Manuscript accepted on : December 13, 2010
Published online on: 28-12-2010
Free Radical Scavenging Activity of Vitex Negundo
G. Mohandass1 and K. Suguna Devi2
1Department of Biomedical Engineering, Sathybama University, Chennai - 119 India.
2Prince Dr. K. Vasudevan College of Engineering and Technology, Chennai India.
Corresponding Author E-mail:arokiyarajs@yahoo.in
ABSTRACT: Natural products have recently become the focus of increased research interest due to their potential pharmacological activities. Therefore, the present investigation was carried out to evaluate Vitex negundo for free radical scavenging activity by diphenyl-picryl-hydrazyl (DPPH) and nitric oxide (NO) radical scavenging assay methods. The results of the present study revealed that Vitex negundo showed remarkable free radical scavenging activity comparable to the standard (Curcumin and BHA).
KEYWORDS: Antioxidant, DPPH, Vitex negundo.
Download this article as:| Copy the following to cite this article: Mohandass G, Devi K. S. Free Radical Scavenging Activity of Vitex Negundo. Biosci Biotech Res Asia 2010;7(2) |
| Copy the following to cite this URL: Mohandass G, Devi K. S. Free Radical Scavenging Activity of Vitex Negundo. Biosci Biotech Res Asia 2010;7(2). Available from:https://www.biotech-asia.org/?p=9678 |
Introduction
Free radicals contribute to more than one hundred disorders in humans including atherosclerosis, arthritis, ischemia and reperfusion injury of many tissues, central nervous system injury, gastritis, cancer and AIDS (Kumpulainen and Salonen 1999; Cook and Samman 1996). Free radicals due to environmental pollutants, radiation, chemicals, toxins, deep fried and spicy foods as well as physical stress, cause depletion of immune system antioxidants, change in gene expression and induce abnormal proteins. Oxidation process is one of the most important routs for producing free radicals in food, drugs and even living systems. Catalase and hydroperoxidase enzymes convert hydrogen peroxide and hydroperoxides to nonradical forms and function as natural antioxidants in human body. Due to depletion of immune system natural antioxidants in different maladies, consuming antioxidants as free radical scavengers may be necessary (Halliwell 1994; Kumpulainen and Salonen 1999; Younes 1981). Antioxidants are the substance that reduce, neutralize and prevent the damage done to the body by free radicals. Vitex negundo (Verbenaceae) an aromatic large shrub/small tree of about 3 m in height with quadrangular branches, leaves opposite, flowers bluish purple, fruits ovoid, four seed drupe, black when ripen traditional used for spleno hepatomegaly, inflammations, ulcers, wounds and arthritis. The present study aimed to evaluate the free radical scavenging potential of Vitex negundo.
Materials and Methods
Chemicals
The chemicals DPPH (1, 1-diphenyl-2-picylhydrazyl), Griess reagent, butylated hydroxyl anisole (BHA), Curcumin, were obtained from Sigma, St. Louis, MO, USA. Sulphanilamide, ferric chloride, phosphoric acid, EDTA (Ethyline diamine tetra acetic acid), dimethyl sulfoxide and naphthyl ethylenediamine dihydrochloride used were of analytical grade.
Plant collection and Extraction
Leaves of Vitex negundo were collected, identified and authenticated by Taxonomist and voucher specimen, was deposited. Shade dried and coarsely powdered leaves of Vitex negundo (1kg) was extracted successively with methanol at room temperature for 48 hrs respectively. The extracts were filtered and concentrated under reduced pressure using rotary evaporator to get complete dried extract (VNM). The yield of the leaves crude extracts were 80 g.
Nitric oxide scavenging activity (Green et al., 1982)
About 3 ml of 10 mM sodium nitroprusside in phosphate buffer was added to the methanol extract (VNM) and reference compound in different concentrations (5, 10, 25, 50 and 100 mg/ml). The resulting solutions were then incubated at 25oC for 60 minutes. A similar procedure was repeated with methanol as a blank, which served as control. To 5 ml of the incubated sample, 5 ml Griess reagent (1% sulphanilamide, 0.1% naphthylethylene diamine dihydrochloride in 2% H3PO4) was added. The absorbance of the chromophore formed was measured using a spectrophotometer (Hitachi 2010) at 546 nm. All tests were performed in triplicate. Percent inhibition of the nitric oxide generated was measured by comparing the absorbance values of control and test preparations. Curcumin was used as a reference material.
DPPH Radical Scavenging Activity
The free radical scavenging activity of methanol extract and standard reference compound was analyzed by the DPPH assay as described by Sanchez-Moreno et al., (1998) with minor modification. In this assay, 1 ml of varying concentrations (5, 10, 25, 50 and 100 mg/ml) of VNM, dissolved in methanol and mixed with 1 ml of methanol solution of DPPH (0.2 mM). The mixture was vortexed and incubated for 30 min. The optical density of the solution was the measured at 517 nm using Hitachi 2050 spectrophotometer. BHA (μg/ml) has been used as standard reference.
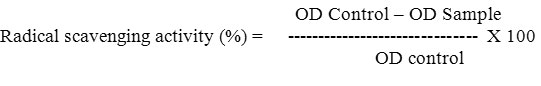
High Pressure Liquid Chromatography (HPLC)
The dried methanol extract of Vitex negundo was prepared at a concentration of 100µg/ml. The extract was then filtered through 0.2 µm syringe filter. The filtered extract was taken for HPLC analysis. An isocratic HPLC (Shimadzu HPLC Class VP series) with one LC-10 AT VP, pump (Shimadzu), variable wavelength UV-Visible Detector SPD-10 A VP, (Shimadzu), and reverse phase Gemini 5u C 18 110A, Phenomenex column (250 x 4.60 mm) was used. The mobile phase components were acetonitrile and water in the ratio of 60:40 was filtered through 0.2 µ membrane filter before use, and pumped from the solvent reservoir at a flow rate of 0.5 ml/min, which yielded column back up, with a pressure of 160-170 kgf/cm2. The column was maintained at 27°C. 20µl of sample was injected into the column using a syringe (Bonaduz Schweiz, Hamilton).
Phytochemical analysis
Phytochemcial screening was carried out by using standard procedure (Edeogal et al., 2005).
Statistical Analysis
Comparison between control and drug treated groups were analyzed by SPSS software package, Version 11.5 with Student t– test. *p<0.05 was considered to be significant.
Results
Free radical scavenging effects of Vitex negundo on DPPH and NO radicals were tested and the results are given below.
Phytochemical analysis
The phytochemical analysis of methanol extract of Vitex negundo showed the presence of flavonoids, terpenoids and carbohydrate (Table 1).
Table 1: Preliminary phytochemical analysis of Vitex negundo Methanol extract
Plant |
Terpenoids | Steroids | Alkaloids | Carbohydrates | Tannins | Flavonoids |
Vitex negundo |
+ | – | – | + | – | + |
Nitric Oxide Scavenging Activity
From the result it was observed that nitric oxide generated from sodium nitroprusside was scavenged by Vitex negundo and the result of this assay showed nitric oxide scavenging effect from 15 mg/ml, moreover the scavenging effect was not dose dependent (Table 2).
Table 2: Scavenging activity of curcumin, VNM on Nitric oxide generated free radicals.
| Concentraton (mg/ml) | % Inhibition of Nitric oxide radical | |
| Curcumin | VNM | |
| 5 | 60.3 ± 6.0 | 47.1 ± 5.2 |
| 10 | 65.1 ± 4.0 | 49.9 ± 5.7 |
| 15 | 65.8 ± 2.0 | 61.5 ± 4.7* |
| 20 | 68.4 ± 5.7 | 63.3 ± 3.2 |
| 25 | 77.2 ± 5.2 | 66.4 ± 3.3 |
(Mean ± S.E) (n = 3); * <0.05 significant, VNM – Vitex negundo Methanol extract
Inhibition of DPPH radical
DPPH is an easy, rapid and sensitive method for the antioxidant screening of plant extracts. The present study investigated the scavenging activity of Vitex negundo, and expressed in percentage of inhibition of DPPH free radicals using BHA as standard reference. Vitex negundo showed significant free radical scavenging activity generated by DPPH. Scavenging activity was observed from 15mg/ml to 25mg/ml (56%, 68%, and 76%). Since more than 50% of DPPH radical inhibition is considered to be significant, the inhibition was observed from 15mg/ml (Table 3).
Table 3: Radical scavenging activity of BHA, VNM on DPPH free radicals.
| Concentraton (mg/ml) | % Inhibition of DPPH radical | |
| BHA | VNM | |
| 5 | 55.6 ± 3.0 | 6.6 ± 1.5 |
| 10 | 71.3 ± 2.0 | 11.3 ± 1.1 |
| 15 | 82.0 ± 3.0 | 56.0 ± 1.0* |
| 20 | 92.0 ± 3.0 | 68.0 ± 2.0* |
| 25 | 91.0 ± 2.0 | 76.6 ± 1.5 |
(Mean ± S.E) (n = 3); * <0.05 significant, BHA – Butalyated hydroxyl anisole, VNM – Vitex negundo Methanol extract
High Pressure Liquid Chromatography
HPLC analysis of Vitex negundo extract showed three distinct peaks (Figure 1). The compounds stick to columns in high aqueous mobile phase and were eluted from columns with high organic mobile phase. The separation of compounds was based on their hydrophobic character.
 |
Figure 1: HPLC analysis of Vitex negundo methanol extract.
|
Discussion
Phytotherapy is based on the active principles contained in plants. In the present study phytochemical analysis of Vitex negundo showed the presence of flavonoids. Flavonoids are a group of polyphenolic compound with free radical scavenging inhibition of hydrolytic and oxidative enzymes (Frankel, 1995). This phytochemical result prompted us to study the free radical scavenging activity. Anti-oxidant plays an important role in inhibiting and scavenging radicals, thus providing protection to humans against infection and degenerative diseases.
DPPH is characterized as stable free radicals by virtue of the delocalization of the spare electron where the molecule as a whole, so that the molecule do not dimerise, as would be the case with most other free radicals. The delocalization gives rise to the deep violet color, characterized by an absorption band (517 nm) in methanol solution. When a solution of DPPH is mixed with a substance of H+ donor, it gets reduced into non-radical state (Diphenyl picryl hydrazine). Hence, the significant decrease in free radical can be attributed to the scavenging ability of Vitex negundo. Similarly Srinivas Reddy et al (2008) reported DPPH scavenging effect from the methanol extracts of Holoptelea integrifolia leaves and stem bark. Ramanathan et al (2006) reported antioxidant and free radical scavenging activities of methanol extracts of Careya arborea by using DPPH, superoxide anion radical, nitric oxide radical and hydroxyl radical scavenging assays. Nitric oxide was generated from sodium nitroprusside and measured by the Greiss reduction. Sodium nitroprusside in aqueous solution at physiological pH spontaneously generates nitric oxide, which interacts with oxygen to produce nitrate ions that can be estimated by use of Greiss reagent. Scavengers of nitric oxide compete with the oxygen, leading to reduced production of nitric oxide (Govindarajan et al 2003).
In our study the generation of free radicals from nitric oxide was significantly inhibited by Vitex negundo. This study indicated that V. negundo has promising free radical scavenging activity hence could be used as antioxidant.
Conclusion
From the investigation it was concluded that the leaf extract of Vitex negundo posses antioxidant properties and further study was aimed to isolate the bioactive compound.
Reference
- Cook C., Samman S., 1996. Flavonoids- chemistry, metabolism, cardio protective effects, and dietary sources. Nutritional Biochemistry, 7, 66- 76.
- Edeogal H.O., OKWU D.E., and Mbaebie Bo., 2005. Phytochemical constituents of some Nigerian medicinal plants. African J. Biotechnol. 4, 685-688.
- Frankel E., 1995. Nutritional benefits of flavonoids. International conference on food factors: Chemistry and cancer prevention, Hamamatsu, Japan. Abstracts, C6- 2.
- Govindarajan R., Rastogi S., Vijayakumar M., Shirwaikar A., Rawat A.K., Mehrotra S., 2003. Studies on antioxidant activities of Desmodium gangeticum . Biol Pharm Bull, 26, 1424-7.
- Green L., Wagner D.A., Glogowshi J., 1982. Analysis of nitrate, nitrite and nitrate in biological fluids. Anal Biochem. 126, 131 – 138.
- Halliwell B., Gutteridge J.M.C., 1989. Free radical in biology and medicine. Oxford: Clerendon.
- Kumpulainen J.T. and Salonen., 1999. Natural Antioxidants and Anticarcinogens in Nutrition, Health and Disease, The Royal Society of Chemistry, UK, pp 178- 187.
- Ramanathan Sambath Kumar., Thangavel Sivakumar., Rajagopal Shanmuga., Dhanapal., Kavimani., Swamy., Malaya, Basu., 2006. Antimicrobial and Antioxidant activities of Careya arborea Roxb. Stem Bark. Iranian Journal of Pharmacology and Therapeutics, 5, 35-41.
- Sanchez-Moreno., Larrauri J.A., Saura- Calixto F., 1998. A procedure to measure the antiradical efficiency of polyphenols. J. Sci. Food Agric., 76, 270-276.
- Srinivas Reddy B., Kiran Kumar Reddy R., Naidu V.G.M., Madhusudhana, Sachin K., Agwane B., Ramakrishna S., Prakash and Diwan , 2008. Evaluation of antimicrobial, antioxidant and wound healing potentials of Holoptelea integrifolia. J. Ethnopharmacol., 115, 249-256.
- Younes M., 1981. Inhibitory action of some flavonoids on enhanced spontaneous lipid peroxidation following glutathione depletion. Planta Medica, 43, 240- 245.

This work is licensed under a Creative Commons Attribution 4.0 International License.





