Manuscript accepted on : September 08, 2010
Published online on: 28-12-2010
Khalid M. Al-Ghamdi*, AbdulRahman A. FarajAllah and Habeeb M. Al-Solami*
Department of Biological Sciences, Faculty of Science, King Abdul-Aziz University, P.O Box: 80203, Jeddah, 21589 Saudi Arabia.
ABSTRACT: The aim of this study is to investigate the effect of two organophosphorous insecticides (Malathion and Chloropyrifos), and a member of the third generation insecticides that belong to the Insect Gross Regulators(IGRs), namely Dimilin (Diflubenzuren, DFB, or TH6040) on the larval stages of the housefly Musca domestica L. which is a chitin synthesis inhibitor replicated three times. Data showed that dimilin gave high larval and pupal mortality rates coupled with pronounced morphological abnormalities and deformations, which are displayed and in immature and adult stages as reduction of thorax and abdominal segments leading to a high rate of emergence inhibition in the adult stage. The high dosages of both organophosphorous insecticides gave 99% larval mortality, however, the recommended low doses gave mortality rates of Malathion and Chloropyrifos as (87% , 72%) and (84%,78%), respectively. Statistical analysis of data(ANOVA) and Latin square showed no significant differences between replicates for the house fly population due to the stability of larval mortality for all applied dosages.
KEYWORDS: Malathion; Cloropyrifos; insecticides; Dimilin (Difluobenzuron DFB); house fly
Download this article as:| Copy the following to cite this article: Al-Ghamdi K. M, FarajAllah A. A, Al-Solami H. M. Evaluation of Two Conventional Insecticides and a Chitin Synthesis Inhibitor against Immature Stages of the House Fly Musca Domestica L. (Diptera: Muscidae). Biosci Biotechnol Res Asia 2010;7(2) |
| Copy the following to cite this URL: Al-Ghamdi K. M, FarajAllah A. A, Al-Solami H. M. Evaluation of Two Conventional Insecticides and a Chitin Synthesis Inhibitor against Immature Stages of the House Fly Musca Domestica L. (Diptera: Muscidae). Biosci Biotechnol Res Asia 2010;7(2). Available from: https://www.biotech-asia.org/?p=8842 |
Introduction
Literature Review
The most preferred foci of infestation for the dipterous flies depend on type of water and habitats where they reproduce and multiply or in adjacent areas in proximity based on the species although most of these environments varies between freshwater and salty (marine, or between pure, contaminated or highly organic water). The house fly M. domestica L. is a well known notorious cosmopolitan pest. It has a worldwide distribution and is found throughout the kingdom of Saudi Arabia in close association with human activities (houses, residential quarters, animal and poultry holdings, drainage and sewage treatment facilities and well-established as widespread pasture and rangeland pests (Tomberlin et al., 2002). The house fly receives the common name by virtue of being the common fly found in and around houses and other domestic dwellings (Moon, 2002). Oviposition occur on places like dumpsites and waste collection or in neighboring suitable habitats, hence, the emerging larvae develop to pupal and adult stages. The favorable habitats for fly multiplication varies from one species to another, though some prefer special requirements distinguished by having thick vegetational cover, however, others prefer organic swamps, or valleys or open desert oases. Some of these flies are diurnal others are crepuscular or nocturnal. It was well known that most female flies especially after mating will be in great need for a diet made of liquid material or decomposing organic matter before they started ovipositiona and deposition off eggs ready for hatching to complete their life cycles. Some of the piercing sucking flies supply their demands of blood by piercing or sucking blood from open wounds of birds or rodents then suck blood from a variety of animals that are prevalent in their local environment (Oldroyd and Smith, 1972; Scott, 1964 and Pedigo, 1989).
In addition to being a nuisance pest, it is a dangerous vector of many parasites and pathogens. It is actually an active carrier of over 100 different pathogenic organisms that cause diseases, especially typhoid, cholera, bacillary dysentry, tuberculosis, anthrax and infantile diarrhea as well as a multitude of a collection of bizarre parasitic worms. Most of the avenues of infection are related to the fact that these dangerous parasitic and/or pathogenic organisms might be picked up by flies from garbage, sewage and other waste sources of filth then brought inside homes ,settle on human food mechanically from contaminated external body parts or through vomiting and defecation while feeding on food.
Most of the dipterous flies have international or global distribution specially the house fly M. domestica L., the stable fly Stomoxyes calictrans L., the flesh fly Sarcophaga sp. albeit there are many genera including Chrysops and Tabanus in the temperate equatorial regions, however the tse tse fly Glossina sp is limited only to equatorial Africa between latitudes 15° North extending to 30° South all along the coastal (Sahelian) region of East Africa (1984).
The order Diptera is comprised from huge groups of fly species and most of these species have their life cycles specially connected with variegated bizarre environments including waste, dump piles rich in organic matter or undecomposed human waste and excreta or on waste and manure of domestic animals or the decayed fruits and vegetables or decomposing dead animal bodies or even stagnant water or waste drainage which is rich with fly larval stages in particular larvae of the house fly M. domestica, Phormia regina, vinegar fly Drosophila spp, the flesh fly Sarcophaga spp., fly Eristakis tenax, the drainage fly Sycholanspp.(Olkowski et al., 1991; Saunders and Hayward, 1998).
The most widespread insecticides applied in combat of these flies included the pyrethroids, organophophorous insectices, usually malathion and diazinon. Special ropes and tapes saturated with chemical insecticides viz. diazinon or parathion normally used where many crowds of flies observed to be stucked dead. Furthermore, some of the chemical population suppression and management of these flies included attractants,poisoned (toxic) baits specifically using malathion, diazinon and dichloropyfos baits. Malathion was most distinguished and used because of its low residual effects with the environment on a long-term basis (Thompson, 1977; Drummond et al., 1988 and Pedigo, 1989), srinivasan et al., 2008).
The chemical control of house flies could be described as fast, rapid causing high degree of mortality on fly populations in short period of time. But unfortunately, these applications of wide spectrum chemicals where not without unwanted unwelcomed negative effects due to the fact that continued on a long term basisof applications has led to the building up of resistance on the following generations and this is further complicated by the chemical pollution specially from those insecticides that has long term effect, i.e: long residual insecticides on the environment and is evidently contaminate the overall ill effects on human health, animals and plants. Therefore it is more apt and advisable to select and use those effective insecticides that are characterized by short residual effects and distinguished by less degree of negative effect in the environment.
Through time many management and control approaches have been adopted to curb the flaring up and outbreaks of house flies population so as not to reach the epidemic levels and with the objective of saving human health and comfort. A wide range of control measures including sanitation use of traps and so many organophosphorous and organochlorine insecticides were usually applied. Some logical rational approaches has yielded an appreciable success by establishing the integrated control of pest management. Due to the onslaughts emanating from blanket spraying with persistent chemicals has led to the development of resistance in the population of horse flies thus ,necessitated and stimulated the evaluation of safer alternatives that put safer environment first and reduce level of toxicity. Traditional or novel methods of control has been enacted and operated including ,biological pest suppression or use of the promising third generation insecticides including the hormones and insect growth regulators (IGRs)or chitin inhibitors that has gained lots of praise due to their positive results after their applications in some successful management programs (Silva et al., 2000, Rina Tilak et al. 2010)
Amongst the Insect Growth Regulators (IGRs, the chitin inhibit Dimillin (Diflubenzuron or Benzoyl-phenylurea) has shown novel mode of action an produced effective results on different house fly strains. It’s inhibition activity known to evoke acceptable ovicidal effect through topical application on adult females or through oral uptake and showed higher rate of early instars mortality or through reproductive inhibition activity (Tomberlin et al., 2007). No wonder it has been accepted by renowned international organizations as an important factor in the total integrated management in fly control .Therefore (Dimilin, diflubenzuron, DFB and TH6040) registered formulations were recommended as efficient fly control methods authenticated by FAO, WHO and EPA (Environmental Protection Agency) of the United States as food additive for larval control in beef and dairy cattle, horses pens as an overall sound strategies and rational logistics for fly control. It has produced 97-100% effectiveness for inhibiting the development of adult houseflies and stable flies in horses treated manure (Anon.2009 a,b,c).
The present study was conducted to evaluate the effect of Dimilin (Diflubenzuron, DFB), a chitin synthesis inhibitor as a house fly larvicide as compared with two organophosphorous insecticides routinely sprayed on house fly populations to control their population numbers.
Materials and Methods
Laboratory investigations were conducted to test the effects of commonly used chemical insecticides against the different stages of the house fly populations. These insecticides include two classical and/or traditional chemical insecticides (malathion and chloropyrifos) where their mode of action is through their contact activity and both insecticidses were applied on house fly larvae with three dosages. The higher recommended dose was 400cm³/100 Liter (water) at the rate of 0.4% and the recommended dose was 200 cm³/liter (water) at the rate of 0.2%. The third lowest dose was at 100cm³/100L (water) at the rate of 0.1%.
The third chemical applied was non-classical non-traditional which is an insect growth regulator and a member of the third generation insecticides with novel mode of action manifested and displayed as a chitin inhibitor during metamorphosis represented by Dimilin/Diflorobenzuron (DFB). Five different dosages were used in this investigation from lowest to highest. These experimental dosages were first prepared by dissolving the active ingredient of Dimilin (Wettable powder) in acetone then water was added to arrive at the desired dosages. The applied dosages were made possible by adding to each respective active ingredient dose 100ml of water.
The lowest dose of toxicity (0.65mg) prepared by adding 6.50 mg of Dimilin to 100 ml of water . The second dose (1.2mg) was prepared by adding 12.50mg to 100 ml of water. The third dose (2.5mg) was prepared by adding 25mg to 100 ml of water. The 4th dose (5.0mg) was prepared by adding 50mg to 100 ml of water. Finally the highest dose (10mg) was prepared by adding 100mg to 100 ml of water.
Dimilin was used against all house fly immature including all larval stages and the pupal stage together with the adult stage by using 3 replications of the desired 5 dosages on 50 house fly larvae replicated 3 times. The overall effects of Dimilin on these larvae was followed up and recorded until the emergence of adult flies.
The 3 insecticides under investigation viz (Malalhion, Chloropyrifos and Dimilin) were all applied using the hand sprayer under high volume spray twice each fortnight for all respective concentration’s. Critical observations were recorded on the (larral, pupal and adult) mortalitiy rates especially those treated with Dimilin applications with the objective of determining its insecticidal activity on all the immature stages. The insecticidal effects of both malathion and chloropyrifus were determined on the house fly adults based on their contact mode of action.
Results and Discussion
Prior to the onset of these laboratory investigations pilot bioassay studies using chemical treatment were conducted using both organophosphate insecticides viz malathion and chloropyrifos. The third insecticide, the member of the novel non-conventional insecticide Dimilin(diflubenzuron) (DFB) an analog or derivative from natural origins as a chitin inhibitor in the cuticle of all arthropods was also bioassyed. The different concentrations of Dimilin were(0.65,1.25,2.5,5.0 and 10.0mg) has shown variable effects as recorded from the over-all effects of Dimilin on the different life stages. The lowest dose of Dimilin viz 1.25mg has yielded/percentage mortality about 52%, however fatality and mortality rate seemed to increase as the concentration of Dimilin increases as shown in Table1. Figure 1. Shows the gradual effects due to the increase in Dimilin concentration on 50 house fly larrval mortality occurred at the concentration 10mg which gave 98% mortality and decreased the total larval population that has been treated with Dimilin. The lowest mortality of house fly larvae by Dimilin was at the lowest dose 0.65mg whereas the mortality rate of the overall larrval population was 50% at the dose 1.25mg. The percentage of larval survival which
Table 1: The Overall Effect of Different Dosages of the (IGR) Dimilin on 50 house fly Larvae in Petri dishes/treatment in 3 replicated.
| Replications | Dosages in Mg | ||||||||||||||||||
| Control | 0.65 | 1.25 | 2.5 | 5.0 | 10 | ||||||||||||||
| L
DL |
P
LP |
A | L
DL |
P
LP |
A | L
DL |
P
LP |
A | L
DL |
P
LP |
A | L
DL |
P
LP |
A | L
DL |
P
LP |
A | ||
| 1 | Effect of Insecticide Dose | 4 | 41 | 5 | 16 | 24 | 10 | 26 | 14 | 10 | 40 | 10 | – | 45 | 5 | – | 50 | – | – |
| Mortality Rate | 8% | 32% | 52% | 80% | 90% | 100% | |||||||||||||
| 2 | Effect of Insecticide Dose | 3 | 44 | 3 | 18 | 22 | 10 | 28 | 12 | 10 | 38 | 6 | 6 | 44 | 6 | – | 48 | 2* | – |
| Mortality Rate | 6% | 36% | 56% | 76% | 88% | 96% | |||||||||||||
| 3 | Effect of Insecticide Dose | 5 | 42 | 3 | 16 | 24 | 10 | 30 | 13 | 7 | 38 | 8 | 4 | 43 | 3 | 4* | 46 | 3* | 1 |
| Mortality Rate | 10% | 32% | 60% | 76% | 86% | 92% | |||||||||||||
| Overall Mortality Rate | 8% | 33% | 56% | 77% | 88% | 96% | |||||||||||||
L: larva DL: dead larva A: adult fly P: pupa LP: live pupa
*Some of the other flies emerge from pupae but showed morphological abnormalities.
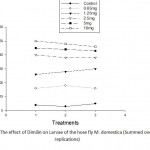 |
Figure 1: The effect of Dimilin on Larvae of the hose fly M. domestica (Summed over replications).
|
 |
Figure 2: Effect of Dimilin on house fly pupae.
|
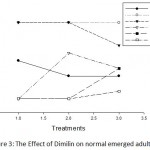 |
Figure 3: The Effect of Dimilin on normal emerged adults.
|
Both Fig.4 and Fig.5 show the effects of Dimilin concentrations 2.5mg and 5mg on the second stage larvae of house flies . These effects were characterized by some black spots on the larval cuticle manifested as cracks on the anterior part of the larval body specially the head and the thorax. Fig.5 shows that increase in the Dimilin rate i.e. 5mg manifested as complete deformation on the larval cuticle which might be due to longer larval survival (larval stage) leading to their fatality or at least being unable to complete their metamorphosis to pupal stage with expected deformations during the pupal stage (Fig.6).
 |
Figure 4: The Effect of Dimilin at 2.5mg on house flies M. domestica.
|
 |
Figure 5: The Effect of Dimilin at 5mg on house flies M. domestica.
|
 |
Figure 6: The Effect of Dimilin at 2.5mg on pupae of house flies deformed pupa M. domestica.
|
Fig.7 shows deformed pupal stage due to the treatment with Dimilin at 5mg dose which led to more deformities characterized by having unsclerotized cuticle due to the inhibition of chitin desposition thus being very thin, transparent and subjected to tear during some pupal activity.
 |
Figure 7: The Effect of Dimilin at 5mg on pupae of house flies M. domestica.
|
Fig.8 shows the deformed folded small size wings on adult house fly emerging for larvae that has been treated with Dimilin at the rate of 2.5mg that will hinder adults ability to fly.
 |
Figure 8: The Effect of Dimilin at 2.5mg on emerging abnormal adult house flies M. domestica.
|
Fig.9. shows the effect of 5mg Dimilin concentration applied to house fly larvae which hampered the emergence of the deformed adult from its pupal coccon. It has been observed that all stages of house fly larvae treated with different rates of Dimilin showed clearly most of the abnormalities and the already reported deformations by many worker in all life stages. The obtained results were in agreement with what was reported earlier from previous studies conducted by many workers on Dimilin on various dipterous flies in a variety of situations on larvae feeding on animal manure treated with diflubenzuron at concentrations 1mg and 5.1mg leading to larval pupal deformations, abnormalities and evidently death (Wright et al., 1975., Gaaboub et al., 1981; Hansen and Gartan, 1982, Grzywinski et al., 1984; Kocisova et al., 200; Silva and Mendes, 2002).
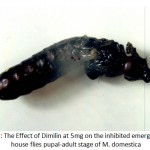 |
Figure 9: The Effect of Dimilin at 5mg on the inhibited emergence of house flies pupal-adult stage of M. domestica.
|
It is evident from this study the positive insecticidal effect of Dimilin which is considered one of the most novel chitin inhibitors that has reached commercial level.
Among many of its advantages that it does not accumulate to high levels in the food chain making it a good candidate to be used widely against dipterous pests in animal and poultry holdings and against the notorious mosquitoes especially at low dosages (Olkoweski et al., 1991; Amondi, 1993, 1997, Anen. 2009 a, b, c).
The effects of the organophosphate insecticides Malathion and chloropyrifos on larvae of house flies which were applied at 3 concentrations (high dose at the rate 0.4%cm³/Liter of water, the recommended dose at the rate of 0.2%cm³/liter of water and the lowest dose at the rate of 0.1% cm³/liter of water), replicated 3 times on 50 larval instars with different ages. The respective dosages of chemical were applied on soil mixed with animal manure.The recovered data showed that the highest dosages of both insecticides has yielded an average mortality rate of 91%, however, the recommended dose for both malathion and chloropyrifors yielded an average mortality rate of 87%, respectively. The lowest doses of both chemicals gave an average of mortality of 72% and 78% respectively (Table 2).
Table 2: The Effect of different dosages of insecticides on 50 house fly larvae in a Petri dish (each treated differently)
| Insecticide Dosages (cm³) | Control | Replicate | Insecticide | ||
| rate
0.01% 400 cm³/100L of water |
rate
0.2% 400 cm³/100L of water |
rate
0.4% 400 cm³/100L of water |
|||
| 68% | 88% | 92% | 8% | I | Malathion |
| 84% | 86% | 90% | 10% | II | |
| 66% | 87% | 91% | 9% | III | |
| 72% | 87% | 91% | 9% | Average Mortality Rate | |
| 76% | 82% | 88% | 6% | I | Chloropyriforus |
| 80% | 86% | 94% | 10% | II | |
| 80% | 85% | 92% | 9% | III | |
| 78% | 84% | 91% | 8% | Average Mortality Rate | |
Figs 10, 11 show the effect of both insecticides on house fly larvae compared with control where the highest dose yielded highest mortality rate, however when compared with the untreated control hence, the natural larval mortality in the mixed soil with manure never exceeds 10%. It is therefore suggested that it would more rational to start applying the lowest dose then a gradual dose increase based on the density of fly population in their habitats and foci of multiplication. Because both insecticides were widely used internationally based on their low effects in the environment when compared with other insecticides but caution must be exercised not to exceed their rate more than 0.4% cm³/ liter of water. More ribbons impregnated and saturated with these chemicals have been commonly used in fly population control situations especially in animal farms.
 |
Figure 10: The Effect of Malathion for 3 replicates on different dosages on 50 housefly larvae
|
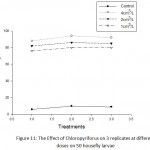 |
Figure 11: The Effect of Chloropyriforus on 3 replicates at different doses on 50 housefly larvae
|
It has been documented that the continued use of insecticides as a unilateral approach for control of pests species has led to the build-up of resistance in the following generations exhibited as resistant strains where their populations cannot be reduced by the same repeated insecticides. However the resistance depends on the dosages of the selective pressure in addition to the biology of the targeted insect and the higher tendency of these strains for building the resistance as a resistant factor in these insect genome (Pap and Farka’s, 1994, Kim-Gil. Hah et al., 1997 and Li-Zhang et al., 1998.
It is recommended that these insecticides should be applied alternatively to reduce the chances for resistant strains to appear. The analysis of variance (ANOVA) and chi square test for the determination of the effect of the different treatments showed that there were no significant differences between replicates which indicated the stability of the larval mortality by using the different dosages for these insecticides (Tables 3, 4, 5).
Table. 3: Statistical Analysis of variance and Latin Square for the effect of different dosages of Dimilin on house fly larvae.
| Source of Variation | DF | SS | MS | F | P |
| Between treatments | 2 | 3.111 | 1.55 | 0.00133 | 0.999 |
| Residual | 12 | 17532.0 | 1168.8 | ||
| Total | 17 | 17535.11 |
Chi square test (c2) = 1.270
P > 0.9
Table.4: Statistical analysis of variance and Latin Square for the effect of different dosages of malathion on house fly larvae
| Source of Variation | DF | SS | MS | F | P |
| Between treatments | 1 | 24.500 | 24.500 | 0.0164 | > 0.902 |
| Residual | 6 | 8919.00 | 1489.833 | ||
| Total | 7 | 8963.500 |
Chi square test (c2) = 1.580
P > 0.66
Table 5: Statistical Analysis of variance and Latin square for the effect of different dosages of chloropyrifus on house fly larvae.
| Source of Variation | DF | SS | MS | F | P |
| Between treatments | 1 | 40.500 | 40.500 | 0.0273 | 0.874 |
| Residual | 6 | 8911.00 | 1485.167 | ||
| Total | 7 | 8951.500 |
Chi square test (c2) = 0.776
P > 0.855
From the statistical analysis, it is rationally advisable to use lower dosages at the onset of chemical application then raise the concentration as the fly season progressed with the buildup of fly population in their habitats and foci of reproduction in waste bins ,dump sites, drainage and cess pools to reduce the housefly population before reaching levels of being problems for human activities.
Acknowledgements
The authors wish to extend their immense gratitude and profound thanks to the Entomology Section Staff at the Department of Biological Sciences, College of Science at King Abdul-Aziz University for their technical cooperation and kind help. Also many thanks go to Sayed Abdul-Aziz Al-Ghamdi, the Head of the protection health the Jeddah Municipality and his technical staff for their expertise supplying the house fly population for the completion of this investigation.
References
- Ali, S.L.; singh, D,P. and Misra, U.S. (1983): Effectiveness of plant oils against pulse beetles, Callosbruchus chinonsis Linn, Indian. Journal of Entomology. (6); 101-105.
- Amoudi, M.A. (1993): New records of some of sarcophagid flies with distribution of all known flesh flies (Diptera: sarcophagidae) of Saudi Arabia. J. Egypt. Soc. Parasitol., 23 91 297-304.
- Anon. 2009.a. Clarify larvicide on an EPA registered feed additive containing diflubenzuron, a larvicide that control house flies and stable flies in areas populated by confined beef and dairy cattle, 2 papers.
- Anon. 2009.b. Simplify ® part of an integrated fly control before fly season begins when first adults are seen 2 papers.
- Anon. 2009. c. Frequently asked questions, Farnam Horse.com
- Drummond, R.O., J.E. Geroge, and S.E. Kunz. (1988). Control of Arthropod pests of Livestock: a review of technology. CRC. Press, Boca Raton, FL. 245 pp.
- Gaaboub, I.A., Rawash, I.A., El gayar, F.H. and Traboulsi, A.F. (1981): The delayed effects and the basal follicle numbers developed by females of Culex pipens L. emerged from treatments of larvae with partially lethal concentration of altosid and Dimilin. Toxicology; 19(3): 269-274.
- Grzywinski, L.; Romaniuk, K. and Siewinski, A. (1984). Effect of the preparation Dimilin on housefly larvae. Wiad parazytol; 30(3): 375-383.
- Hansen , S.R. and Gartan, R.R. (1982): The effects of diflubenzuron on complex laboratory stream community. Arch. Enviran. Cantam. Toxical; 11(1): 1-10.
- Kim-Gil-Hah; Choi-Yong, Ho; Kim, Jeong-Hwa; Cho-Kwang-Yun. (1997). The resistant level of the house fly, Musca domestica L. to several insecticide from various localities in Korea. Korean J. of Entomology. 27(4): 305-312.
- Kocisova, A.; Venglovsky, J.; Para. L. and Petrovsky, M. (2000). Control of the house flies (Musca domestica) using the insect growth regulator diflubenzuron. Czech. Journal of Animal Science, 45(10): 475-480.
- Li-Zhang; Harda, Kenji and Shono, Toshio. (1998). Cross resistance to insect growth regulators in pyriproxy for resistant housefly. Applied entomology and zoology . 33(1): 195-197.
- Moon, R. 2002. Muscid Flies (Muscidae) In: Mullen, G.R. Durden, L.A. editors. Medical and Vetrinary E Entomology. San Diego: Academic Press, 2002; P. 279-301.
- Oldroyd, H. and Smith, K.G.V. (1973). Chapter 6 “Eggs and Larvae of flies”, 289-323 In: Insect and other Arthropods of Medical Importance, K.G.V. Smith (ed.), (British Museum) Nat: Hist., London, 561 pp.
- Olkowski, W., S. Daar, S. and H. Olkowski. (1991). Common-sense pest control: least-toxic solutions for your home garden, Pets and communit. Taurton Press, Newtown, CT. 715 pp.
- Pap, L., and R. Farkas. (1994). Monitoring resistance of insecticides in housefly (Musca domestica) populations in Hungary. Pesticide Science 40(4): 245-258.
- Pedigo, L.P. (1989). Entomology and pest management. McMillian Publishing Co. New York. 646 pp.
- Rina Tilak, A.K; Verma and Urmila B. Wankhade.2010. Effectiveness of diflubenzuron in control of house flies. J. V Vector Borne Dis. 47, pp97-102
- Sannders, D.S. and Hayward, S.A.L. (1998). Geographical and diapause-related cold tolerance in the blow fly, Calliphora vicina. J. Insect Physiol. 1998, 44: 7-8, 541-551; 24 ref.
- Scott, H.G. (1964). Human myiasis in North America, Fla. Ent. 47. house fly Musca domestica (Diptera. muscidae). J. Am Mosq Control Assoc. 8: 268-272.
- Silva, J.J. and Mendes , J. (2002): Effect of diflubenzuron on immature stages of Haematobia irrituans (L.) Diptera: muscidae) in UberLandia, State of Mians gerais brazil. Men. Inst. Oswaldo. Cruz. Jul; 97(5) 679-82.
- Silva, G.S; Costa, J.; Rocha, U.F, Soares, V.E; Mendes, J. and Yoshida, L. 2000. The efficacy of 25% Dif fed to pop; to control synanthropic flies in the dung. Brazil. J. vet. Parasitol;: (2): 119-23
- Srinivason, R.; Jambulingam, P.; Gunasekaran, K. and Boopathi doss, P.S. 2008. Tolerance of house fly, Musca d domestica L. (Diptura: Muscidae) to dichlorros (76%EC) an insecticide used for fly control in the t tsunami –hit coastal village of Southern India. Acta Trop; 105 (2): 187-90.
- Thomson, W. T . (1977). Agricultural Chemicals, Book 1. Insecticides. Thomson Publications, Fresno, California.
- Tomberlin, J.K; Lohmeyer, K.H. and D. Kattes. 2007. Treatment of pastures with diflubenzuron suppress horn fly Haematabia irritans (Diptera: Muscidae) development. J. Agric. And Urban Entomel. 24 (@): 95-101
- Wright, J .E.; Oehler, D.D. and Johnson, J.H. ( 1975). Control of house fly and stable fly breeding in rhinoceros dung with an insect growth regulator used as a feed additive. J. Wildi Dis. Oct; 11 ( 4): 522-4.

This work is licensed under a Creative Commons Attribution 4.0 International License.





