Manuscript accepted on : September 26, 2010
Published online on: 28-12-2010
Protective Effect of Marjoram Plant against the Toxicity of Cadmium Chloride in Testes of Albino Rat
Samira Omar Abu Bakr Balubaid
Department of Biology, Faculty of Science, King Abdul Aziz University, Jeddah Saudi Arabia.
ABSTRACT:
Due to the extensive use of the marjoram plant in the treatment of some diseases, with a lack of its scientific research , there for the aim of this study was to identify the extent of safety when used and to study its protective effect against the toxicity of cadmium chloride on testicular tissue. Thirty male albino rats ( 60- days) age divided into four groups, the first group ( control group ) , group II treated with cadmium chloride (2mg/kg), the third group treated with marjoram (2mg/kg), the fourth group treated with both cadmium(2mg/kg) and marjoram(2mg/kg). Cadmium treated group showed imbalance in both structure and physiology of the testis .Also, an obstruction of spermatogenesis and spermiogenesis process appeared as degradation of spermatocytes, decrease in their number and distort their structure with low testosterone level . The third group treated with marjoram only showed similarity to the control structure with increased density of spermatocytes and sperm, also the testosterone level was comparable to the control group. Co- administration of cadmium and marjoram lessened most histopathological changes in tissue structure and balance the testosterone level . Thus it was recommend : Non-use of cadmium metal and try to find a replacement for it in industry because of its adverse effect on male fertility .Also ,we hope of using marjoram as protective agent for its safe on tissue and increases the rate of male fertility
KEYWORDS: cadmium chloride; albino rat-testis; marjoram; testosterone
Download this article as:| Copy the following to cite this article: Balubaid S. O. A. B. Protective Effect of Marjoram Plant against the Toxicity of Cadmium Chloride in Testes of Albino Rat. Biosci Biotech Res Asia 2010;7(2) |
| Copy the following to cite this URL: Balubaid S. O. A. B. Protective Effect of Marjoram Plant against the Toxicity of Cadmium Chloride in Testes of Albino Rat. Biosci Biotech Res Asia 2010;7(2).Available from: https://www.biotech-asia.org/?p=9015. |
Introduction
Cadmium (Cd) was an environmental toxicant and an endocrine disruptor in humans and rodents. Also, Cd was known to be a carcinogenic metal that especially its compounds had sufficient evidence in both humans and experimental animals beneath its environmental effects (Ates et al .,2004). Several organs (e.g., kidney, liver) were affected by Cd and recent studies had illustrated that the testis was exceedingly sensitive to Cd toxicity (Ates et al .,2004, Martin et al .,2007 and Sadik,2008). More important, Cd might account for the recent declining fertility in men among developed countries by reducing sperm count and testis function (Siu et al 2009 ). Clinical and animal studies indicated that abnormalities of spermatogenesis resulted from exposure to three toxic metals (lead acetate, cadmium chloride, and arsenic trioxide) (Nava et al .,2009) because the testes were one of the most sensitive organs to acute cadmium toxicity ( Kim and Soh 2009). Sadik (2008 ) showed decreased body weight, paired testicular weight, relative testicular weight, serum testosterone when male adult Wistar rats treated with cadmium (2.5 mg/kg body wt, five times a week for 4 weeks) where the testicular toxicity by Cd appeared as inhibition of androgen production in adult male rats probably by affecting pituitary gonadotrophins.So, Cd was considered gonadotoxic and spermiotoxic factor (Ola et al ., 2008). Ates et al .,(2004) proposed that cadmium also increased oxygen derived free radicals and lipid peroxidation which led to DNA damage.
The most important attention was paid to the search of natural dietary antioxidants and their evaluation in medicinal and food raw materials of plant origin. A number of plants, their extracts, food products, and medicinal preparations appeared to be the objects of scientific research (Raudonis et al .,2009 ) .They were studied for their ability to protect cells from miscellaneous damages. Marjoram volatile oil was potent antioxidants ( El-Ashmawy et al ., 2007) and was known to possess various therapeutic properties (Aristatile et al .,2009) . Al-Howiriny et al .,(2009) used Marjoram at doses of 250 and 500 mg/kg of body weight and found significantly decreased the incidence of ulcers, basal gastric secretion and acid output. Furthermore, the extract replenished the ethanol-induced depleted gastric wall mucus. Ulcer preventing potential was further confirmed by histopathological assessment. An acute toxicity test showed a large margin of safety of the extract in mice.
El-Ashmawy et al., (2005) illustrated that the administration of Origanum majorana (volatile oil, alcoholic and aqueous extracts) with oral lead acetate in the diet of mice at concentration of 0.5% (W/W) for one month showed improvement in the liver and kidney histology in comparison with lead acetate treated group. Alcoholic extracts of majorana significantly reduced the rate of micronucleus, number of aberrant cells and different kinds of chromosomal aberrations .Aqueous extract and volatile oil of majorana significantly reduced number of gaps, ring chromosome and stickiness. It could be concluded that majorana played an important role in ameliorating liver and kidney functions and genotoxicity induced by lead toxicity.
Dorman et al .,(2004) found that the Syrian oregano extract was the most effective chelator of iron(II), while Spanish and Turkish oregano extracts were the most effective inhibitors of non site-specific hydroxyl radical-mediated 2-deoxy-d-ribose degradation. All the extracts contained Folin-Ciocalteu reagent-reactive substances, which was confirmed by the presence of polar phenolic analytes.
Botoglou et al., (2002) showed that as oregano oil increased in the diet, malondialdehyde values decreased in tissue samples, suggested that the oil, particularly at 100 mg/kg of feed, exerted an antioxidant effect on chicken tissues against iron where Iron-induced lipid oxidation.
All the previous researches confirmed that the cadmium metal was highly toxic and affected both plant and animal organisms .If human exposed to very low dose of cadmium metal for long periods of time, whether through drink or food or by inhalation of polluted air, this might cause chronic toxicity led to weaken of the immune system, as a result of the accumulation within the tissues, leading to infection Therefore the objective of this research ,shed light on the effects of cadmium on tissue composition of birth testicle and then to reduce and mitigate them by using the Marjoram plant, where few previous studies indicated its great protective role.
Materials and Methods
Materials
30 male albino rats ( 60- days) age were reared in animal house at the center of King Fahad of Medical Researches in Saudi Arabian, divided into four groups were maintained in polypropylene cages of 50x33x20 cm at a constant temperature (21±1ºC) and 62% relative humidity, were given all the daily dose orally for 10 days as follows: the first group ( control group), the second group treated with cadmium chloride (2mg/kg)( Fouad et al., 2009), the third group treated with a similar dose of aqueous extracts of Marjoram plant ( Ashmawy et al., 2005), the fourth group treated with both cadmium chloride (2mg/kg)and aqueous extracts of Marjoram plant(2mg/kg) .
Methods
Both the control and experimental animals were killed by cervical decapitation and dissected. Testes were extracted and histological preparation were carried according to (El- Banhawy and El- Gansory, 1989).
Specimens were fixed in 10% neutral formalin and the standard procedures of dehydration, clearing and embedding in wax were followed. They were sectioned at 3-5 µm and stained with Hematoxylin and eosin(Drury and Wallington1980).
The animals at the time of tissue collection were slightly anaesthetized , bled within two minutes by eye using a heparinized syringe, plasma samples were separated by centrifugation, frozen and stored at -85ºC until all samples have been collected for the determination of testosterone. Plasma testosterone levels were measured by radioimmunoassay (RIA) according (Chang et al. 1995).
Statistical analysis
The body and tests weight in both control and experimental groups were analyzed by using the program SAS (Institute Inc.1988, Cary, NC, USA) where t-test was used to assess the significance of changes between control and treated mice (Sokal & Rohlf 1981).
Results
During the experiment, all animals survived and this means that the doses administered, the laboratory conditions of water, food and shelter were appropriate.
The group treated with Marjoram plant
From table (1) homogeneity marjoram and control groups in both testes weight and body weight were noticed , where testes weight in marjoram group reach ( 0 .957±0.62 g) and their body weight reach ( 251.7±4.41g).
Examination of testicular sections of Marjoram treated group showed that the tests take circular shape surrounded by testicular capsule consisting of collagen fibers have spindle shape fibroblasts followed by internal vascular layer containing blood vessels. The testis consists of a large number of round or oval seminiferous tubules and majority of these seminiferous tubules filled with large numbers of spermatozoa in the lumen and there were an increase in epithelium height of the tubules (Fig.2) .Each seminiferous tubule had pyramidal Sertoli cells with pale triangular nucleus alternated with many spermatogonia appeared as smaller dark cells rest on the basal lamina and followed by primary spermatocytes with large nuclei and dense chromatin followed by secondary spermatocytes appeared small in size and has a faint nucleus . It was also observed that the spermatozoa filled the lumen of these tubules with high intensity (Fig.3 ) compared with the control group(Fig.1).
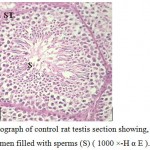 |
Figure 1: Light micrograph of control rat testis section showing, seminiferous tubule (ST) with central lumen filled with sperms (S) ( 1000 ×-H α E ). |
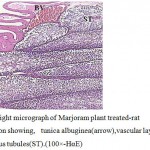 |
Figure 2:Light micrograph of Marjoram plant treated-rat testes section showing, tunica albuginea(arrow),vascular layer(BV)and seminiferous tubules(ST).(100×-HαE) |
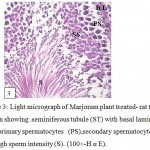 |
Figure 3: Light micrograph of Marjoram plant treated- rat testes section showing . seminiferous tubule (ST) with basal lamina (BL),primary spermatocytes (PS),secondary spermatocytes (SS) and high sperm intensity (S). (100×-H α E). |
The level of blood testosterone reached (24.42 ± 0.257 ng / mg) that approximated the hormone level of control group (24.45 ± 0.275 ng / mg).
The group treated with cadmium
From table (1) cadmium group showed a clear reduction in both testes weight (0.620±.012 g.) and body weight (185.0±2.89 g.)
The volume of testes became smaller in compared with the control group. From histological examination of testicular sections in this group ,it found that sexual fertility delayed in comparison to control and other experimental group where the germ cells sloughed into the tubular lumen and the germinal epithelium was distributed (Fig.4). Also, the tunica albuginea was disrupted, more lytic intestinal tissue with few Leydig cells were observed, the seminiferous tubules suffered from low epithelium height and surrounded by irregular basal lamina (Fig.5). This accompanied with retardation and alteration in the histological structure of the tubules, this retardation that recognized by the separation of the necrotic dense germinal cells and a sharp decrease in both the mature spermatozoa and primary spermatocytes with central eosinic material .Sertoli cells could not be detected but Liquid infiltration was detected in interstitial tissue (Fig.6). Also, the usage of cadmium showed low testosterone level as it reached (13.03± 0.125 ng / mg).
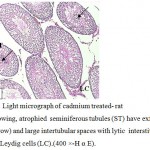 |
Figure 4: Light micrograph of cadmium treated- rat testis showing, atrophied seminiferous tubules (ST) have exfoliated cells (arrow) and large intertubular spaces with lytic interstitial tissue (IT) and Leydig cells (LC).(400 ×-H α E). |
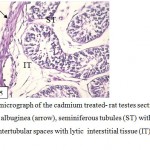 |
Figure 5: Light micrograph of the cadmium treated- rat testes section showing, disrupted tunica albuginea (arrow), seminiferous tubules (ST) with deformed germ cells and large intertubular spaces with lytic interstitial tissue (IT) . (400 ×-H α E ). |
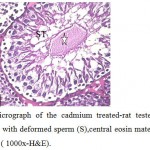 |
Figure 6: Light micrograph of the cadmium treated-rat testes section showing, seminiferous tubule with deformed sperm (S),central eosin material(star) and liquid infiltration (arrow). ( 1000x-H&E). |
The group treatment with cadmium and Marjoram plant
The testes weight (0.850±.029 g.) and body weight (228.3±6.009 g.) in this group increased than their comparable in cadmium group .
The testes sections treated with cadmium and Marjoram showed improvement in the structural organization of testes where the seminiferous tubules took the round or oval shape and the connective tissue between them appeared normal in quantity and contained leydig cells and blood vessels (Fig.7).With high power ,presence of well-arranged stages of spermatogenesis included spermatogonia, large primary spermatocytes (with central rounded nuclei, small size secondary spermatocytes) , round spermatids and moderate amount of spermatozoa directed to Sertoli cells were recorded. (Fig.8).Still, there was low liquid infiltration level in compare with cadmium treatment group. Also, noted the high level of testosterone hormone comparable to the cadmium treatment group (22.88±0.176 ng/mg) .
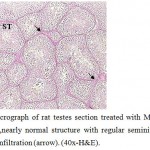 |
Figure 7: Light micrograph of rat testes section treated with Marjoram plant and cadmium showing ,nearly normal structure with regular seminiferous tubule (ST) suffer from liquid infiltration (arrow). (40x-H&E). |
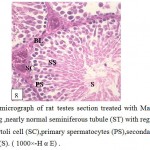 |
Figure 8: Light micrograph of rat testes section treated with Marjoram plant and cadmium showing ,nearly normal seminiferous tubule (ST) with regular basal lamina (BL), healthy Sertoli cell (SC),primary spermatocytes (PS),secondary spermatocytes (SS) and sperms (S). ( 1000×-H α E) . |
Discussion
The present results showed homogeneity Marjoram and control groups in both testes and body weight but cadmium group showed a clear reduction in them , this consistent with the results of Sadik (2008 ) who showed that cadmium lowered rat body weight and testis, while cadmium and Marjoram group showed improvement and that may be due to the presence of the antiradical activity of Marjoram (Dambolena et al.,2010 and Botoglou et al., 2002).
The present findings indicated that exposure to cadmium affected primary spermatocytes and this agreed with ( Nava- Hernández et al .,2009) who found that the chronic exposure (13 weeks) to toxic metals, affected primary spermatocytes DNA and were suggested of possible direct testicular toxicity.
From the present research, Cd intoxication significantly decreased testicular sperm and this agreed with (Ola- Mudathir et al ., 2008 ) who recorded decreased epididymal sperm concentration and sperm progress motility, increased percent total sperm abnormalities and live/dead count .
In between seminiferous tubules, there were interstitial connective tissue and some Leydig cells which secrete the male hormone testosterone (Junqueira et al., 1998) and this might explained the low level of testosterone in cadmium treated group as it was noticed that there were a few number of Leydig cells in the interstitial tissue and this agreed with Fouad et al .,(2009) who found that testicular damage was induced by a single i.p. injection of cadmium chloride (2mg/kg) in which cadmium reduced testosterone level glutathione, catalase and superoxide dismutase activities .
Sertoli cells had many functions as feeding, supporting spermatids; hormones secreation which regulated Leydig cells function, spermatocytes division and spermatozoa formation . Also, Sertoli cells had an important role in the formation of testicular blood barrier which provided good circumstances for the developed spermatocytes and these cells were undividable so they had resistance to the harmful ecological factors as food shortage (Junqueira et al., 1998).
Cd-induced toxicity to the testis was probably the result of interactions of a complex network of causes. This was likely to involve the disruption of the blood-testis barrier (BTB) via specific signal transduction pathways and signaling molecules, such as p38 mitogen-activated protein kinase (MAPK) (Siu et al., 2009 ).Also, Kaisman et al .,(2009) indicated that Clusterin, is a glycoprotein produced abundantly by Sertoli cells lining the walls of the seminiferous tubules which associated with either apoptosis or cell survival, Cd(2+) influx induce expression and secretion of Clusterin, thereby linking metal homeostasis and germ cell fate. So the defects of Sertoli cells in cadmium treated group resulted in loss of spermatic cells and might lead to the destruction of this tissue and infertility as indicated before (Monsees et al., 2000).
It was summarized in this work that the harmful effect of cadmium on testis was known to be germ cell degeneration (Oner et al .,2005) , Sertoli cell damage and impairment of testicular steroid genesis as indicated before (Sadik,2008).Also, Gurel et al .,(2007) investigated that the seminiferous tubules and Leydig cells were damaged by cadmium. Martin et al .,(2007) recorded At high doses (5 mg/kg cadmium chloride or higher), testicular damage in mice, rats, and other rodents included interstitial edema, hemorrhage, and changes in the somniferous tubules affecting spermatogenesis . Yang et al .,(2006) found that ,when animals received cadmium at 2 mg/kg, undifferentiated spermatids and dead Sertoli cells increased in the seminiferous tubules while interstitial cells decreased and inflammatory cells increased in the interstitial tissues. On flow cytometric analysis, the numbers of elongated spermatids and round spermatids decreased .Oner et al .,(2005) found that spermatic cells in seminiferous tubules of CdCl2 treated animals displayed necrosis and homogenous pink particles were present in spermatic places. Also, interstitial areas were edematous and intertubular vessels were plugged.
El-Demerdash et al.,( 2004) found that treatment with CdCl2 caused a significant decrease in sperm concentration, motility (%), weight of testes and epididymis, and increase in dead and abnormal sperm.
The present results suggested that Marjoram plant affords a significant testoprotective and hypolipidemic effect against Cd and this agreed with Aristatile et al .,(2009) who found that the administration of Marjoram for 21 days prevented and improved the histopathological parameters of Cd toward normalcy.
It may be concluded that marjoram plant was useful herbal remedies, especially for controlling oxidative damages in which, co-administration of protective plants resulted in minimizing the hazard effects of Cd toxicity on male fertility .
Acknowledgment
I am very grateful to Dr.Awatef M. Ali for her continuous encouragement whilst completing this manuscript.
References
- Al-Howiriny T, Alsheikh A, Alqasoumi S, Al-Yahya M, Eltahir K, Rafatullah S.(2009). Protective Effect of Origanum majorana L. ‘Marjoram’ on Various Models of Gastric Mucosal Injury in Rats. Am J Chin Med.;37(3):531-45
- Aristatile B, Al-Numair KS, Veeramani C, Pugalendi KV.(2009). Antihyperlipidemic effect of carvacrol on D-galactosamine-induced hepatotoxic rats. J Basic Clin Physiol Pharmacol.;20(1):15-27
- Ates I, Suzen HS, Aydin A, Karakaya A.(2004).The oxidative DNA base damage in testes of rats after intraperitoneal cadmium injection Biometals. Aug;17(4):371-7
- Botoglou NA, Florou-Paneri P, Christaki E, Fletouris DJ, Spais AB (2002). Effect of dietary oregano essential oil on performance of chickens and on iron-induced lipid oxidation of breast, thigh and abdominal fat tissues. Br Poult Sci. May;43(2):223-30
- Chang CF, Lau EL & Lin BY .(1995). Estradiol-17B suppresses testicular development and stimulates sex reversal in protandrous black porgy, Acanthopagrus schlegeli.Fish Physiol .Biochem. 14: 481-488.
- Dambolena JS, Zunino MP, Lucini EI, Olmedo R, Banchio E, Bima PJ, Zygadlo JA (2010).Total phenolic content, radical scavenging properties, and essential oil composition of origanum species from different populations. J Agric Food Chem. Jan 27;58(2):1115-20
- Dorman HJ, Bachmayer O, Kosar M, Hiltunen R.(2004). Antioxidant properties of aqueous extracts from selected lamiaceae species grown in Turkey. J Agric Food Chem. Feb 25;52(4):762-70.
- Drury, R. A. and Wallington, E. A. (1980). Carleton’s Histology Technique,4th Ed.Oxford University Press. New York, Toronto.
- El-Ashmawy IM, El-Nahas AF, Salama OM. (2005). Protective effect of volatile oil, alcoholic and aqueous extracts of Origanum majorana on lead acetate toxicity in mice. Basic Clin Pharmacol Toxicol. Oct;97(4):238-43.
- El-Ashmawy IM, Saleh A, Salama OM.( 2007 ). Effects of marjoram volatile oil and grape seed extract on ethanol toxicity in male rats. Basic Clin Pharmacol Toxicol. Nov;101(5):320-7
- El-Banhawy, M.and El-Gansory, M. (1989).Microscopical technich.1st edd.Cairo, Al-maarf.
- El-Demerdash FM, Yousef MI, Kedwany FS, Baghdadi HH.(2004). Cadmium-induced changes in lipid peroxidation, blood hematology, biochemical parameters and semen quality of male rats: protective role of vitamin E and beta-carotene Food Chem Toxicol. Oct;42(10):1563-71
- Fouad AA, Qureshi HA, Al-Sultan AI, Yacoubi MT, Ali AA.(2009) .Protective effect of hemin against cadmium-induced testicular damage in rats. Toxicology. Mar 29;257(3):153-60. Epub 2008 Dec 30.
- Gurel E, Caner M, Bayraktar L, Yilmazer N, Dogruman H, Demirci C.(2007) .Effects of artichoke extract supplementation on gonads of cadmium-treated rats. Biol Trace Elem Res. Oct;119(1):51-9
- Junqueira, L. C.; Carneiro, J. and Kelley, R. O. (1998).Basic histology.8thed.Prentice-Hall International, Inc 301-407 .
- Kaisman T, Sekler I, Fishman D, Karol N, Forberg M, Kahn N, Hershfinkel M, Silverman WF.(2009). Cell death induced by zinc and cadmium is mediated by clusterin in cultured mouse seminiferous tubules. J Cell Physiol. Jul;220(1):222-9
- Kim J and Soh J.(2009). Cadmium-induced apoptosis is mediated by the translocation of AIF to the nucleus in rat testes. Toxicol Lett. 10;188(1):45-51.
- Martin LJ, Chen H, Liao X, Allayee H, Shih DM, Lee GS, Hovland DN Jr,
- Monsees TK, Franz M, Gebhardt S, Winterstein U, Schill WB, Hayatpour J.( 2000 ). Sertoli cells as a target for reproductive hazards. Andrologia. Sep;32(4-5):239-46.
- Nava-Hernández MP, Hauad-Marroquín LA, Bassol-Mayagoitia S, García-Arenas G, Mercado-Hernández R, Echávarri-Guzmán MA, Cerda-Flores RM.(2009). Lead-, cadmium-, and arsenic-induced DNA damage in rat germinal cells. DNA Cell Biol. May;28(5):241-8.
- Raudonis R, Jakstas V, Burdulis D, Benetis R, Janulis V.(2009). Investigation of contribution of individual constituents to antioxidant activity in herbal drugs using postcolumn HPLC method. Medicina (Kaunas).;45(5):382-94
- Sadik NA. (2008 ).Effects of diallyl sulfide and zinc on testicular steroidogenesis in cadmium-treated male rats. J Biochem Mol Toxicol. Sep;22(5):345-53
- Siu ER, Mruk DD, Porto CS, Cheng CY.(2009). Cadmium-induced testicular injury. Toxicol Appl Pharmacol.Aug 1;238(3):240-9. Epub Feb 21.
- Ola-Mudathir KF, Suru SM, Fafunso MA, Obioha UE, Faremi TY.(2008). Protective roles of onion and garlic extracts on cadmium-induced changes in sperm characteristics and testicular oxidative damage in rats. Food Chem Toxicol. Dec;46(12):3604-11. Epub Sep 12
- Oner H, Karatepe M, Karatas F, Oner J, Yilmaz I, Cukurovali A.(2005). Effects on rat testes of the thiosemicarbazone derivative Schiff base (4-(1-phenylmethylcyclobutane-3-yl)-2-(2-hydroxybenzylidenehydrazino)thiazole) and its cadmium(II) complex. Cell Biochem Funct. Nov-Dec;23(6):427-33.
- Yang HS, Han DK, Kim JR, Sim JC.(2006) . Effects of alpha-tocopherol on cadmium-induced toxicity in rat testis and spermatogenesis J Korean Med Sci. Jun;21(3):445-51

This work is licensed under a Creative Commons Attribution 4.0 International License.





