Manuscript accepted on : October 27, 2009
Published online on: 28-12-2009
In Vitro Callus Culture of Centella Asiatica L. (Urb.) and its HPLC Analysis
C. M. Shashikala1*, S. Shashidhara2 and P. E. Rajshekharan2
1Department of Pharmacognosy, Govt. College of Pharmacy, Bangalore India
2Indian Institute of Horticulture Research, Bangalore India.
Corresponding Author E-mail: shashimetri@rediffmail.com
ABSTRACT: Different explants of Centella asiatica were excised and cultured on Murashige & Skoog (MS) media supplemented with different growth hormones at different concentrations. It has been found that the frequency of callus induction was more on MS media supplemented with Naphthalene acetic acid (NAA) (0.5 mg-1) + kinetin (2.0 mg-1) and NAA (0.5mg-1) + Benzyl Amino Purine (BAP) (2.0 mg-l). Among the leaf, petiole and root explants, petiole explants showed more frequency of callus induction followed by leaf and root explants. Qualitative chemical tests of the methanolic extracts of callus showed the presence of saponins, triterpenes, flavonoids but the absence of alkaloids and qualitative analysis by thin layer chromatography (TLC), ultra violet (UV) and infra red (IR) analysis confirm the presence of asiaticoside. Quantitative High performance liquid chromatography (HPLC) analysis reveals that the leaf callus contains significantly more amounts of asiaticoside (1.1%) and madecassoside (2.4%)) compared to the root and petiole callus on dry weight basis.
KEYWORDS: Centella asiatica; callus culture; asiaticoside; HPLC
Download this article as:| Copy the following to cite this article: Shashikala. C. M, Shashidhara. S, Rajshekharan. P. E. Raghava. Allelopathic Substances of Parthenium Weed and Interactions of Solanum Melongena. Biosci Biotechnol Res Asia 2009;6(2) |
| Copy the following to cite this URL: Shashikala. C. M, Shashidhara. S, Rajshekharan. P. E. Raghava. Allelopathic Substances of Parthenium Weed and Interactions of Solanum Melongena. Biosci Biotechnol Res Asia 2009;6(2). Available from: https://www.biotech-asia.org/?p=9089. |
Introduction
Centella asiatica L. (Urb.) belongs to the family Apiaceae is slender, prostrate, creeping perennial herb that flourishes in and around damp-swampy area. It contains triterpene saponins such as asisaticoside, madecassosode, brahmoside, triterpenic acids etc1,2. It proved to have antileprotic, antibacterial antiulcer, and wound healing properties and useful in the treatment of skin diseases and mental disorders3. Ritu et el., achieved shoot bud regeneration from callus of Centella asiatica through organogenesis4. Shashikala et el., carried out in vitro regeneration of Centell asiatica5. There were fewer reports regarding the callus culture of Centella asiatica using different explants but no reports regarding presence and determination of secondary metabolites. So this paper through a light on the callus culture showing the effect of various growth regulators and identification and quantification of presence of asiaticoside and madecassoside in the callus by HPLC, obtained from different explants.
Materials and Methods
Surface sterilization and inoculation of explants
Plant material was collected from the Indian Institute of Horticulture, Bangalore and identified by Regional Research Institute (Ay), Bangalore. Plants were washed under running tap water followed by distilled water. Than soaked in bavistan, a fungicide (BASF India, Ltd., Bombay.) 150 mg-l for 25-30 min. and washed with distilled water. The leaf, petiole and root explants (1.0-1.7 cm) were excised and transferred to sterilized conical flask containing 70% (v/v) alcohol and surface sterilized for 1-2 min followed by treatment with 0.1% (w/v) mercuric chloride for 2 min. Then explants were washed thoroughly with sterile distilled water to remove excess of mercuric chloride. The sterilized explants were trimmed from both the ends and inoculated aseptically on Murashige and Skoog (MS, 1962) media with 3% (w/v) sucrose and 0.8 % (w/v) agar. MS media was supplemented with different concentrations of (0.5- 2.0 mg–1) of Naphthalene acetic acid (NAA ) and 2,4- Dichloro acetic acid(2,4-D) alone and in combination with different concentration (0.5-2.0mg-l) of kinetin and Benzyl amino purine (BAP) for callus induction6. (Ref table 1&2).
The pH of the media was adjusted to 5.7±0.1 with 0.1 N NaOH or 0.1N HCL. The cultures were maintained at 24±0.5oC with 16 hrs photoperiod.
Root and petiole explants were inoculated horizontally on the medium and the leaves were placed so that dorsal side in contact with the medium. All the treatments were replicated thrice with 20 culture tubes in each set. The responses of explants were represented in the form of frequency (% response) of callus induction.
The growth study of leaf, petiole and root callus were carried out by inoculating one month old callus on MS media supplemented with NAA( 0.5mg-l)+kinetin (2.0 mg-l) by fresh weight method. The culture tubes were incubated under the same conditions as mentioned above. The weights of callus were taken in the intervals of 8 days for 2 months7.
Qualitative analysis
Callus dried at 600 C in an incubator was macerated with methanol for 10 hrs and filtered under vacuum and concentrated. The residue was subjected to standard chemical tests for saponins, triterpenins, alkaloids and flavonoids, and also to thin layer chromatography (TLC), ultra violet (UV) and infra red (IR) analysis8, 9.
TLC
The residue obtained above was dissolved in methanol and spotted on precoated silica gel GF 254 TLC plate (E. Merck) and developed using n-Butanol: Glacial Acetic Acid :Water(5:1:4) as a mobile phase. The spots were visualized by spraying with Anisaldehyde-Sulphuric acid reagent and RF values were compared with reference standard. (Fig no.3)
UV and IR analysis
Asiaticoside was isolated from the residue obtained above, by two major steps, viz., fractional separation of saponins and preparative TLC.
The residue was suspended in the warm water and extracted with n-Butanol. The n-Butanol layer was concentrated and dissolved in methanol and poured into excess of acetone to precipitate saponins. The precipitate is dissolved in methanol to carry out preparative TLC.
Preparative TLC was carried out according to the method mentioned in TLC of callus using reference standard asiaticoside. The band corresponding to asiatcoside was scarped off and extracted with methanol and filtered. The filtrate was subjected to UV and IR analysis.
For UV analysis methanolic solution was scanned at 200-400 nm using UV spectrometer (Shimadzu UV-1601). For IR the solution was concentrated and evaporated to dryness. The residue was subjected to IR spectral studies using KBR pellet technique (Shimadzu-FTIR, 8900).
High performance liquid chromatographic (HPLC) analysis
5 g of dried leaf, petiole and root callus were macerated with methanol for 10 hrs, filtered and evaporated to dryness. The residue obtained were dissolved in HPLC grade methanol individually and analysed using HPLC model Shimadzu LC-2010A with RP8 lichrosphere column length of 30 cm, internal diameter of 4 mm and particle size of 5 µ. The mobile phase was Acetonitrile: Water (3:7) with a flow rate of 1 ml/min detected at 210 nm10.
Results and Discussion
Callus induction
Variable responses were observed when the leaf, petiole and root explants were cultured on MS media supplemented with various concentrations (0.5, 1.0, 2.0 mg-1) of NAA and 2-4-D alone and in combination with various concentrations (0.5, 1.0, 2.0 mg-1) of BAP and kinetin for the callus induction. In all cultures the formation of callus initiated after 15 days of incubation.
When auxins used alone the highest (50-59%) frequency of callus induction of all the explants was observed in the media with 2.0 mg-1 of NAA and 2, 4-D at the end of fourth wk. The nature of leaf and petiole callus was pale green and spongy where as root callus was creamy and compact. But the callus on media with 2, 4- D was become brown after 40 days of incubation showing no further growth.
Table 1: Effect of auxins on the frequency of callus induction.
| Growth regulators (mg l-1) | Frequency (% response) and the nature of the callus | ||
| Leaf | Petiole | Root | |
| NAA | |||
| 0.5 | 30
PG, S, a
|
34
PG, S, c |
28
Cr, C, c |
| 1.0 | 52
PG, S, b
|
55
PG, C, c
|
50
Cr, C, c
|
| 2.0 | 55
PG, S, b
|
59
PG, C ,c
|
53
Cr, C, c |
| 2, 4-D |
|
||
| 0.5 | 20
PG, S, c
|
25
PG, S, c
|
19
Cr, S, c |
| 1.0 | 49
PG, S, c
|
56
PG, S, c
|
43
Cr, S, c |
| 2.0 | 52
PG, S, c
|
55
PG, S, c
|
50
Cr, S, c |
PG: pale green; S: spongy; C: compact; Cr: creamy; a rooting to small extent,; b average rooting; c no rooting 20 explants were maintained in each treatment data were recorded up to 6 wks
Amongst the different concentrations of auxins in combination with different concentrations of cytokinins tested, NAA (0.5mg-1) + kinetin (2.0mg-1) and NAA (0.5 mg-1) + BAP (2.0 mg-1) found to be effective. In case of leaf explants the callus formed at the edges initially followed by more callus formation near the vascular tissue due to the availability of more nutrients around the conducting tissue11 and in petiole and root explants the callus initiated at only one end. The nature of leaf callus was pale green and spongy on the media with NAA (0.5 mg-1) + BAP (2.0 mg-1). Even though the media with NAA (0.5mg-1) + kinetin (2.0mg-1) showed similar response but rooting of the callus was observed after 25 days of incubation. The frequency of callus induction was 75- 88% at the end of fourth week. Even media with 2,4-D (0.5 mg-1) + kinetin (2.0 mg-1) found to be effective (80-84%) but the callus become brown after 40 days of incubation showing no further growth as in case of 2,4- D alone. The browning of the callus was influenced by the accumulation of phenolics in the cytoplasm which undergo oxidation and polymerization and oxidized products appears dark brown12. (Table 2) and (Fig 1, A- C).
 |
Figure 1: A – C: Effect of auxins in combination with cytokinins on LEAF callus induction.
|
Rooting of callus on MS media with NAA (0.5 mg l-1) +Kn (2.0 mg l-1)
Callus on MS media with NAA (0.5 mg l-1) + BAP (2.0 mg l-1)
Browning of callus on MS with 2, 4-D (0.5 mg l-1) + Kn (2.0.mg l-1)
Table 2: Effect of auxins + cytokinins on the frequency of callus induction.
| Growth regulators (mg l-1) | Frequency (% response) and nature of the callus | ||
| Leaf | Petiole | root | |
| NAA + kinetin | |||
| 0.5 + 0.5 | 35
PG, S, a |
40
PG, C, c |
32
Cr, C, c |
| 0.5 + 1.0 | 60
PG, S, b |
63
PG, C, c |
60
Cr, C, c |
| 0.5 + 2.0 | 86
PG, S, b |
88
PG, C, c |
78
Cr, C, c |
| 1.0 + 0 5 | 30
PG, S, a |
34
PG, C, c |
30
Cr, C, c |
| 1.0 + 1.0 | 40
PG, S, a |
30
PG, C, c |
32
Cr, C, c |
| 2, 4-D + kinetin | |||
| 0.5 + 0.5 | 30
PG, C, c |
35
PG, C, c |
32
PG, C ,c |
| 0.5 + 1.0 | 50
PG, S, c |
55
PG, C, c |
60
Cr, C, c |
| 0.5 + 2.0 | 80
PG, C, c |
84
PG, C, c |
80
Cr, C, c |
| 1.0 + 0.5 | 40
PG, C, c |
45
PG, C, c |
60
Cr, C, c |
| 1.0 + 1.0 | 50
PG, C, c |
55
PG, C, c |
23
Cr, C, c |
| NAA +BAP | |||
| 0.5+0.5 | 35
PG, S, c |
42
PG, C, c |
35
PG, C ,c |
| 0.5 + 1.0 | 60
PG, S, c |
64
PG, C, c |
58
PG, C ,c |
| 0.5 + 2.0 | 82
PG, S, c |
84
PG, C, c |
75
PG, C ,c |
| 1.0 + 0.5 | 40
PG, S, c |
45
PG, C, c |
35
PG, C ,c |
| 1.0 + 1.0 | 55
PG, S, c |
52
PG, C, c |
45
PG, C ,c |
PG: pale green; S: spongy; C: compact; Cr: creamy; a rooting to small extent,; b average rooting; c no rooting 20 explants were maintained in each treatment, data were recorded up to 6 wks.
The callus produced from petiole, root explants was light green and compact without rooting. (Table2) and (Fig 2, a-c).
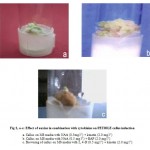 |
Figure 2: a-c: Effect of auxins in combination with cytokinins on PETIOLE callus induction.
|
Callus on MS media with NAA (0.5mg l-1) + kinetin (2.0 mg l-1)
Callus on MS media with NAA (0.5 mg l-1) + BAP (2.0 mg l-1)
Browning of callus on MS media with 2, 4-D (0.5 mg l-1) + kinetin (2.0 mg l-1)
From this it has been concluded that if only auxins are used, media requires high concentrations of auxins (up to 2.0mg–1) to get high frequency of callus induction where as if it is supplemented with cytokinins, the media requires less concentrations of auxins. Further, the media supplemented with auxins in combination with cytokonins is effective for the induction of the callus.
The callus obtained above was proliferated on media with NAA (0.5 mg-1) + BAP (2.0 mg-1). The callus was light green spongy without rooting after 6 weeks of incubation and it was used for the qualitative and quantitative analysis.
Growth studies of callus
Leaf and petiole callus show same long lag phase, whereas the root shows a shorter lag phase during 8 days of incubation. The exponential phase of the petiole deviates from the leaf and root calli and it was long during 16, 24 and 32 days of incubation. The nature of the callus was light green and compact. The petiole, leaf and root show the stationary phase turning to light yellow and then to the brown colour.
The weight of petiole callus was 1.64±0.03 g, leaf callus, 1.36±0.10 g and root callus, 1.34±0.91 g.
Qualitative analysis
Qualitative chemical tests of all three callus confirm the presence of
carbohydrates, proteins, sterols, saponins, triterpenes, flavanoids but the absence of alkaloids. The
results obtained were compared with methanolic extract of the natural plant.
The TLC studies of the petiole, leaf and root callus showed two major spots of bluish violet having the Rf values of 0.42 and 0.33. The Rf value, 0.42 was similar to that of reference standard, asiaticoside. Thus the callus was found to contain asiaticoside as major active constituent. (Fig3).
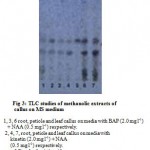 |
Figure 3: TLC studies of methanolic extracts of callus on MS medium.
|
1, 3, 6 root, petiole and leaf callus on media with BAP (2.0 mg l-1) + NAA (0.5 mg l-1) respectively.
2, 4, 7, root, petiole and leaf callus on media with kinetin (2.0 mg l-1) + NAA (0.5 mg l-1) respectively.
5 Standard asiaticoside
UV spectra of isolated asiaticoside showed λmax at 204.4 which was similar to that of reference standard and IR spectra was also similar to that of reference standard and reported data. The major peaks observed were 2923.9 (C-H stretching), 1651.0 (C=C stretching), 1720.4 (C=O stretching), and 3409.9 cm -1 (-OH stretching) 13.
HPLC analysis
The % of asiaticoside and madecassoside in the methanolic extract of leaf callus was
found to be 1.1and 2.4, in petiole callus 0.68 and 1.31 and in root callus 0.93 and 1.65 respectively. It
showed that the leaf callus contains significantly more amount of asaiticoside and madecassoside as the
leaves are the major sources. Increase in the secondary metabolites through tissue culture work will be
the criteria for further study.
Acknowledgement
Authors are thankful to Dr. Deepak. Manager, Phytochemistry, Natural Remedies, R&D center, Bangalore for providing reference standards and HPLC facilities.
References
- Kirtikar K. R., Basu B. D., (Eds.), Indian medicinal palnts, Vol. II, L.M Basu, Allabad, India, 1193-1195 (1975).
- Rao P. S., Seshadri T. R., Variation in chemical composition of Indian samples of Centella asiatica. Current science, 20 (4), 77-79 (1969).
- Aziz Z. A., Lowe R. C., Micropropagation of medicinal species of Centella asiatica. J. of Experimental Biology, 59, p2– 39, 19-20 (1999).
- Ritu S., Sukla Y. N. and Sushil K., Chemistry and Pharmacology of Centella asiatica: a review. J. of Medicinal and Aromatic Science, 19, 1049-1056 (1997).
- Shashikala C. M., Shashidhara S. and Rajshekharan P.E., In vitro regeneration of Centella asiatica. Plant Cell Biotechnology and Molecular Biology, 61 (1&2), 53-56 (2005).
- Murashige T., Skoog F. A., Revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiology plant, 15, 473-497 (1962).
- Gamborg O. L., Philips G. C., (Eds.), Plant Cell Tissue and Organ Culture, Narosa publishing house, Springer- Verlag Berlin Heidenburg, 71-72 (1995).
- Kokate C. K., Practical pharmacognosy. Vallabh prakashan, 107-111 (1991).
- Wagner H., Bladt S. and Zginski E. M., Saponin drug – in plant drug analysis. Publications, Springer- Verlag Berlin Heidenburg, Newyork, 225-227 (1984).
- Indian Herbal Pharmacopoeia, a joint publication of Regional Research Lab., and Indian Drug manufacturer’s associations, Bombay, 47-55 (1998).
- Teixeria J. B, Sondahl M. R. and Kirby E. G., somatic embryogenesis from immature inflorescence of oil plam. Plant Cell Rep., 13, 247-250 (1994).
- Lukas A. M., Christoper D. G., Rebecca A. S and Virgnia W. AN9- a petunia glutathione s-transferase required for anthocyanin sequestration, is a flavonoid binding protein. Plant physiol., 123, 1561-1570 (2000).
- Mishra T. N., Singh R.S., Upadyay J. and Srivastava, R., Chemical constituents of Vernonia cinerea, part-II isolation spectral studies of triterpenes. J. of natural products. 47, 368-372 (1984).

This work is licensed under a Creative Commons Attribution 4.0 International License.





