on EcoRI-digested self-ligated B230 genomic DNA with PCR primers xfor3 (50-CCG CGC TTA CGG TAG AAG GC) and xrev2 (50-CGT TCC CGT CGG TCT GTA GGT GC). The PCR products were cloned and sequencing revealed a known part of the Xyn11X coding region, 114 bp coding for the last 38 amino acids of Xyn11X, plus an intergenic region of 190 bp and the start of an ORF which showed 85% identity with the B. subtilis PPS (phosphoenolpyruvate synthase) gene. To confirm the data obtained from these three cloning steps, primers to the end of the xynB gene; 50-AAT GATAAG TGT GGT TCG TAA TGG) and the start of the PPS gene (PEP-rev1; 50-TCC AGT TCA TGT AAA CCA AGTACC) were used for PCR on B230 genomic DNA. (Oakley et al, 2003). P.Chang et al, 2004 has reported the cloning that is done with the E-coli as a host and is maintained on Birchwood xylan LB agar. The cloned product is confirmed by the xylan–Congo red clearance plate assay (where the clones with Xyn10A gene showed more zone of clearance than the clones containing Xyn11A gene) also the Restriction enzyme analysis is done to identify positive clones harbored different recombinant plasmid DNAs containing 4.0kb (pI:4.5) and 2.0 kb inserts pI(9.6), respectively.
The nucleotide sequences was inferred to be 4kb insert of length 204 amino acids, having three open reading frames (ORF) and the proteins that does not have cysteine residues in the Xyn10A, While, the Xyn11A the nucleotide sequence was inferred to be 2kb insert of length of 134 amino acids, having two ORF and the protein with one cysteine residue (Cys-20) and thus can be further read for better understanding and to find out the homology with other organisms. Deduced amino acid sequence of Xyn11A gene for the family 11 xylanase of various bacteria and fungi are also studied. The sub cloning has is done for the ORF of the inserts, followed by the zymographic analysis by SDS PAGE. The following report has showed the activity bands at 45kDa and 23kDa for the Xyn10A and Xyn11A. (P.Chang et al, 2004). The heterogeneous nature of xylan, along with the multiplicity of xylanases in microorganisms may be caused by a redundancy in gene expression. Generally, a single xylanase gene encodes multiple xylanases, and xylanase multiplicity may arise from posttranslational modifications, such as differential glycosylation, proteolysis or both (Juturu et al, 2012).
The Xylanase producing gene Xyn10N18 was screened and obtained from the rumen of the grass/ hay fed bovine, is identified by metagenomic library to have a gene cluster that is involved in the xylose metabolism. The xylanolytic gene cluster shuffling encodes a putative α-L-arabinofuranosidase, a putative xylanase that encodes for a protein of molecular mass 54.5 kDa, similar to the class GH10 and the carbohydrate active enzymes and thus reported to secrete only xylanase enzyme without the cellulase enzyme (Gong et al,2012). The same is done with the compost soil and a xylanase producing gene xyn10J was obtained and the site directed mutagenesis is done to enhance the production (Jeong et al, 2012). Similarily, the thermostable xylanase encoding gene xyn10CD18 was directly cloned from the metagenomic DNA of cow dung compost and expressed in bacillus megaterium MS941, showed the extra-cellular xylanase activity at 106 IU/ml. This proves that the xyn10CD18 is a Endoxylanase and has efficient usage in biomass conversion and oligosaccharide production (sun et al,2015). Based on the genomic DNA sequence, the 2 xylanase producing genes, JX030400 and JX030401 were identified from Caldicellulosiruptor sp. F32, which shows close homology of 97% with Csac 0696 of C. saccharolyticus DSM 8903 and 94% with Athe 0089 of C. bescii DSM 6725. This was not observed in the Caldicellulosiruptor saccharolyticus DSM 8903, which showed up 99% close homology with the Caldicellulosiruptor sp. F32. The gene JX030400 is reported to show 9 folds high specificity than the other gene and the strain F32 is found to produce 2.5 times more xylanase activity than its close homology DSM 8903 (ying et al, 2013).
The Xylanase gene XynAM6 is isolated from Streptomyces megasporus DSM 41476 using colony PCR screening method. This xylanase comes under the class 10 and is 1440 base pair long contributing 479 amino acid peptide molecules. The matured peptide of xynAM6 is expressed in Pichia pastoris GS115. This resulted in the xylanase with good temperature adaptability and resistance to proteases (qiu et al, 2010). The gene M2C38 endo xylanase from the Trichoderma Reesei is also responsible for xylanase production. By adding glycosylation site to this gene by replacing the amino acids like Asn at position 131 and Ser/Thr at position 133, the enzyme production can be enhanced (Juturu et al, 2012). The novel xylanase gene Xyn11E which is identified in the Paenibacillus barcinonensis BP-23, is reported to belong to the class 11 GH with a predicted molecular weight of 20.65kDa. It is also observed that the highly active xylanase enzyme does not depend on its own LppX in active form, for its expression. The Co-expression with E-coli chaperone has substituted this LppX necessity while acting as the specific chaperone for correct folding. Thus is said to play major role in paper-pulp industries (Valenzuela et al, 2014). Another novel xylanase producing gene Auxyn11D is obtained from aspergillus usamii E001. The whole cDNA is studied using the Rapid Amplifier cDNA Ends (RACE) and the cloning and expression studies are done by doing recombination with pichia pastoris (Zhang et al,2012). The gene axy43A from Paenibacillus curdlanolyticus B-6 encodes for a trifunctional xylanolytic enzyme which after recombination exhibited endo-xylanase, β-xylosidase, and arabinoxylan 31 arabinofuranohydrolase activities. It is also reported that the crude P.curdlanolyticus B-6 endo-xylanase Xyn10C and the recombinant Asy43A works synergistically in combination, to efficiently produce arabinose and xylose (Teeravivattanakit et al, 2016). Similarily, another novel, alkali tolerant, thermostable xylanase gene Svixyn10A is identified from the actinomycetes Saccharomonospora viridis and has 1,374 bp. It is reported to show 57% close identity with Streptomyces lividans. The further recombination is done by tagging histone to the xylanase, cloned and expressed in E-coli strain (Wang et al, 2012).
Isolation of Xylanase Producing Microorganisms
Thermostable Bacillus subtilis sp. B230 which has the thermostability upto 343K is isolated from white-rotted karri wood collected near Walpole, Western Australia and reported to secrete the type 2 xylanase (Xyn11X) (Oakley et al, 2003). Bacillus circulans are again a type of bacillus, that is reported to secrete xylanase enzyme at an optimized condition (Pithadhia et al,2016). Another novel producer of xylanase: Bacillus pumilus SY30A is identified and id reported to serve as a great use in pretreatment of the unbleached oil palm soda-anthraquinone pulp (Bakri et al,2016). Fungi are also a well known organism to produce the xylanases as well. Different samples of decomposing manures, dead and decaying wood and soil enriched with lignocelluloses from Uttarpradesh, Uttarkhand and Rajasthan were collected and the isolation of xylanase producing fungi was done by dilution plate method. Once the strains are identified, they are maintained on the potato dextrose agar slants at 4°C (Gautam et al, 2015). The Trichoderma species are also the type of fungi that is widely used as biocontrol against the plant pathogen that causes disease to plants. Chitinases, Xylanases Glucanases, and proteases, are major lytic enzymes that are secreted by biocontrol agents. There are about 41 species in Trichoderma genus. One of such Trichoderma species was isolated from the soil sample in Uttarpradesh. The isolated fungi strain was maintained on the potato dextrose agar medium while having Birchwood xylan as the carbon source (Pandey et al, 2014). Aspergillus Fumigatus is known to secrete cellulase free xylanase and it is obtained from the effluent samples from the paper mill in Cheranmahadevi, India. The sample is plated on potato dextrose agar medium and after growth only the necessary colonies are transferred to the CD (Czapek Dox) medium with oatspelt xylan as the substrate. The growth of A.fumigatus was observed only in the CD medium and not in the CMCellulose medium (Antony et al, 2003). Similarly, the Streptomyces sp. QG-11-3 is isolated from the decaying coconut fibers, by the selective screening, from the coastal areas of Goa. The isolate is maintained on the actinomycete isolation agar medium (beg et al, 2000). P.Chang et al, 2004 has characterized the thermostable xylanase from the Alkaliphilic Bacillus Firmus and cloned them into E-coli vectors. The Bacillus sp. AR-009 since being an alkalophile is isolated from the naturally occurring alkaline habitat in Addis Abba, Ethiopia (A. gessesse et al, 1999). Thermoanaerobacterium sp. strain JW/SLYS485 was isolated from a small hot spring pool in the West Thumb Basin near the Vandalized Pool in Yellowstone National Park in Wyoming during a survey of anaerobic thermophiles that are able to grow with xylose as a carbon source at pH values below 4.5. The organism’s physiological sequence and the result of 16sRNA sequencing help to identify the organism. Later the organism is maintained on the agar supplement medium with birch xylan as substrate at low pH conditions (SHAO et al, 1995). Microbial community EMSD5 was isolated from compost soil sample from Shandong province, east of China and stored at 4°C. This was maintained on PCS medium (z.Lv et al, 2008). T. longibrachiatum was initially isolated from paper sludge and obtained from Dr. C. J. Wang at SUNY College of Environmental Science and Forestry, Syracuse, Newyork and is maintained on potato dextrose agar medium that contains either larch, oatspelt xylan or aspen as the substrate (Nakas et al, 1989). T. lanuginosus strain SSBP was isolated from soil sample in Durban, South Africa and is found by the CSIR team from Pretoria, is maintained on the Potato Dextrose Agar medium (Singh et al,2000). A Bacillus strain with xylanolytic properties was isolated during a screening of water samples from a hot spring in Azores (Portugal) and was characterized as Bacillus subtilis by Microbial I.D., (USA) using gas chromatography of cellular fatty acids. It is maintained on the liquid LB Media (spereira et al, 2002). Bacillus Firmus was isolated from a wastewater treatment plant of a pulp and paper manufacturing industry in Bangkok, Thailand and is maintained on Berg’s mineral salts medium supplemented with Birchwood xylan medium (Tseng et al,2002). The novel anaerobic thermostable xylanase obtained from Caldicoprobacter algeriensis sp. TH7C1T also called as XYN35 is isolated from a hot spring in Algeria (B.D-Amel et al,2016). Another novel xylanase producing fungi Cladosporium oxysporum GQ-3 which is isolated from the decaying agricultural waste is identified based on the morphology and the internal transcribed spacer comparison (ITS) by rDNA gene sequence. The Cladosporium, which was earlier found as producer of antiproliferative taxol against human pathogenic bacteria and human colon cancer cell line HCT 15 was now identified to produce xylanase (Guan et al, 2016).
One of the interesting phenomenons lies in the red algae, Rhodymenia Palmata commonly known as Dilisk or dulse is immersed in dilute Hydrochloric acid for 24 hours incubation. Later a viscid liquid is obtained, which when mixed with alcohol gives solid white precipitate. This white pellet when put in the water, swells up and obtains optical rotation. This when mixed with dilute nitric acid, gives the crystalline xylose. This is the first record of isolation of xylan from the Marine Algae (Barry et al, 1940). The same work on the red seaweed polysaccharides revealed the red algae producing water soluble xylan Chaetangium erinaceum (J.R Nunn et al, 1972).
Xylanase Assay
As discussed, the xylans get hydrolyzed by the enzyme xylanase. The xylanase can be assayed by measuring the amount of reducing sugar released during the hydrolysis by DNS method or Nelson Somogyi method. There is no proper standardization as different assay results are obtained from different laboratories. This was explained by Bailey et al, 1992 as the difference is due to the type of substrate used. After the standardization of the substrate, very less mean error has occurred. Researchers use the 4-o-methylglucuronoxylan covalently dyed with Remazol Brilliant Blue (RBB xylan) as substrate or the Congo-Red (Tseng et al,2002) There are certain other techniques like fluorescence-based method EnzChek® Ultra Xylanase Assay Kit (Invitrogen, Carlsbad, CA) or the Xylazyme tablet (Megazyme, Bray, Ireland), which employs azurine-crosslinked arabinoxylan (AZCL Arabinoxylan) as substrate and its hydrolysis by xylanase produces water soluble dyed fragments and the xylanase is assayed based on the release of the dyed fragments. Most of the assays are done with Larchwood or beech wood or birch wood xylan as the substrate (Tseng et al, 2002; Singh et al, 2000; lv et al, 2008; knob et al, 2008), while some of them use oatspelt xylan (Shao et al, 1995) or aspen xylan (Royer et al, 1989) as substrates.
The Zymogram analysis is another method to identify the activity of the xylanase. In this method, SDS PAGE was run with gel containing 0.1% xylan in it. Thus after the electrophoresis, the gel is soaked into the Congo-Red dye and the bands are observed (Tseng et al, 2002). Another method to check the activity of xylanase includes the carboxy-methyl cellulase assay. So finally, one unit of enzyme activity is defined as the amount of enzyme producing 1_mol of xylose equivalents per min under the given conditions.
Production of Xylanase
Since micro organism exhibit high catalytic activity and a high degree of substrate specificity, they can be produced in large amounts, they are highly biodegradable, they pose no threat to the environment and they are economically viable. Microbial Xylanases are the preferred catalysts for xylan hydrolysis, due to their high specificity, mild reaction conditions, negligible substrate loss and side product generation. These Xylanases have applications in food, feed and paper-pulp industries (Collins et al, 2005). Some of the most important xylanolytic enzyme producers include Aspergillus, Trichoderma, Streptomyces, Phanerochaetes, Chytridiomycetes, Ruminococcus, Fibrobacteres, Clostridia and Bacillus.
The production can be improved by obtaining the potent fungal and bacterial strains and even the mutant strains to obtain high yield of enzymes. The Production also depends on the carbon and nitrogen supply, physical circumstances and chemical conditions. The production can be enhanced by optimizing the nutrition, temperature, pH parameters. The xylanase activity under optimized conditions is observed and it is concentrated by either using ammonium sulphate or acetone precipitation method or by using the dialysis tubing placed against the polyethylene glycol or by using the cation exchanger (Khasin et al, 1993). The dialysis tubing is one of the efficient ways to concentrate the enzyme with no activity loss.
Xylanase production can be done either solid state fermentation or submerged state fermentation depending on the type of organism and substrate used. Most of the thermophilic xylanase are prepared with the solid state fermentation which has high moisture content but is free from flowing water. The advantage of SSF processes over SMF include smaller volumes of liquid required for product recovery, cheap substrate, low cultivation cost for fermentation, higher enzyme yield and lower risk of contamination. The overall cost of fermentation is very low as the agricultural residues are used up as substrates and is eco-friendly. The fermentation medium allows the micro-organism to grow under the optimized conditions. The Xylanase production is done in the solid state fermentation with wheat bran (gautam et al, 2015), Birchwood xylan, Nicotiana tabaccum leaf dust (komal et al, 2016), Corn cob, Carboxy methyl cellulose (Pandey et al, 2014), Eucalyptus craft pulp (beg et al, 2000). The SMF (Submerged state fermentation) can be used to produce the cellulase and xylanase from the aerobic microorganisms. The effect of different moisture levels on xylanase production in SSF was determined by varying the solid substrate-to-moisture ratio in the range of 1:1 to 1:4. The stock can be inoculated with 5% seed culture for 24 hours at 32°C. The highest xylanase production was observed in a wheat bran-to-moisture ratio of 0.5 to 1.5. With increasing moisture level, the level of productivity was decreasing (gessesse et al, 1999). The highest extracellular xylanase production was obtained in 8 and 5 days old cultures with oat spelt xylan served as carbon source (knob et al, 2008). The xylitol and bioethanol are the two byproducts of this fermentation process that has high market value. Xylitol is the low-caloric sweetener and a preventative agent against dental caries. It is used in making chewing gums, toothpastes and diabetic products (deutschmann et al, 2012). The Agitation system in the fermenter is normally used to maintain the medium homogeneity, but the shearing forces in the fermenter can disrupt the fragile fungal biomass, leading to the reported low productivity. Higher rates of agitation may also lead to hyphal disruption, further decreasing the xylanase activity (Subramanian et al, 2000).
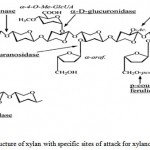 |
Figure 1: The structure of xylan with specific sites of attack for xylanolytic enzymes
|
Immobilization of Xylanase
Immobilization of the enzyme is done to improve its activity. For example, the Polyurethane foams (PUF) are the inert polymers, which is used as a source of immobilizing the enzyme. These Polyurethane foam particles along with the unwoven fabrics (like silk, cotton, polyester etc…) and proper substrate serves as the support material for the mycelia. The mycelia spores grow over the fabric material and PUF to get immobilized. This technique has proven many fold increase in the enzyme production. Unless the PUF technique, the active immobilization technique needs the growth of the cells before the immobilization. The fermenter is sterilized along with these inert particles and is inoculated in the usual way. In the case of unwoven fabrics, the polyester supports high xylanase yield followed by silk and cotton. This is a widely used technique (Beg et al, 2000). Another example can be by using the methyl-methacrylate/ EudragitTM L-100, which is a reversible-soluble polymer that binds only to xylanase and immobilize it by adsorption of the enzyme. Eudragit TM L-100 provides the extra stability to the enzyme and does not alter its pH. The leeching of xylanase can also be prevented. By this method, cellulase free xylanases can be obtained efficiently (Sardar et al, 2000). Polymethyl methacrylate (PMMA) Nano fibres (NFM) are produced by elecrospinning technique and are used to immobilize xylanase enzyme. The PMMA NFM is put to function by phenylenediamine and activated with glutaraldehyde that yields an aldehyde group on its surface for covalent immobilization of xylanase. The immobilization efficiency is much high and this can be confirmed by the Fourier transformation analysis (Kumar et al, 2013). Similar to Eudragit TM L-100, the chitosan can also be used to immobilize xylanase enzyme as it is biocompatible, hydrophilic and easy to be linked. The Dialdehyde starch (DAS) cross linked chitosan beads can be used instead of cross linked chitosan-glutaraldehyde and pure chitosan beads for the high xylanase activity recovery. The chitosan can also be coupled with the zirconium oxide for higher efficiency. This kind of immobilization of xylanase yields high thermal and storage stability (Chen et al, 2010). Dealloyed Nanoporous gold, a nanostructured metallic porous sponge is a highly active and very stable biocatalyst. It can also be used to immobilize the xylanase enzyme efficiently. In this technique, when the sulfur end groups react with the gold surface atoms, the activity can be retained and this type of immobilization has a very high efficiency (Yan et al, 2012). The multi walled carbon nano tubes are another source for immobilization. The enzyme can be refolded with 8M urea for higher activity in commercial production. The hydrophobic surface of the tube binds, refolds, purifies and simultaneously immobilizes the enzyme. The refolded xylanase decreases its alpha groups, increases its beta groups and decreases the beta turns in its structure and is observed under Fourier transformation analysis method. Thus the xylanase structure is modified by immobilization for the high productivity (shah et al, 2008). Nagar et al, 2012 has suggested the use of aluminium oxide pellets, which can be activated upon coupling with glutaraldehyde, can be used for immobilization of xylanase. This can also be referred to as response surface methodology. By this technique, the pH and temperature stability was also enhanced. The stability and reusability is its major advantage. This type of immobilization would improve the digestibility of the poultry feed (Nagar et al, 2012). Apart from this, there are many other methods like magnetic latex beads, Glutaraldehyde activated alginate beads (pal et al, 2011), reversibly soluble–insoluble polymer Eudragit S-100 and Eudragit L-100 (Sardar et al, 2000), chitosan–xanthan hydrogel (Dumitriu et al, 1997), Duolite A147 pretreated with glutaraldehyde, cellulose acetate membrane grafted with acrylamide etc. as the source of immobilization (Chen et al, 2009). Landaraniisfahani et al, (2015) has proposed another novel method of immobilizing xylanase on multifunctional hyper branched polyglycerol grafted (HPG) magnetic nano particles.
 |
Figure 2: Pie chart depicting the total microorganism that produces xylanase.
|
Application of Xylanase
Xylanase has a widespread usage in paper-pulp industries, poultry feed, textiles, cosmetics etc. Its main application includes bioconversion of lignocelluloses and agricultural wastes into the fermentation products, the improvement in brewer industry and digestibility in poultry feedstock. It is most widely used in the paper-pulp industries for the biobleaching process.
The Paper Pulp industry
The enzymes used in the paper-pulp bleaching has caught the attention of the researchers worldwide, for the production of stable, economic and eco-friendly xylan degrading enzymes like xylanase. They reduce lignin content and increase the brightness of the paper (Sunna et al, 1997). The usage of xylanase in the industry can reduce the level of lignin and also the usage of chlorine in the bleaching process. The biobleaching is a process of removing the lignin content and making the pulp white and clear, by using the lignolytic enzymes (beg et al, 2000). The usage of chlorine and other sodium hydrosulfite in the biobleaching process that release the toxins, mutagens as the byproduct causes environmental pollution. The cellulose is the primary product of the paper industry. Thus a cellulose free xylanase should be used to prevent damage to the pulp fibers. The desirable characters include the stability in high temperature and pH.
Pharmaceutical and Poultry feed industry
The xylanase can be used together with pectinase, carboxy methyl cellulase and amylase, for the identification of turbidity level and for the clarification of juices. Xylan gives xylose on depolymerization and this xylose can be further converted to xylitol, which can be used as a sweetener in the drugs and food industry (Beg et al, 2001). The quality of the bread can be increased by using the xylanases and can be further enhanced in combination with amylase. According to Collins et al, 2006 psychrophilic enzymes like xylanase is suitable for use in the baking industry as they are optimally active at the temperature required for dough preparation (at or below 35 °C). These enzymes could also be used as more efficient baking additives than the currently used commercial mesophilic enzymes, which are optimally active at higher temperatures.
Xylanases are also said to improve the nutritional quality of the silage and grain feed. They mainly improve the digestibility of the broiler chickens by decreasing the viscosity level in their intestines and improving the weight gain of the cattle and the feed conversion efficiency. (Nagar et al, 2012). The arabinoxylans are soluble in water and thus makes the water viscous, causing trouble in the brewing system like forming the haze in the beer. This can be prevented by adding the xylanase enzyme (Qiu et al, 2010). The xylooligomers (XO), a product of xylan hydrolysis, is not hydrolyzed or absorbed in the gastrointestinal tract. They directly interact with the bacteria in the colons and improve the health, thus used as a prebiotic in the food. They are also reported to reduce cholesterol levels, improve the gastrointestinal health and provide surplus amount of calcium to the body (Alonso et al, 2002).
Bioconversion of lignocelluloses in biofuel
The bioconversion product of lignocellulose like ethanol can be used as a biofuel in the industry. Lignocellulose contain lignin, cellulose, protein, ash and pectin in different propositions based on its source (Behera et al,2014). The first generation conversion can be done by converting sugar and starch to ethanol while the second generation converts lignocelluloses and other agricultural residues to ethanol. This provides strategic benefits for the fuel production. Apart from this, the water hyacinth is also said to produce biofuel efficiently (Uday et al, 2016). In the second generation conversion, the enzyme hydrolysis is a major cost factor in bioconversion of lignocellulose (Nigam et al, 2011). In bioethanol fuel production, the first step is the delignification of lignocellulose, to liberate cellulose and hemicellulose from their complex with lignin. The second step is a depolymerization of the carbohydrate polymers to produce free sugars, followed by the fermentation of mixed pentose and hexose sugars to produce ethanol (Mehdi et al,2009). This is one of the most promising feature to significantly improve the overall bioethanol production (Huang et al,2011).
Conclusion and future aspects
The xylanase, due to its enormous ability to hydrolyze xylan, has increasing demand in the industries. These xylanases are required in large quantities to do trial in the industries. Native xylanases are produced in very low quantities due to lack of proper conditions for the growth of microbes or incompatible fermentation parameters. Large scale production at a low cost is still an existing problem. Thus cloning can be done to enhance the productivity of the industrially important enzyme: xylanase. The cloning of the fungal xylanase gene can be done into the E. coli for the gene manipulation and enhanced production.
The xylanase has to be free from cellulase for efficient xylan hydrolysis. The Aspergillus fumigates, Bacillus pumilus and Bacillus firmus are known to produce this type of xylanases. The optimum temperature, pH stability and proper carbon source would help in better utilization of the xylanase. Moreover, when the xylanase producing gene is cloned by the recombination technology, production of xylanase can be enhanced and also that the properties of the recombinant strain is better than the native or crude enzyme. The immobilization techniques would further enhance the production of the xylanase enzyme.
The Thermophilic or thermostable xylanases are on very high demand on the industries. This rare and unique property of the xylanase makes it more temperature tolerant and is most needed in the industries for production under high temperatures. But the drawback is set as such that it cannot be produced in a large scale due to the high fermentation conditions. Maintaining a high temperature in the fermenter would be difficult. The Trichoderma reesei or Bacillus firmus is said to produce such thermostable xylanases that can tolerate a very high temperature.
Since the xylanases that are high demand on the thermostable xylanase in the industries, the further advancement in this would do miracles in the industrial sector. The low cost, high quality, thermostable xylanase can be found in the future by exploring new micro-organisms that are capable of xylanase secretion. There is a high chance of the producing improved xylanase production by using genetic engineering where the recombination and cloning techniques would help to induce desire qualities into the strain. Thus on further technical advancement, the microbial expression system can be developed and the production of xylanase can be done upto industrial level.
Acknowledgements
The authors would like to thank SRM University, Kattankulathur, India for supporting and performing the study.
References
- Ahmed S., Riaz S., Jamil A. Molecular cloning of fungal xylanases an overview. Applied Microbial Biotechnology. 2009;84:19–35.
CrossRef - Dahlberg L., Hoist O., Jakob K. K. Thermostable xylanolytic enzymes from Rhodothermus marinus grown on xylan. Appl Microbiol Biotechnol. 1993;40:63-68.
CrossRef - Thomas W. J. Biochemistry and genetics of microbial xylanases. Current Opinion in Biotechnology. 1996;7:337-342.
CrossRef - Shao W., Deblois S and Wiegel J. A High-Molecular-Weight, Cell-Associated Xylanase Isolated from Exponentially Growing Thermoanaerobacterium sp. Strain JW/SL-YS485. Applied and Environmental Microbiology. 1995;937–940.
- Verma T. S. Cloning expression and applicability of thermo-alkali-stable xylanase of Geobacillus thermoleovorans in generating xylooligosaccharides from agro-residues. Bioresourse. Technology. 2012;107:333–338.
CrossRef - Khasin A., Alchanati I and Shoham Y. Purification and characterization of a thermo stable xylanase from Bacillus stearothermophilus t-6. Applied and Environmental Microbiology. 1993;1725-1730.
- Khalil Q. B., Bhushanb B., Kapoora M., Hoondala G. S. Enhanced production of a thermostable xylanase from Streptomyces sp. QG-11-3 and its application in biobleaching of eucalyptus kraft pulp. Enzyme and Microbial Technology. 2000;27:459–466.
CrossRef - Yang Q.,Yan Q. J., Jiang Z. Q., Li L.T., Tian H. M., Wang Y. Z. High-level of xylanase production by the thermophilic Paecilomyces themophila J18 on wheat straw in solid-state fermentation. Bioresource Technology. 2006;971794–1800.
CrossRef - Min-Jen T., Mee-Nagan Y., Ratanakhanokchai K., Lay K. K., Shui-Tein C. Purification and characterization of two cellulase free xylanases from an alkaliphilic Bacillus firmus. Enzyme and Microbial Technology. 2002;30590–595.
CrossRef - Liu B., Zhang N., Zhao C., Lin B., Xie L., Huang Y. Characterization of Recombinant Thermo stable xylanase from hot spring the rzmophilic Geobacillus sp. TC-W. J. Microbiol. Biotechnol. 2012;22:1388–1394.
CrossRef - Wu S., Liu B & Zhang X. Characterization of a recombinant thermostable xylanase from deep-sea thermophilicGeobacillus MT-1 in East Pacific. Applied Microbiology and Biotechnology. 2006;72:1210.
CrossRef - Bhalla A., Kenneth M. B., Uppugundla N., Balan V., Rajesh K. S. Novel thermostable endo-xylanase cloned and expressed from bacterium Geobacillus sp. WSUCF1. Bioresource Technology. 2014.
CrossRef - Shi H., Zhang Y., Li X.,Huang Y., Wang L., Wang Y., Ding H and Wang F. A novel highly thermostable xylanase stimulated by Ca2+from Thermotoga thermarum: cloning, expression and characterization. Biotechnology for Biofuels. 2013;6:26.
CrossRef - Winterhalter C And Liebl W. Two Extremely Thermostable Xylanases of the Hyperthermophilic Bacterium Thermotoga maritima MSB8. Applied And Environmental Microbiology. 1995;1810–1815.
- Gomes J., Gomes I.,Steiner W. Thermolabile xylanase of the Antarctic yeast Cry ptococcus adeliae production and properties. Extremophiles. 2000;4:227–235.
CrossRef - Saha B. Hemicellulose bioconversion. J Ind Microbiol Biotechnol. 2003;30:279–291.
CrossRef - Haltrich D., Nidetzky B., Klaus D. K., Steiner W & Zupan S. Production Of Fungal Xylanases. Bioresource Technology. 1996;58:137-161.
CrossRef - Anand I., Krishnamurthy S., Vithayathil P. J. Purification and properties of xylanase from the thermophilic fungus Humicola lanuginose. Arch Biochem Biophys. 1990;257:546–53.
CrossRef - Khucharoenphaisan K., Tokuyama S., Ratanakhanokchai K and Kitpreechavanich V. A comparative study of Thermomyces lanuginosus strains on thermostable xylanase production. African Journal of Biotechnology. 2009;8(8):1608-1614.
- Kitpreechavanich V., Hayashi M., Nagai S. Production of xylan degrading enzymes by thermophilic fungi Aspergillus fumigatus and Humicola lanuginose. J Fermentation Technology. 1984;62:63–69.
- Beg K.,Kapoor M.,Mahajan L., Hoondal G. S. Microbial xylanases and their industrial applications. Applied Microbial Biotechnology. 2001;56:326–338.
CrossRef - Chanda K., Hirst E. L., Jones J. K. N and Percival E. G. V. The Constitution of Xylan from Esparto Grass (Stipatenacissima, L.). Journal of the Chemical Society. 1950;1289-97.
- Shigeru E. D. A., Ohnishi A and Kato K. Xylan Isolated from the Stalk of Nicotiana tabacunzt. Agricultural and Biological Chemistry. 1976;40(2):359-364.
CrossRef - Vincent C. B., Dillon T. Occurance Of Xylan In Marine Algae. Nature. 1940;146:620.
CrossRef - Juturu V., Chuan J. W. Microbial xylanases: Engineering production and industrial applications. Biotechnology Advances. 2012;30:1219–1227.
CrossRef - Verma D., Satyanarayana T. Molecular approaches for ameliorating microbial xylanases. Bioresource Technology. 2012;117:360–367.
CrossRef - Subramaniyan S., Prema P. Biotechnology of microbial xylanases enzymology molecular biology and application. Critical Reviews in Biotechnology. 2002;22:33–64.
CrossRef - Kucharoenphaisan S. T.,Ratanakanokshai K.,Kitpreechavanich V. Induction an repression of beta xylanase from Thermomyces lanuginosus TISTR 3465. Pakistan Journal Of Biological Sciences. 2010;13(5):209-215.
CrossRef - Aaron J. O., Heinrich T., Colin A. T and Matthew C. J. W. Characterization of a family 11 xylanase from Bacillus subtillis B230 used for paper bleaching. Acta Cryst. 2003;59:627-636.
- Henrissat B and Coutnho P. M. Classification of Glycoside Hydrolases and Glycosyltransferases from Hyperthermophiles. Methods In Enzymology. 2001;330:183.
CrossRef - Vidyasagar P. M., Jayalakshmi S. K., Sreeramulu K. Purification and some properties of low-molecular-weight extreme halophilic xylanase from Chromohalobacter sp. TPSV 101. Journal of Molecular Catalysis B: Enzymatic. 2012;74:192–198.
CrossRef - Collins T., Marie-Alice M.,Stals I., Claeyssens M. Georges Feller and Charles Gerday. A Novel Family 8 Xylanase, Functional and Physicochemical Characterization. THE JOURNAL OF BIOLOGICAL CHEMISTRY. 2002;277(38):35133–35139.
CrossRef - Davies J., Wilson K. S and Henrissat B. Nomenclature For Sugar-Binding Subsites In Glycosyl Hydrolases. Journal of Biochemistry. 1997;321:557-559.
CrossRef - Torronen A.,Mach R. L ., Messner R., Gonzalez R., Kalkkinen N., Harkki A and Kubicek C. P. The two majour xylanase from Trichoderma Reesei characterization of both enzymes and genes. Nature Biotechnology. 1992;10: 1461-1465.
CrossRef - Chang P., Wein-Shiang T., Chia-Liang T and Min-Jen T. Cloning and characterization of two thermostable xylanases from an alkaliphilic Bacillus firmus. Biochemical and Biophysical Research Communications. 2004;319: 1017–1025.
CrossRef - Jeong Y. S., Na B. H., Kim K. S., Kim H. Y. et al. Characterization of Xyn10J, a Novel Family 10 Xylanase from a Compost Metagenomic Library. Appl Biochem Biotechnol. 2012;166:1328–1339.
CrossRef - Gong., Gruniniger R. J., Forster R. J.,Teather R. M and McAllister T. A. Biochemical analysis of a highly specific, pH stable xylanase gene identified from a bovine rumen-derived metagenomic library. Appl Microbiol Biotechnol. 2013;97:2423–2431.
CrossRef - Ming-zhe S., Hong-chen Z ., Ling-cai M., Jun-she S., et al. Direct cloning, expression of a thermostable xylanase gene from the metagenomic DNA of cow dung compost and enzymatic production of xylooligosaccharides from corncob. Biotechnol Lett. 2015;37(9):1877–1886.
- Ying Y., Meng D., Chen X., Li F. An extremely thermophilic anaerobic bacterium Caldicellulosiruptor sp. F32 exhibits distinctive properties in growth and xylanases during xylan hydrolysis. 2013;53:194–199.
CrossRef - Valenzuela S. V., Diaz P., and Pastor F. I. J. Xyn 11E from Paenibacillus barcinonensis BP-23 a LppX-chaperone-dependent xylanase with potential for upgrading paper pulps. Appl Microbiol Biotechnol. 2014;98(13):5949-57.
CrossRef - Zhang H.,Wu M., Li J.,Gao S., et al. Cloning and Expression of a Novel Xylanase Gene (Auxyn 11 D) from Aspergillus usamii E001 in Pichia pastoris. Appl Biochem Biotechnol. 2012;167:2198–2211.
CrossRef - Teeravivattanakit T.,BarameeS., Phitsuwan P.,Waeonukul R., et al. Novel Trifunctional Xylanolytic Enzyme Axy43A fromPaenibacillus curdlanolyticus Strain B-6 Exhibiting Endo-Xylanase, β-D-Xylosidase and Arabinoxylan Arabinofuranohydrolase Activities. Environ. Microbiol. 2016;82:236942-6951.
- Wang Z., Jin Y., Wu H., Tian Z.,Wu Y. et al. A novel, alkali-tolerant thermostable xylanase from Saccharomonospora viridis: direct gene cloning, expression and enzyme characterization. World J Microbiol Biotechnol. 2012;28:2741–2748.
CrossRef - Pithadiya D.,Nandha D., Thakkar A. Partial purification and optimization of xylanase from Bacillus circulans. Archives of Applied Science Research. 2016;8(2):1-10.
- Bakri Y., Ammouneh H., Harba M., Akeed Y., et al. Xylanase production by a new bacillus pumilus sy30a under solid state fermentation and its application in oil palm biomass pulp bleaching. Journal of Sustainability Science and Management. 2016;11(2):49-56.
- Qiu Z., Shi P., Luo H., Bai Y., Yuan T., Yang P., Liu S., Yao B. A xylanase with broad pH and temperature adaptability from Streptomyces megasporus DSM 41476 and its potential application in brewing industry. Enzyme and Microbial Technology. 2010;46:506–512.
CrossRef - Gautam A., Kumar A and Dutt D. Production of Cellulase-Free Xylanase by Aspergillus flavus ARC- 12 Using Pearl Millet Stover as the Substrate Under Solid-State Fermentation. Journal of Advanced Enzymes Research. 2015;1:1-9.
- Pandey S., Shahid M., Srivastava M., Sharma A., Singh A., Kumar V and Srivastava Y. Isolation and Optimized Production of Xylanase under Solid State Fermentation Condition from Trichoderma sp. International Journal of Advanced Research. 2014;2(3):263-273.
- Anthony K., Raj C., Rajendran A., Gunasekaran P. High molecular weight cellulase-free xylanase from alkali-tolerant Aspergillus fumigatus AR1. Enzyme and Microbial Technology. 2003;32:647–654.
CrossRef - Lv Z., Yang J., Yuan H. Production, purification and characterization of an alkaliphilic endo 1,4-xylanase from a microbial community EMSD 5. Enzyme and Microbial Technology. 2008;43:343–348.
CrossRef - Royer C and Nakas J. P. Xylanase production by Trichoderma long ibrachiatum. Enzyme Microbial Technology. 1989;11:405-410.
CrossRef - Singh S., Pillay B. Bernard Alexander Prior. Thermal stability of b-xylanases produced by different Thermomyces lanuginosus strains. Enzyme and Microbial Technology. 2000;26:502–508.
CrossRef - Sa´-Pereiraa P., Mesquitaa A., Duartea C. J. Maria Raquel Aires Barrosb, Maria Costa-Ferreiraa. Rapid production of thermostable cellulase-free xylanase by a strain of Bacillus subtilis and its properties. Enzyme and Microbial Technology. 2002;30:924–933.
CrossRef - Bouanane-Darenfed A., Nawel B., Khelifa B., Mohammed G., et al. Characterization of a purified thermostable xylanase from Caldicoprobacter algeriensis nov. strain TH7C1T. Carbohydrate Research. 2016;419:60–68.
CrossRef - Guo-Qiang G., Peng-Xiang Z.,Zhao J., Mei-Juan W., et al. Production and Partial Characterization of an Alkaline Xylanase from a Novel Fungus Cladosporium oxysporum. BioMed Research International. 2016;2016.
- Nunn R., Parolis H and Russell I. Polysaccharides of the red alga Chaetangium erinaceum. Carbdhydrate Research. 1973;26:169-180.
CrossRef - Bailey J. M., Biely P and Poutanen K. Interlaboratory testing of methods for assay of xylanase activity. Journal of Biotechnology. 1992;23:257-270.
CrossRef - Knob A and Carmona C. E. Xylanase Production by Penicillium sclerotiorum and its Characterization. World Applied Sciences Journal. 2008;4(2):277-283.
- Collins T., Gerday C., Feller G. Xylanases, xylanase families and extremophilic xylanases. FEMS Microbiology Reviews. 2005;29:3–23.
CrossRef - Matte A and Forsberg W. C. Purification characterization and mode of action of endoxylanases 1 and 2 from Fibrobacter succinogenes s85. Applied and Environmental Microbiology. 1992;58:157-168.
- Belancic A., Scarpa J., Peirano A., Diaz R., Steiner J., Eyzaguirre J. Penicillium purpurogenum produces several xylanases Purification and properties of two of the enzymes. Journal of Biotechnology. 1995;41:71-79.
CrossRef - Antoine A. A., Jacqueline D., Thonart P. Xylanase Production by Penicillium canescens on Soya Oil Cake in Solid-State Fermentation. Applied Biochemistry and Biotechnology. 2010;160:50–62.
CrossRef - Nair G. S., Sindhu R.,Shashidhar S. Fungal xylanase production under solid state and submerged fermentation conditions. African Journal of Microbiology Research. 2008;2:082-086.
- Pandey A., Kumar P. S., Soccol R. C.,Nigam P. Solid state fermentation for the production of industrial enzymes. Current science. 1999;77(1):149-162.
- Bocchini A., Oliveira O. M. M. F.,Gomes E., Silva D. R. Use of sugarcane bagasse and grass hydrolysates as carbon sources for xylanase production by Bacillus circulans D1 in submerged fermentation. Process Biochemistry. 2005;40:3653–3659.
CrossRef - Pellerin P., Gosselin M., Jean-Paul L., Samain E and Debeire P. Enzymic production of oligosaccharides from corncob xylan. Enzyme Microbial Technology. 1991;3:617-621.
CrossRef - Damaso M. C. T., Castro A. M. D., Castro R. Q., Maria C., Andrade M. C and Periera N. Application of Xylanase from Thermomyces lanuginosus IOC-4145 for Enzymatic Hydrolysis of Corncob and Sugarcane Bagasse. Applied Biochemistry and Biotechnology. 2004;113–116.
- Arabi I. E., Jawhar M and Bakri Y. Effect of Additional Carbon Source and Moisture Level on Xylanase Production by Cochliobolus sativus in Solid Fermentation. Microbiology. 2011;80(2):150–153.
CrossRef - Gessesse A., Mamo G. High-level xylanase production by an alkaliphilic Bacillus by using solid-state fermentation. Enzyme and Microbial Technology. 1999;25:68–72.
CrossRef - Komal P., Acharya and Shilpkar P. Production partial purification and characterization of xylanase using Nicotiana tabacum leaf dust as substrate. Journal of Environmental Biology. 2016;37:297-303.
- Deutschmann R., Dekker R. F. H. From plant biomass to bio-based chemicals: Latest developments in xylan research. Biotechnology Advances. 2012;30:1627–1640.
CrossRef - Sardar M.,Roy I., Gupta M. N. Simultaneous purification and immobilization of Aspergillus niger xylanase on the reversibly soluble polymer EudragitTM L-100. Enzyme and Microbial Technology. 2000;27:672–679.
CrossRef - Kumar P., Gupta A., Dhakate S. R.,Mathur R. B., Nagar S., GuptaV. K. Covalent immobilization of xylanase produced from Bacillus pumilus SV-85S on electrospun polymethyl methacrylate nanofiber membrane. International Union of Biochemistry and Molecular Biology Inc. 2013;60(2):162–169.
- Chen H., Liu L., Lv S., Liu X.,Wang M., Song A.,Jia X. Immobilization of Aspergillus niger Xylanase on Chitosan Using Dialdehyde Starch as a Coupling Agent. Applied Biochem Biotechnol. 2010;162:24–32.
CrossRef - Yan X., Wang X., Zhao P., zhang Y., Xu b. P.,Ding Y. Xylanase immobilized nanoporous gold as a highly active and stable biocatalyst. Microporous and Mesoporous Materials. 2012;161:1–6.
CrossRef - Shah S., Gupta M. N. Simultaneous refolding, purification and immobilization of xylanase with multi-walled carbon nanotubes. Biochimica et Biophysica Acta. 2008;1784:363–367.
CrossRef - Nagar S., Mittal A., Kumar D.,Gupta K. V. Production of alkali tolerant cellulase free xylanase in high levels by Bacillus pumilus SV-205. International Journal of Biological Macromolecules. 2012;50:414–420.
CrossRef - Nagar S.,Mittal A ., Kumar D., Kumar L.,Gupta K. V. Immobilization of xylanase on glutaraldehyde activated aluminum oxide pellets for increasing digestibility of poultry feed. Process Biochemistry. 2012;47:1402–1410.
CrossRef - Pal A., Khanum F. Covalent immobilization of xylanase on glutaraldehyde activated alginate beads using response surface methodology: Characterization of immobilized enzyme. Process Biochemistry. 2011;46:1315–1322.
CrossRef - Dumitriu S and Chornet E. Immobilization of Xylanase in Chitosan-Xanthan Hydrogels. Biotechnology Prog. 1997;13:539-545.
CrossRef - Buchert J., Tenkanen M., Kantelinen A & Viikari L. APPLICATION OF XYLANASES IN THE PULP AND PAPER INDUSTRY. Bioresource Technology. 1994;50:65-72.
CrossRef - Landarani-Isfahani A., Taheri-Kafrani A., Amini M., Mirkhani V.,et al. Xylanase Immobilized on Novel Multifunctional Hyperbranched Polyglycerol-Grafted Magnetic Nanoparticles: An Efficient and Robust Biocatalyst. Langmuir. 2015;31(33):9219–9227.
- Sunna and Antranikian G. Xylanolytic Enzymes from Fungi and Bacteria. Critical Reviews in Biotechnology. 1997;17( 1):39-67.
CrossRef - Collins T., Hoyoux A., Dutron A., Georis J., Genot B., Dauvrin T. Use of glycoside hydrolase family 8 xylanases in baking. Journal of Cereal Science. 2006;43:79–84.
CrossRef - Va´ zquez J., Alonso J. L., Domı´nguez H., and Parajo´J. C. Enzymatic Processing Of Crude Xylooligomer Solutions Obtained By Autohydrolysis Of Eucalyptus Wood. Food Biotechnology. 2002;16(2):91–105.
CrossRef - Bhalla A.,Bansal N., Kumar S., Bischoff K. M., Sani R. K. Improved lignocellulose conversion to biofuels with thermophilic bacteria and thermostable enzymes. Bioresource Technology. 2013;128:751–759.
CrossRef - Kumar A., Gupta R., Shrivastava B., Khasa Y. P., Kuhad C. R. Xylanase production from an alkalophilic actinomycete isolate Streptomyces sp. RCK-2010, its characterization and application in saccharification of second generation biomass. Journal of Molecular Catalysis B: Enzymatic. 2012;74:170–177.
CrossRef - Morosoli R., Durand S and Moreau A. Cloning and expression in Escherichia from the yeast Cryptococcus albidus coli of a xylanase-encoding gene. Gene. 1992;117:145-150.
CrossRef - Behera S., Arora R., Nandhagopal N., Kumar S. Importance of chemical pretreatment for bioconversion of lignocellulosic biomass. Renewable and Sustainable Energy Reviews. 2014;36:91–106.
CrossRef - Uday S. P., Choudhury P., Bandopadhyay T. K., Bhunia B. Classification, mode of action and production strategy of xylanase and its application for biofuel production from water hyacinth. International Journal of Biological Macromolecules. 2015;82:1041-54.
CrossRef - Nigam S. P., Singh A. Production of liquid biofuels from renewable resources. Progress in Energy and Combustion Science. 2011;37:52-68.
CrossRef - Dashtban M., Schraft H., Qin W . Fungal Bioconversion of Lignocellulosic Residues; Opportunities & Perspectives. Int J Biol Sci. 5(6):578-595.
- Huang R.,Su R.,Qi W & He Z. Bioconversion of Lignocellulose into Bioethanol: Process Intensification and Mechanism Research. Bioenerg. Res. 2011;4:225–245.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





