Manuscript accepted on : 19 June 2015
Published online on: --
E.B. Ezenwanne* and R.O. Aikpitanyi-Iduitua
Department of Physiology, School of Basic Medical Sciences, College of Medical Sciences, University Of Benin, Benin City, Edo State – Nigeria.
DOI : http://dx.doi.org/10.13005/bbra/2167
ABSTRACT: It was the aim of this studyto comparethePeak expiratory flow rate (PEFR) and some hematological indices in pregnant women withthose of non-pregnant women.This study was carried out in University of Benin Teaching Hospital Antenatal Clinic and St Philomena’s Catholic Hospital, Benin City, Edo State, Nigeria. A total of 118 women were engaged for this study. These were made up of 84 pregnant women,out of which 10 women were in their first trimester, 40 in their second trimester and 34 in their third trimester. Ethical approval for this study was obtained from the two hospitals. Another 34 non pregnant women were used as control group. All the participating women were within the age ranges of 25 to 35years. Determination of PEFR wasdone using mini Wright peak flow meter. Venous blood samplesof each subject were collected, and packed cells volume (PCV), total white cells count (WBC), platelet count and erythrocyte sedimentation rate (ESR)of each subject was determined.The PEFR was found to be significantly lower (p<0.05) compared with those of control subjects. The PEFR of the control women was 483.75±13.1,that ofthe first trimester was 462.25 ± 8.71, second trimester was 423.75± 10.51 and third trimester was 391.25 ± 7.81, respectively. Comparatively the PEFR was significantly lower (p<0.05) in the order ofthe trimesters, thus: first trimester < second <third. The Packed cell volume was also significantly decreasedacross the trimesters (p<0.001). The erythrocyte sedimentation rate wassignificantly higher across the trimesters (p<0.001). Platelet count value wassignificantly decreased in the first and second trimesters compared to the control subjects (p<0.001). Total white cells count increased significantly in the second and third trimesters (p<0.05). In conclusion, this study has drawn attention to thePEFR lowering effect of the gravid uterus on the pulmonary function of pregnant women. It can also be concluded that a decrease in packed cells volume and platelet countvaluesaccompanied with increase in erythrocyte sedimentation rate and total white cells count are features of normal pregnancy.
KEYWORDS: Hematological parameters; Peak flow rate; Comparative study; Pregnant women; Trimesters
Download this article as:| Copy the following to cite this article: Ezenwanne E. B, Aikpitanyi-Iduitua R. O. Comparative Study of Some Hematological Parameters and Peak Expiratory Flow Rate in Pregnant and Nonpregnant Women in Benin City, Nigeria. Biosci Biotech Res Asia 2015;12(spl.edn.2) |
| Copy the following to cite this URL: Ezenwanne E. B, Aikpitanyi-Iduitua R. O. Comparative Study of Some Hematological Parameters and Peak Expiratory Flow Rate in Pregnant and Nonpregnant Women in Benin City, Nigeria. Biosci Biotech Res Asia 2015;12(spl.edn.2). Available from:https://www.biotech-asia.org/?p=12758 |
Introduction
Peak expiratory flow rate (PEFR) was designated as the maximal air flow rate achieved while forcefully expelling from the lungs following maximal inspiration (Pocock and Christopher, 1999). The use of PEFR as a parameter for assessing respiratory function was first introduced by Hadon in 1942. From a position of maximum inspiration, the PEFR represents the largest expiratory flow rate expressed in litres per minute and has remained a simple effective tool for the assessment of ventilatory function (Wright and McKerrow, 1959;Pocock and Christopher, 1999;Dikshit et al., 2005). The inexpensive nature of the peak expiratory flow rate makes it a suitable test of ventilatory function for use in many parts of Africa where medical facilities are still poor and hence represents a simple, easy and portable test for lung function (Cotes, 1993; Quanjer et al., 1995; Radoes and Camargo, 2004). PEFR can be affected by a number of factors including age, sex, body mass index (BMI), daily activities, nutrition, pregnancy, and smoking. Thus, the PEFRmainly depends on the voluntary and muscular effort of the subject (Daniel et al., 2011).
Hematology is the branch of internal medicine and allied fields concerned with the study of blood, including the blood forming organs and diseases. Hematological parameters are those related to blood and the blood forming organs. The values of these parameters, some of which includes Packed Cells Volume (Hematocrit), Red and White Blood Cells counts, Platelet count, Erythrocyte Sedimentation Rate, Differential Leukocytes count, etc., enables the health worker to reach one of several diagnosis anddecisions.The fact remains that some hematological parameters are bound to show alterations in certain conditions, including pregnancy. For example, it is known that hematocrit value may be employed to evaluate anemic condition; erythrocyte sedimentation rate (ESR) value maybe employed as a non-specific measure of inflammation, sickle cell anemia or rheumatoid arthritis, hereditary spherocytosis and congestive heart failure (Pisetsky, 2007), platelet count value have been employed to monitor the cause of excess bleeding (Campbell and Neil, 2008; Schmaier, 2008), white blood cells count (which are the cells of the immune system) is areliable indicator that can be used to track several disease conditions, etc. Pregnancy, the state of retaining the developing embryo within the uterus of the female, is typically conveniently broken down into three periods or trimesters of the average duration of about 42 weeks (Cunningham et al., 2010). Maternal physiology undergoes considerable changes during pregnancy, and these are even secondary to the effects of progesterone and estrogen which are products of the ovary in the first 12 weeks of pregnancy, and thereafter are produced by the placenta. These changes enable both the fetus and placenta to develop and prepare the mother and baby for delivery (Bernhard and John, 2003). All the aforementioned changes are known as adaptations to the pregnant state which begins after conception. For example, maternal endocrine system adaptation to pregnancy state involves the hypothalamus, pituitary, parathyroid, thyroid, adrenal glands and the ovary, and all these are linked to varying degrees of interactions with fetal-placental-maternal units. Thus, there is no doubt that changes in hematological parameters are bound to occur in parallel with the adaptations to the pregnant state. It was the aim of this study to assess possible alterations in some of the vital hematological parameters in pregnancy state and compare these with those of non-pregnant state.
Materials and Method
Eighty-four (84) pregnant women between the ages of 20-40 years were enrolled in the Antenatal Clinic of the University of Benin Teaching Hospital (UBTH) and St. Philomena’s Catholic Hospital, Benin City, Nigeria, were engaged for this study. The studygroupings of the pregnant women were: ten (10) in the first trimester, forty (40) in the second trimester and thirty-four (34) in the third trimester. There was another group of thirty-four (34) non-pregnant age-matched women who also served as control subjects. Ethical approval was obtained from the Research and Ethics Committee of both Hospitals for the study. The women were screened with some exclusion criteria including those involving some types previous medical history and treatments, smokers, cases involving heavy regular consumption of alcohol, etc., all of which served as exclusion criteria for the study. Height and weight of each participating woman wasdetermined using standard anthropometric protocol (International Society for the Advancement of Anthropometry, 2001). The blood pressure of each subject wasalso measured.The BMI was calculated from the formula: weight (kg)/height (meters2).The peak flow rate was determined using the Wright’s mini peak flow meter while subject was in sitting position. Informed consentthat also met the inclusion criteria was obtained from each subject, and the purpose of the research and details of the procedures were explained to each subject.
Subjects were required to make maximal expiratory effort into the peak flow meter after an initial maximum inspiration. Three readings were taken with the highest value recorded.With the use of syringes and needle, hand gloves and methylated spirit, some hematological values were measured from the blood sample of each subject. The blood specimen bottles containing k EDTA (anticoagulant), cotton wool and tourniquet of about three milliliters of venous blood was collected from the median cubital vein with minimum stasis, while subject was seated. The blood samples were immediately transferred into the k EDTA containing tubes and properly mixed before they were transferred to a valid clinical laboratory whereupon, each was analyzed for Packed Cell Volume(PCV), Total White Cells Count, Platelets Count, Erythrocyte Sedimentation Rate (ESR), using automated haematological analyzer (Sysmex-k 10001VB=15.20, Japan). The results of the analysis of each of the blood samples were generated by the analyzer, and displayed by a paper speed on thermal printing copy. All statistical analysis were performed by the statistical package for the social science (SPSS), using the paired simple t-test with probability level p< 0.05 regarded as statistically significant.
Results
As presented in Table 1 werethe mean ±SEM values of the anthropometric characteristics and hematological parameters of the subjects. The results show that the gestational age increased significantly across the three trimesters (p< 0.001), the increase in the maternal weight was significant only in the third trimester, while the mean values of the maternal age and height remained unchanged. The determined values of the packed cell volume as noted in Table 2 revealed a consistently significantlydecreased across the three trimesters (p<0.001). Similarly, the platelets count showed notable decrease (p<0.005) in the second and third trimesters compared to the control. Values of the ESR increased significantly across the three trimesters (p<0.001), while the observed significant increase in WBC (p<0.005) was noted only in the second and third trimesters compared tothose of the control subjects. However, it is noteworthy that a significant increase was recorded when the result of the WBC of the first and third trimesters were compared.
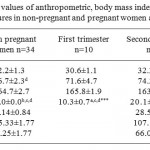 |
Table 1: Mean values of anthropometric, body mass index, systolic and diastolic blood pressures in non-pregnant and pregnant women across the trimesters.
|
*Significant values are Mean± SEM compared to the control (***, ** and * denotep<0.001, p<0.01and p<0.05 respectively). a represents the non-pregnant group, b represents women in first trimester, c represents women insecond trimester, drepresents women in third trimester.
Table 2: Peak expiratory flow rate (PEFR) and some haematological parameters as determined in the non-pregnant and pregnant women across the three trimesters
|
Non-Pregnant women n=34 |
First trimester n=10 |
Second trimester n=40 |
Third trimester n=34 |
|
|
PEFR |
483.75±0.37 |
462.25±0.65* |
423.75±0.87* |
391.25±7.81* |
| PCV (%) | 37.6±0.7b,c,d | 33.4±0.7a** | 31.8±1.0a** | 32.6±1.2a** |
| WBC(103) | 5.05±0.34c,d | 5.76±0.47d | 6.59±0.58* | 7.51±0.48a,b* |
| Platelets(103) | 255.40±24.5c,d | 203.7±14.3 | 182.56±9.61a* | 166.40±12.8a* |
| ESR(mm/hr) | 09.6±1.0b,c,d | 29.2±3.5a,d*** | 33.1±2.4a*** | 40.3±6.2ab*** |
*Significant values are Mean ± SEM compared with control (*** ,**and *denotep<0.001, p<0.01 and p<0.05 respectively).arepresents the non-pregnant group,b represents women in first trimester,c represents women in second trimester, drepresents women in third trimester.
Discussion
As was noted in some earlier report, pregnancy and advancing gestation were associated with physiological changes in ventilatory function (Pernoll et al, 1975). In this study, PEFR showed a fairly consistent decrease acrossthe three trimesters of pregnancy (Table 2). Furthermore, the result revealed that the decrease in mean PEFR value ofthe pregnant womenwas lowest in the third trimester (391.25±7.81/min). Thus, significant decrease in PEFR in the three trimesters of pregnancy with the highest decreaseoccurring in the third trimester havebeen confirmed in the present study.
Theobserved progressive decrease in PEFR values in the two trimesters of pregnancy in this study can be attributed to the mechanical effects of the enlarged uterus caused by upwards thrusts (pushing) applied to the diaphragm,and which obviously limits its movements. Thus, it can be argued that there was also the resultant airways obstruction translated into the reduced PEFR valuesespecially in the late pregnancy (third trimester)where thehighest decline in PEFRin this study (391.251/min) was observed (Table 2).
Amongst the hematological parameters examined in this study, thepacked cell volume (PCV) of the pregnant women showed a clear decrease across the three trimesterscompared to the non-pregnant subjects (Table 2). This result isconsistent with the reports of earlier workers who similarly noted a decline in PCV in women in various states of pregnancy (Osoagbaka et al., 2000; Imam and Yahaya, 2008; Osonoga et al., 2011). James et al. (2008)also reported a significance difference in PCV across the trimesters. However, Dapper et al.(2006) on the other hand made some contrary observation, in which case the PCV was found to be lowest in the second trimester.
It is note worthy that the observed decrease in the PCV value was not statistically significant in the first and second trimesters and there was a slight increase in the third trimester (Table 2). These findings appear to be largely in agreement with those of Akingbola et al.(2006) and the reports of Akinsegun et al.(2013). Decreases in the PCV may be due to the vasodilatation activities of progesterone which ultimately leads to a reduction in blood pressure, thereby triggering the release of rennin andaldosterone through the renin-angiotensin system (RAS). The RAS on the other hand go through the normal tendency forthe retention of renal sodium thereby resulting into an increase in plasma volume (45%). Since the increase in plasma volume does not normally affect red cell mass, a physiological anaemia in pregnancy is obviously bound to result (Bernard and John, 2003). In this study, there was a slight but significant rise in PCV in the third trimester (Table2) which might be as a result of the slower increase in plasma volume, characteristic of late pregnancies. Also, it is known that in late pregnancy, the turn-over rate of progesterone and oestrogen occur and which eventually lead to the decrease in plasma volume with resultantslight rise in PCV value.
The increase in erythrocyte sedimentation rate (ESR) observed in this study was largely consistent with thefindings of earlier workers (Osoagbaka et al., 2000; Sonuga et al., 2011). It is possible that this can also be attributed to increases in plasma volume anemia and marked increase in fibrinogen as known to normally occur in pregnancy (Manten et al., 2004).
Earlier reportshave noted increases in total white blood cells (WBC) count in the state of pregnancy as was similarlyobserved throughout the three trimesters in this study (Table 2). While Pitkin and Witte (1979) generally reported increases in WBC in the state of pregnancy,it was observed in one other report that such increases in WBC may have been as the result ofincreases in neutrophils(Akinsegun et al., 2013). On the other hand, pain, nausea and vomiting have been reported and attributed to leukocytosis in pregnancy without infection. In late pregnancy however, a gradual reduction was observed. This report is consistent with thework of Akingbola et al.(2006). It is believed that this may be as a result of gestational thrombocytopenia, although absolute WBC count tends to remain within the normal range for most women throughout the trimesters.
Conclusion
The results of this study have clearly demonstrated the possible effect of the gravid uterus on the peak expiratory flow rate which was noted to decrease progressively in the trimesters. Thus, it can be concludedthat decrease in packed cell volume and platelet count withconcomitant increase in erythrocyte sedimentation rate and total white cell count are tfeatures ofnormal pregnancy, andthis is alikelytenable hypothesis.
References
- Akingbola, T.S., Adewole, F., Adesina, K.A., Fehintola, F. S., Bamgboye, E.A., Haematological profile of healthy pregnant women in Ibadan, south-western Nig. J. Obstet Gynecol; 2006; 26(8):763-769.
- Akinsegun, , Sarah, O. A., Kabiru, A.R., Adeniyi, A. A., Adedoyin O.D.,Adewumi, A., Vincent, O.O., Bodurin, I., and Kamal, A.I., Haematological profile of normal pregnant women in Lagos, Nig. Intwomens Health, 2013; 5: 227-232.
- Bernhard, , John, H. Changes in maternal physiology during pregnancy. British Journal ofAnaesthasia; 2003; 3(3):65-68.
- Campbell, , Neil, A, (2008): Biology(8th edition) London Pearson education. Pp912.
- Cotes E. Lung function throughout life. Oxford Blackwell scientific, 1993; 5: 445-513
- Cunningham F., Leveno , Haulth J., Touse D. and Spong C. Williams textbook of obstetrics 23rd ed. McGraw Hill professional publishing., 2010; Pp 282
- Daniel R. Neuspiel D., Courtland T. Michael R.F. Peak flow measurement. J Med Drugs Dis prol, 2001; 12: 139-145.
- Dapper, V., Ibe. C.J., Nwauche, C.A. Haematological values in pregnant women in Port Harcourt, Nigeria. Nig. J Med, 2006; 15(3): 237-240.
- Dikshit B., Raje S. and Agrawal M.J. Lung functions with spirometry: An Indian perspective, peak expiratory flow rates. Indian J. physiol. , 2005; 49(1): 8-18.
- Imam, T.S., Yahaya,A. Packed cell volume of pregnant women attending Dawakin Kudu General Hospital, Kano State, Int. J. P App Scs, 2008; 2(2): 46-50.
- James, T. , Reid,H.L., Mullings, M.A. Are published standards for haematological indices in pregnancy applicable across populations: an evaluation in healthy pregnant Jamaican women. BMC pregnancy Childbirth; 2008; 8:8-11.
- Manten, T.R., Franx,A., Sikkema, M., Hameeteman, T.M., Viser, G.H., Fibrinogen and high molecular weight fibrinogen during and after normal pregnancy. Throm Res, 2004; 114(1):19-23.
- Osoagbaka, U., Haruna-Rasheed, U.H., Anokwuru, O.C. Observations of some haematological parameters of some Nigerian women during pregnancy. Journal of Medical Investigation and practice; 2000; 1: 45-48.
- Osonuga, O., Osonuga, O.A., Onadeko, A.A., Osonuga, A., Osonuga, A.A., Haematological profile of of pregnant women in south-west of Nig. Asian Pac J. Trop.Dis, 2011; 1: 232-234.
- Pisesky, S. Laboratory testing in the rheumatic diseases.In: Goldman, I., Ausiello, D. eds. Cecil Medicine. 23rd ed. Philadelphia, Pa: Saunders Elsevier; 2007; p 952.
- Pitkin, M., Witte, D. Platelet and leucocyte count in pregnancy. JAMA; 1979; 242(24): 2696-2698.
- Pocock G and Christopher R. (1999): Human Physiology, the Basis of Medicine. BM Oxford University Press, 337.
- Quanjer P.H., Borsboom G.J., Brunekreef , Zach M., Forche G., Cotes J.E., Sanchis J., Reference values for white Europeann children and adolescents: Polgar revisited. Pediatrpulmol 1995; 19:135-42
- Radoes S., and Camargo C.A. Predicted peak expiratory flow: Differences across formulae in the literature, Am J Emerg. Med. 2000; 22(7):516-21.
- Schmaiser, H. Laboratory evaluation of hemostatic and thrombotic disorders.Hoffman heamatology basic principles and practice.5th ed. Philadelphia, Pa Churchill Livingstone Elsevier; 2008; pp 122.
- Wright A. and McKerrow B. Maximum forced expiratory flow as a measure of ventilator capacity. British Med J, 1985; 2: 1041-7.
(Visited 376 times, 1 visits today)

This work is licensed under a Creative Commons Attribution 4.0 International License.





