Manuscript accepted on : May 22, 2008
Published online on: 05-02-2016
Histological evaluation of daily oral iron administration in anemic rabbits
Abuzinadah1* And A. Ali2
1Department of Biological Science, Faculty of Science,King Abdul Aziz University, Jeddah Saudi Arabia.
2Department of Zoology, Faculty of Medicine, King Abdul Aziz University, Jeddah Saudi Arabia.
ABSTRACT: Iron deficiency, anemia is a common feature of may targets of human living in developing world. For this respect this work was designed to evaluate the daily iron supplementation in treatment of certain types of anemia due to severe shortage of food as well as study the fate of excess iron supplementation within the bodies as chronic iron-overload promotes oxidative stress and organ failure. Three groups of rabbits, one as control, the second severely starved and the third was well fed. The last two groups were both supplemented by iron (as Furmarate tablets) equivalent to those of anemic human therapies, daily till three weeks. The signs of anemic animals were developed in those starved ones such as weakness and size loss, out look, the organs were pall due to the shortage of blood supply. The hepatic and renal cells seemed to be empty with clear cytoplasm some of cells were being degenerated. After daily supplementation of iron the structures of the organs were restored to normal, however, certain amounts of iron accumulations were observed. The well-fed group appeared to be normal with excess iron accumulation in the form of stainable granules in a dose-dependent manner. It is easy to say that iron is important supplement to overcome certain type of anemia by daily doses to a limited time. This supplement, if increased, will accumulate and adverse the biological mode of cells and cause a significant hepatotoxic and nephrotoxic effects. Excess oral iron intake should be avoided unless prescribed by a physician.
KEYWORDS: Iron deficiency; daily intake; rabbits
Download this article as:| Copy the following to cite this article: Abuzinadah O, Ali A. Histological evaluation of daily oral iron administration in anemic rabbits. Biosci Biotechnol Res Asia 2008;5(1) |
| Copy the following to cite this URL: Abuzinadah O, Ali A. Histological evaluation of daily oral iron administration in anemic rabbits. Biosci Biotechnol Res Asia 2008;5(1). Available from: https://www.biotech-asia.org/?p=6415 |
Introduction
Iron deficiency and anemia are prevalent among infants, children, and women in all ages, and adult men in whom parasites induced blood losses, in developing and even in the industrial world (Hallberg et al., 1995 and WHO, 1992,1999).
This implies that iron supplements are needed across the life span. Prusak and Grzegorzewska (2002) summarized the factors leading to absolute iron deficiency. The factors are 1) loss of iron as a result of blood loss by gastrointestinal tract, 2) enhanced use of iron during intensive erythropoiesis, 3) dietary iron deficiency or impaired iron uptake from gastrointestinal tract, 4) other forms of gastrointestinal tract impairment,5) pharmaceutical substances forming in absorbable iron complexes and/or diluting the acidity of gastric juice,6) certain demographic factors. However, iron supplementation remains an important strategy for the prevention and treatment of iron deficiency anemia and can produce substantial improvement in the functional performance of iron deficient individuals (Allen, 2002). In this review, the adverse effects on iron deficiency anemia increases the risk of both child and maternal mortality that iron deficiency anemia impairs work productivity and child development.
In a report published by Allen (2001) to explain how anemia and/or iron deficiency could cause low birth weight and preterm delivery. Anemia (by causing hypoxia) and iron deficiency (by causing increase neither serum nor epinephrine concentrations) can induce maternal and fetals stress, which stimulates the synthesis of corticotrophin releasing hormone, intern, a major risk factor for preterm labor. This hormone also increases fetal cortisole production, the cortisole may inhibit longitudinal growth of fetus. Iron deficiency increases oxidative damage to erythrocyte and fetoplacental unit, and also increases the risk of maternal infection.
Iron is both an essential nutrient and a potential toxicant to cells, it requires a highly sophisticated and complexes set of regulatory approaches to meet the demands of cells as well as prevent excess accumulation (Beard, 2001).
The normal total circulating iron pool is 3 to 4 mg (Ponka et al., 1998), iron is bounded and transported in plasma by the non-heme and 1-globulin transferrin. During normal erythropoiesis, all of the circulating iron is bounded to transferrin and iron is turned over 6 to 10 times daily (Cavill, 1982) deposited wide variation in iron stores, the iron pool remain remarkable stable. Functional deficiency is defined by the delivery of less iron to the developing erythron than that required for optimal epoetin driven erythropoiesis (Besarab
et al., 1999). The provision of adequate iron to support the erythropoiesis in iron deficient patients is a time-consuming process. In a study aimed to evaluate the safety and tolerability of intravenous high dose iron sucrose therapy in-patient with iron deficiency anemia due to gastrointestinal blood loss (Schroder et al., 2004) concluded that this dose appears to be safe and therefore is therapeutic option which may save time and improve patient compliance. Also in a patient with chronic kidney disease and severely anemic and iron deficient, an aggressive regimen of multiple high dose iron sucrose infusions may be both safe and effective (Schwenk and Blaustein, 2004).
Many studies indicated that adequate iron stores critically necessary to achieve optimal responses to epoetin. If iron released from its ferritin core, it may catalyze a variety of deleterious reactions (Besarab et al., 1999) the result is the highly reactive hydroxyl radicals. A variety of methods are available for diagnosing iron over load, of these, assessment of hepatic iron index in a liver biopsy specimen (Bassett et al., 1986).
Efforts regarding dietary iron supply focused mostly on the prevention of iron deficiency anemia. The aim of this study is to evaluate iron supplementation as a quick aid to overcome certain types of anemia developed due to the severe shortage of food. In the meantime study the effect short-term administration of iron on starved and well feed groups of animals by using routine histological examination.
Material and Methods
Premature rabbits (Oryctolagus cuniculus) weighing about 0.750k gm were obtained from a private farm in Jeddah district, Saudi Arabia. Twenty-four animals were housed in stainless steel cages under standard laboratory condition (14hr light: 10hr darkness) at 25 ± 3°C with constant humidity 40-60%. They were fed commercial diets, vegetables, crushed wheat and corn as a complete diet. They left for a week to be acclimatized.
Then they divided into 3 groups (8 each): one control group and two experimental groups, one well fed and that other starved as fed small amount of crushed wheat and corn (low iron diet) ones every morning and the rest of food removed quickly for two weeks, these two experimental groups (8 each) were supplemented daily by iron in a dose equivalent to the human therapies for three weeks later.
Therapeutic dose of human (33 mg/kg/twice/day), according to Paget and Barnes formula (1964) the equivalent dose of Fumarate was calculated (0.7mg/rabbit/day). Fumarate tablets were crushed and dissolved in distilled water to form a suspension, suspension were force-fed by stomach tube to experimental groups, daily till three weaks. After each week one control animal and 2 experimental ones were sacrificed. An out took of the organs, samples of livers and kidneys were put in 10% neutral formalin for fixation, embedded in paraffin wax, sectioned, stained by hematoxylin and eosin stains, diagnosed and photographed.
Results
Control group
In this group, animals were normal active, their size increase with the normal ratio with time. From the outlook, the mentioned organs were red in color.
This control group exhibited the general structure of liver as hepatocytes arranged in the form of branching and anatomizing plates forming lobules surrounding the central vein and separated by blood sinusoid (Fig. 1).
Histological examination of control kidney revealed that it was distinguishable into an outer cortical region and an inner modularly region. The parenchyma of the cortex is formed of large number of renal tubules and Malpighian corpuscle consists of Bowman’s capsule surrounding the glomeruli and the urinary space is found in between the two parietal layers (Fig. 1*).
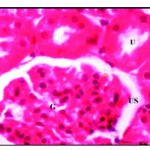 |
Figure 1: Normal renal tissues showing normal uriniferous tubules (U) and glomeruli (G) with urinary space (US) X 750.
|
Starved group
In this group, animals were less active, their size decreased at the end of the second week about 30% from the beginning size. From the outlook, the organs were decolorized and became very pall at the end of the experiment. Histopathological examination of the liver rabbit’s revealed drastic alteration in his-architecture. The hepatocytes were disrupted, vacuolated with hyper chromic nuclei and lost their polyhedral shape. Vacuolization was severe especially in the centrilobular region which showed widespread necrosis, also cellular infiltration was detected around the blood vessels (Fig. 2). In kidney, bale renal tissues showing disrupted small uriniferous tubule and
small Bowman’s capsule with dilated urinary space (Fig. 2*).
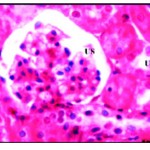 |
Figure 2: Pal renal tissues showing disrupted small uriniferous tubule (U) and dilated urinary space (US) X 750.
|
After one week of iron supplementation, the starved animals showed a moderate activity, their organs looked fewer palls, the liver and kidney showed moderate recovery form starving induced liver and kidney damage as was evident from nearly recovery of some liver cells with round nuclei and control amount of chromatin, no cellular infiltration, and certain iron deposition around the central veins but still another disrupted cells show vacuolated cytoplasm and necrosis (Fig. 3), also kidney showed improvement in all microscopic examination but some glomerular tufts was seen with evidence of vacuolation of mesangial cells accompanied with moderate vascular degenerative changes in few renal tubules as the cytoplasm of the tubular cells contained large vacuoles and the nuclei with condensed abnormal shape (Fig. 3*)
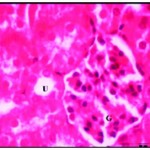 |
Figure 2: Renal tissues after one week of iron supplementation showing small uriniferous tubule (U) and small glomeruli (G) X750.
|
After three weeks of iron supplementation, the animal’s activity nearly normal and their internal organs looked pall red, and by time, the sections looked to be restoring the normal appearance, the deposition of iron or accumulation, was increased by time in dose-dependent manner as shown in the liver which showed well defined hepatic cords with polyhedral hepatocytes and normal appearing round nuclei (Fig. 4) and in kidney the accumulation expanded to the whole section (Fig. 4*).
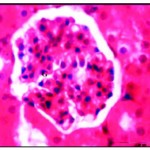 |
Figure 4: Renal tissues after three week of iron supplementation showing iron accumulation in cytoplasm and nearly normal glomeruli (G) X750.
|
Well fed group
Normal higher animal’s activity and more body size gains, the organs, seemed to be normal in shape and red colour. The sections of liver and kidney showed certain iron accumulation, which looked darkly, stained. After one week the nuclei of the liver cells were densely stained and the cytoplasm showed iron deposition, the cells arranged regularly in lobules and central vein was congested, with shrinkage of blood sinusoides (Fig. 5). Also kidney was completely blocked by darkly stained iron deposition which in the form of granules scattered in the cytoplasm and increased in volume and number by time and the Lumina of many renal tubules and were occupied by cellular casts hyaline material with disrupted brush border (Fig. 5*). This accumulation exceeding a high rate by time and at the end of third week causing Kupffer cell proliferation and some hepatic cells with ruptured cell membrane were observed (Fig. 6) and renal tubules appeared irregular, dilated with flattened.
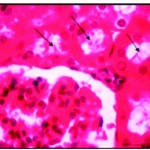 |
Figure 5: After one week of well fed with iron supplementation the renal lumina were occupied by cellular casts (Ø) and hyaline materisl with disrupted brush border X750.
|
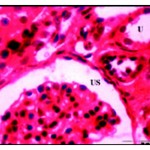 |
Figure 6: After three weeks of well fed with iron supplementation. Dialted renal tubules with flattened epithelial lining (U) and dilated urinary space (US) X 750.
|
Discussion
On supplementation, as known before, requires a highly complex set of regulatory approaches to meet demands of cells. In our experiment, iron is supplemented daily to the starved animals. This supplementation restores the moderate activity of these animals and the organs, which affected due to the shortage of food, are also appeared to be normal in colour and shape. In the meantime, the sections also restores the normal appearance. However, certain accumulation of stainable fine granules takes face. In this respect, it was concluded that body daily and weekly iron supplementation reduced the prevalence of iron deficiency and anemia (De Marshi et al., 1997).
The daily supplementation was found to be more effective than weekly for increasing hemoglobin and ferritin and caused greater reduction of the risk of anemia (Auerbach et al., 1998). The author’s depicted that the magnitude of hemoglobin response iron dependent on the amount of iron consumed. Current wideness suggested that supplementation should be taken daily to treat anemia, because the daily doses improved iron status. Whereas weekly ones was an effective way to prevent iron deficiency. Also, they reduced the side effects of excess iron accumulation (Schafer and Schaefer, 1992 and MacDougall et al., 1996) and kidative stress (Navarro et al., 1996) and maintained iron store for a long period then supplementation was stopped (MacDougall et al., 1996).
Homeostatic mechanisms increase intestinal iron absorption in iron deficiency, but its down regulation at high intake levels seems insufficient to prevent accumulation of high iron store at high intake. There is no regulation of iron excretion overload. Excess of pharmaceutical iron may cause toxicity and therapeutic doses may cause gastrointestinal side effect. Chronic iron excess amy lead to hepatic abrosis due to iron amplification of oxidative stress and increase of DNA damages Schumann, 2001).
The accumulation of iron in the starved animal’s liver observe here, may caused cytotoxicity due to delivery of free radical or may be reflected after opping supplementation the cells release the excess by certain ways. Basarab et al., 1999) demonstrated that, to counteract the free radical and/or excess iron the cells use a primary line of defense involving supraoxide dismutase, catalase, glutathione peroxidase. Phospholipids hydroxide glutathione peroxide provides a secondary line of defence, limiting membrane lipoperoxidation. Antioxidant such as vitamin E and C may limit the chain reaction formation of free radicals.
In contrast Viteri et al., (1999) depicted in his review article that the significant differences in the distribution of hemoglobin and plasma ferritin argue in favor of long-term weekly supplementation rather than repeated short-term daily supplementation periods for improving iron reserves and controlling mild moderate anemia.
The excesses accumulation of iron in well-fed animal’s liver was is dose-time dependent manner observed in this study. It was an important observation to pay attention to those who have no iron deficiency and take such drugs without may physician advice. This accumulation was found by Apple et al., (2001) who concluded that under administration of daily diet containing sufficient iron in different forms, iron was accumulated from the diet in liver, spleen and kidney in dose-dependent. Iron in its free ferrous and ferric forms may serve as a physiological regulator of normal intracellular functions but can be linked to several pathways of cellular toxicity (Tavill and Qadri 2004), in particular oxidative stress induced cytotoxicity leading to necrosis which may be promoted by increased intracellular free ion (Mohamed et al., 2006).
When hepatocyte-iron accumulates to excess induces liver damage and promoting sub cellular organelles damage leading to cell death. Patients with iron overload frequently suffer from hemochromatosis of major organs, such as the heart and liver (Emara, et al., 2006).
The accumulation which was shown here in the liver may be due to the link of excess iron with certain types of proteins, transferrin ferritin, heme-iron and non-heme free iron molecules. Knutson et al., (2002) found that the daily supplemented iron to normal and deficient rats had liver non-heme iron concentration that were 1.8-2.7 fold higher receptivity than unsupplemented normal rats.
The accumulated iron reported here was found to be very fine granules which enlarged by time. This observation agrees with certain evidence (Cazzola et al., 2003) who suggested that most of iron deposited in the cells in the form of mitochondrial ferritin. Magens et al., (2005) demonstrated that the nuclear deposits of stainable iron in hepatocytes are a sign of liver iron overload in mice. The nuclear iron deposits were most abundant in all mice fed carbonyl iron (2.5% w/w) for 52 weeks, almost irrespective of their genetic back ground. As the nuclear iron deposition corresponded the electron microscopically to aggregated ferritin molecules, the represent a non-immunoreactive form of presumably denaturated ferritin.
References
- Allen, L.H., Biological mechanisms that might underlie iron’s effects on fetal growth and preterm birth. J. Nutrit., 131: 581-589 (2001).
- Allen, L.H., Iron supplements: Scientific issues concerning efficacy and implications for research and programs. J. Nutrit., 132: 813-819 (2002).
- Allen, M.J., Cuper, C.F. and Woutresen, R.A., Disposition, accumulation and toxicity of iron fed as iron (II) sulfate or as sodium iron EDTA in rats. Food Chem. Toxical., 39(3): 261-269 (2001).
- Auerbach, M., Winchester, J. and Wahab, A., A randomize-trial of three iron dextran infusion methods for anemia in EPO-treated dialysis patients. Am. J. Kidney Dis., 31:
81-86 (1998). - Bassett, M.L., Halliday, J.W. and Powell, L.W., Value of hepatic iron measurements in early hemochromatosis and determination of critical iron level associated with fibrosis. Hematology., 6: 24-26 (1986).
- Beard, J.L., Iron biology in immune function musde metabolism and neurobal functioning., J. Nutrit., 131: 568-579 (2001).
- Besarab, A., Frinak, S. and Yee, J., Reviews: An Indistinct Balance, The safety and efficacy of parenteral iron therapy., J. Am. Soc. Nephrol., 10: 2029-2043 (1999).
- Cavill, I., Disorder of iron metabolism: Diagnostic methods. Clin. Hematol., 11:
259-273 (1982). - Cazzola, M., Invernizzi, R., Bergamashi, G., Levi, S., Corsi, B., Travaglino, E., Rolandi, V., Biasiotto, G., Drysdale, J. and Arosino, P., Mitochondrial ferritin expression in erythroid cells from patients with sideroblastic anemia. Blood, 101(5) : 1996-2000 (2003).
- De Macrhi, S., Ceechin, E., Falleti, E., Giacomello, R., Stel, G., Sepiacci, G., Bortolotti, N., Zanello, F., Gonano, F. and Batroli., Long-term effets of erythropoietin therapy on fistula stenosis and plasma concentrations of PDGE and MCP=1 in hemodialysis patients., J. Am. Soc. Nephtol., 18: 1147-1156 (1997).
- Emara, A.M., Kelany, R.S. and Moustafa, K.A., Comparative study of protective Effect between Deferoxamine and Deferiprone on Chronic Iron Overload Induced Cariotoxicity in Rats. The 30 conference of the Egyptian Society of Histology and Cytology (2006).
- Hallberg, L., Hulten, L., Bengtsson, C., Lapidul, L., and Lindstedt, G., Iron absorption from whole diet in men: how effective is the regulation of iron absorption? Am. J. Chin. Nutrit., 66: 347-356 (1995).
- Knutson, MD., Walter, PB., Ames, BN. and Viteri, FE., Article: Both iron Deficiency and Daily iron supplements increase lipid., J. Nutrit., 130: 621-628 (2000).
- Macdougall, IC., Tucker, B., Thomson, CR., Baker, LR. and Raine, AE., A randomized controlled study of iron supplementation in patients treated with erythroprotein. Kidney Ist., 50: 6194-1699 (1996).
- Magens, B., Dullmann, J., Schumann, K., Wulfheke, U. and Nielsen, P., Nuclear iron deposits in hepatocytes of iron-loaded HFF knock-out mice: a morphometric and immunocyto chemical analysis. Acta Histochem., 107(1): 57-65 (2005).
- Mohamed, F.H., Amal, M.M., Amany, S.E., Azza, R.E., and Nasra, N.A., Effect of Chronic Overload on the Liver, Kidney and Panreas of Albino Rat and the Possible protective Role of Vitamin-E, An Ultrastructural study. The 30 conference of the Egyptian Society of Histology and Cytology (2006).
- Navarro, JE, Teruel, JL., Liano, F., Marcen, R. and Ortuno, J., Effectiveness of intravenous administration of Fe-gluconate-Na complex to maintain adequate body iron stores in hemodialysis patients. AM. J. Nephrol., 16: 268-272 (1996).
- Ponka, P., Beaumont, C., and Richardson, DR., Function and regulation of transferrin and ferritin. Semin. Hematol., 35: 35-54 (1998).
- Prusak, M., Grzegorzewska, AE., Causes of disturbances of in iron turnover in chronic renal failure. Pol. Merkuriusz lok, 12(70):
309-313 (2002). - Schaefer, R. and Schaefer, L., Management of iron substitution during hu EPO therapy in chronic renal failure patients., Erythropoiesis., 3: 71-75 (1992).
- Schroder, M., Blumenstein, I., Jahnel, H., Dignass, AV., Stein, J., A study for evaluation of safety and tolerability of intravenous high-dose iron sucrose in patients with iron deficiency anemia due to gastrointestinal bleeding Z. Gastroenterol., 42(8): 663-667 (2004).
- Schemann, K., Safety aspects of iron in food., Ann. Nutv. Metab., 45: 91-101 (2001).
- Schwenk, MH, Blaustein, DA., Rapid high-dose intravenous iron sucrose therapy in 2 Jehovah’s witness patients with severe anemial iron deficiency and chronic kidney disease. Clin. Nephrol., 62(2): 116-120 (2004).
- Tavill, AS. and Qadri, AM., Alcohol and iron Semin. Liver Dis., 24(3): 317-325 (2004).
- Viteri, FE., Ali, F. and Tujague, J. Article: Long-term weekly iron supplementation improves and sustains non-pregnant women’s iron status a well or better than recommended short term daily supplementations. J. Nutrit., 129: 2013-2020 (1999):
- WHO. The prevalence of anemia in women: a tabulation of avilable information. WHO. Maternal Health and Safe Motherhood Programme, Nutrition Programme. Geneva, Switzer Land, PP 100 (1992).
- WHO., UNICEF/UNU (United Nations University). Consultation on the control of iron deficiency. WHO. Geneva, Switzer., (1999).

This work is licensed under a Creative Commons Attribution 4.0 International License.





